Abstract
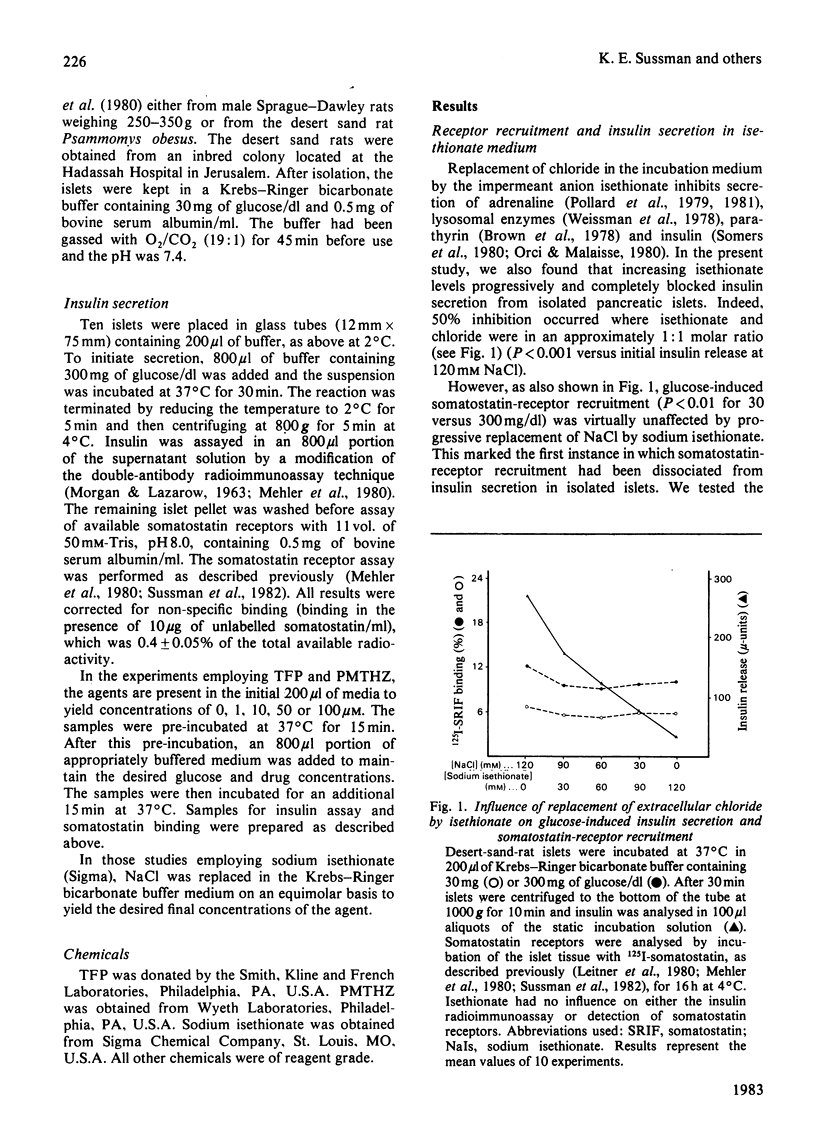
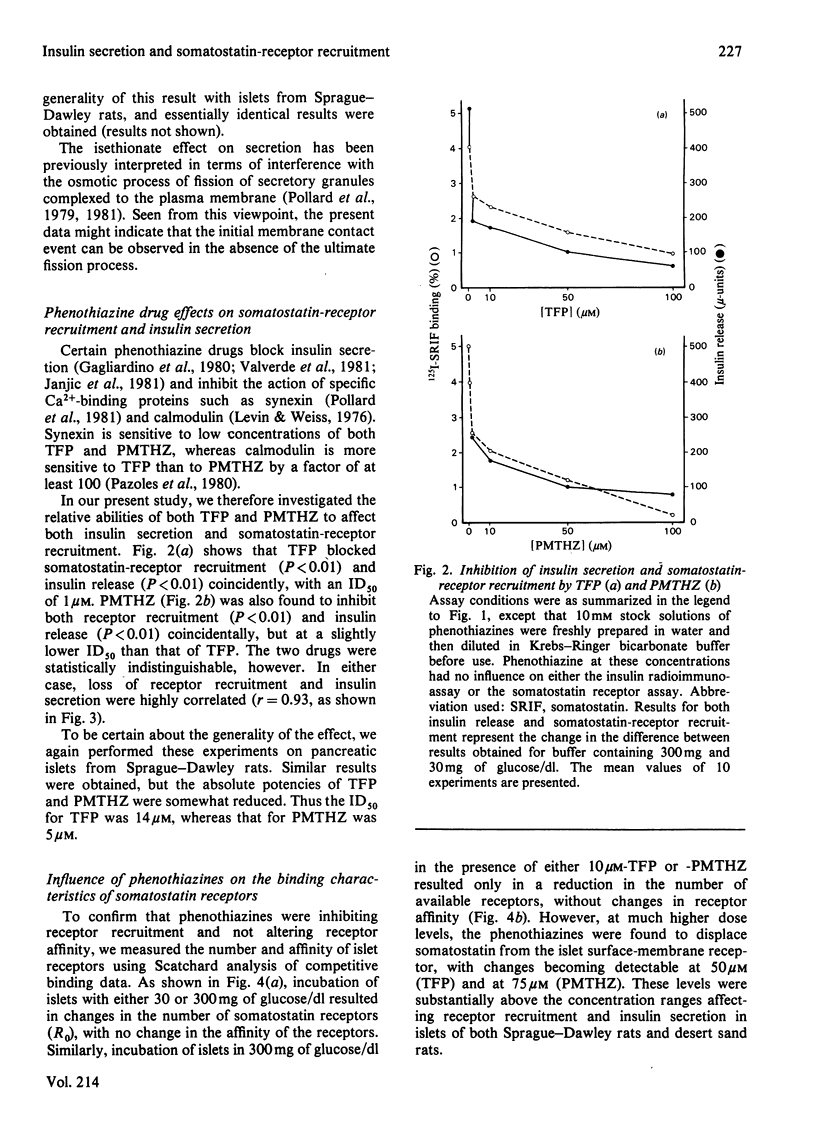
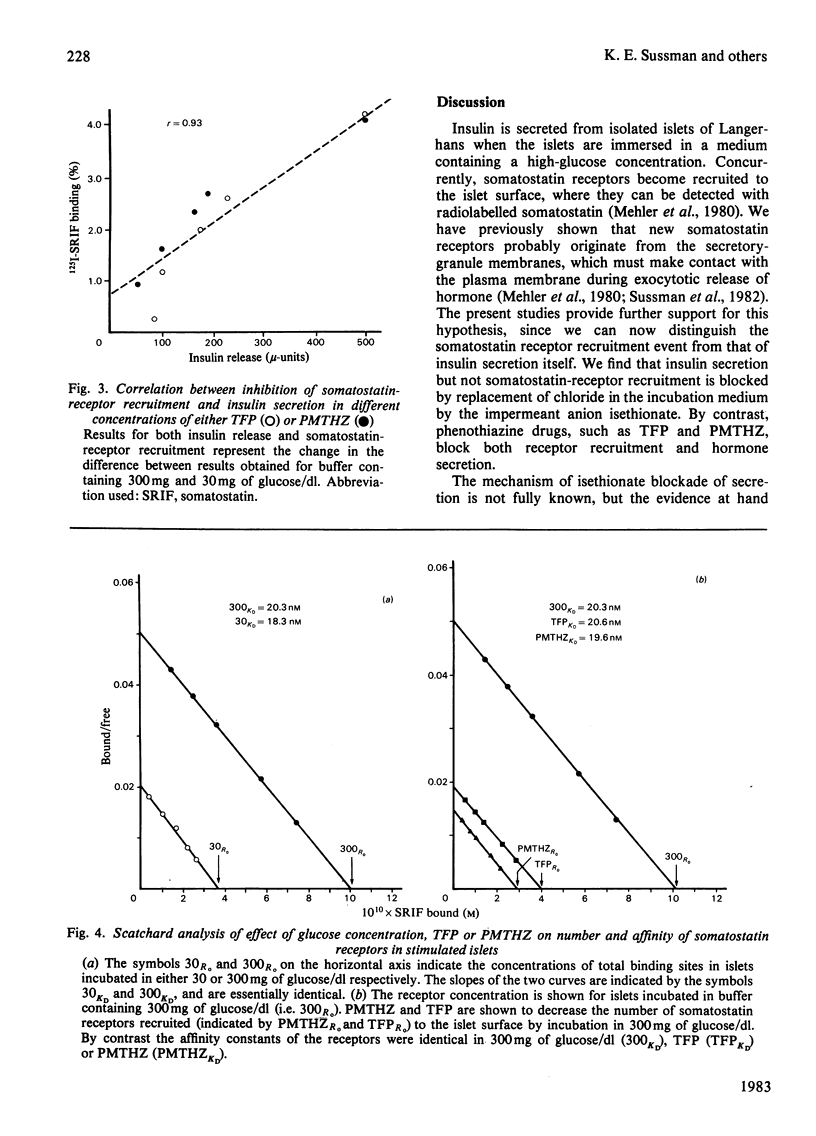
Somatostatin receptors appear to be localized to secretory granules in pancreatic islet homogenates. Recruitment of these receptors to the islet-cell surfaces may mark the contact event between secretory granules and plasma membranes before release of insulin by fission. Isethionate, an impermeant anionic replacement for chloride, blocks the release step but does not affect receptor recruitment. By contrast, low concentrations of phenothiazine drugs, such as trifluoperazine and promethazine, inhibit both receptor recruitment and secretion. Scatchard analysis of phenothiazine effects on somatostatin receptors reveals that these drugs reduce the number of receptors but do not affect the affinity of the receptor for somatostatin. These data indicate that membrane contact and fission steps during exocytosis can be biochemically separated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown E. M., Pazoles C. J., Creutz C. E., Aurbach G. D., Pollard H. B. Role of anions in parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):876–880. doi: 10.1073/pnas.75.2.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Creutz C. E., Scott J. H., Pazoles C. J., Pollard H. B. Further characterization of the aggregation and fusion of chromaffin granules by synexin as a model for compound exocytosis. J Cell Biochem. 1982;18(1):87–97. doi: 10.1002/jcb.1982.240180108. [DOI] [PubMed] [Google Scholar]
- Draznin B., Leitner J. W., Sussman K. E. Kinetics of somatostatin receptor migration in isolated pancreatic islets. Diabetes. 1982 May;31(5 Pt 1):467–469. doi: 10.2337/diab.31.5.467. [DOI] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazum E., Meidan R., Keinan D., Okon E., Koch Y., Lindner H. R., Amsterdam A. A novel method for localization of gonadotropin releasing hormone receptors. Endocrinology. 1982 Dec;111(6):2135–2137. doi: 10.1210/endo-111-6-2135. [DOI] [PubMed] [Google Scholar]
- Janjic D., Wollheim C. B., Siegel E. G., Krausz Y., Sharp G. W. Sites of action of trifluoperazine in the inhibition of glucose-stimulated insulin release. Diabetes. 1981 Nov;30(11):960–966. doi: 10.2337/diab.30.11.960. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leitner J. W., Rifkin R. M., Maman A., Sussman K. E. The relationship between somatostatin binding and cyclic AMP-stimulated protein kinase inhibition. Metabolism. 1980 Oct;29(11):1065–1074. doi: 10.1016/0026-0495(80)90218-8. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Mehler P. S., Sussman A. L., Maman A., Leitner J. W., Sussman K. E. Role of insulin secretagogues in the regulation of somatostatin binding by isolated rat islets. J Clin Invest. 1980 Dec;66(6):1334–1338. doi: 10.1172/JCI109986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malaisse W. Hypothesis: single and chain release of insulin secretory granules is related to anionic transport at exocytotic sites. Diabetes. 1980 Nov;29(11):943–944. doi: 10.2337/diab.29.11.943. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pazoles C. J., Creutz C. E., Ramu A., Pollard H. B. Permeant anion activation of MgATPase activity in chromaffin granules. Evidence for direct coupling of proton and anion transport. J Biol Chem. 1980 Aug 25;255(16):7863–7869. [PubMed] [Google Scholar]
- Pollard H. B., Creutz C. E., Fowler V., Scott J., Pazoles C. J. Calcium-dependent regulation of chromaffin granule movement, membrane contact, and fusion during exocytosis. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):819–834. doi: 10.1101/sqb.1982.046.01.077. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Zinder O. The chromaffin granule and possible mechanisms of exocytosis. Int Rev Cytol. 1979;58:159–197. doi: 10.1016/s0074-7696(08)61475-8. [DOI] [PubMed] [Google Scholar]
- Poste G., Reeve P. Inhibition of cell fusion by local anaesthetics and tranquillizers. Exp Cell Res. 1972 Jun;72(2):556–560. doi: 10.1016/0014-4827(72)90029-8. [DOI] [PubMed] [Google Scholar]
- Rosenzweig L. J., Kanwar Y. S. Dopamine internalization by and intracellular distribution within prolactin cells and somatotrophs of the rat anterior pituitary as determined by quantitative electron microscopic autoradiography. Endocrinology. 1982 Dec;111(6):1817–1829. doi: 10.1210/endo-111-6-1817. [DOI] [PubMed] [Google Scholar]
- Somers G., Sener A., Devis G., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XLV. The anion-osmotic hypothesis for exocytosis. Pflugers Arch. 1980 Dec;388(3):249–253. doi: 10.1007/BF00658490. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Mehler P. S., Leitner J. W., Draznin B. Role of the secretion vesicle in the transport of receptors: modulation of somatostatin binding to pancreatic islets. Endocrinology. 1982 Jul;111(1):316–323. doi: 10.1210/endo-111-1-316. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Valverde I., Sener A., Lebrun P., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XLVII. The possible role of calmodulin. Endocrinology. 1981 Apr;108(4):1305–1312. doi: 10.1210/endo-108-4-1305. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Finkelstein M. C., Csernansky J., Quigley J. P., Quinn R. S., Techner L., Troll W., Dunham P. B. Attack of sea urchin eggs by dogfish phagocytes: model of phagocyte-mediated cellular cytotoxicity. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1825–1829. doi: 10.1073/pnas.75.4.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]



