Abstract
Current guidelines recommend reflex testing for hepatitis D virus (HDV) coinfection in hepatitis B surface antigen (HBsAg)-positive patients over risk-factor based screening. We aimed to evaluate the feasibility and diagnostic yield of reflex anti-HDV testing at a Central European tertiary care center. We retrospectively included 560 consecutive patients who had a recorded (first) positive HBsAg test result at the Vienna General Hospital between 2018 and 2022. While reflex anti-HDV testing had been implemented in our hepatitis outpatient clinic (n = 153, ‘reflex testing cohort’), HDV screening needed to be manually ordered in the remaining patients (n = 407, ‘standard testing cohort’). Overall, 98.0% and 65.1% of patients in the reflex and standard testing cohort were screened for anti-HDV, respectively, and the overall seroprevalence of anti-HDV among screened patients was 6.7% (n = 28, reflex testing cohort: 9.3%, standard testing cohort: 5.3%). Risk factors for HDV were present in 49.1% of all included and in 89.3% of anti-HDV positive patients, respectively. Anti-HDV positive patients showed higher ALT (54 [33–83] vs. 29 [19–49] U/L; p = 0.005) and a higher proportion of low-to-undetectable HBV-DNA (61.5% vs. 33.2%; p < 0.001), as compared to anti-HDV negative patients. HDV-RNA PCR was ordered in n = 21/28 (75.0%) of anti-HDV positive patients, and 76.2% had detectable HDV-RNA. Among viremic patients, 75% and 37.5% had significant fibrosis (≥ F2) or cirrhosis (F4), respectively. The prevalence of anti-HDV among HBsAg-positive patients is considerable in a large hospital located in Central Europe. Double reflex testing, i.e., anti-HDV being triggered by the presence of HBsAg and HDV-PCR bring triggered by the presence of anti-HDV, seems warranted to increase the diagnostic yield.
Keywords: Hepatitis delta, Epidemiology, Antivirals, Hepatology, Virology
Subject terms: Viral hepatitis, Viral hepatitis
Introduction
Coinfection with the hepatitis D virus (HDV) can only occur in patients who are infected with the hepatitis B virus (HBV)1. Chronic hepatitis D (CHD) has been associated with a particularly progressive disease course, causing a higher incidence of adverse hepatic outcomes as compared to HBV monoinfection2. From a global perspective, CHD remains an orphan disease, despite its high prevalence in endemic areas, e.g., Eastern Europe, Central Asia, sub-Saharan Africa, and the Amazonas Basin, although data is scarce for some countries in these and other regions, and its quality heterogenous3.
CHD is defined by the presence of antibodies against HDV (anti-HDV). Active infection must then be further evaluated by HDV-RNA PCR to identify patients who are at the highest risk for deleterious outcome4,5. Austria is a low-endemic country with a previously estimated anti-HDV seroprevalence among persons infected with HBV of approximately 1%5. Importantly, however, many patients are never screened for CHD, even though the European Association for the Study of the Liver (EASL) endorsed universal screening among HBsAg-positive patients already in 20176, a recommendation that was upheld in the more recent EASL guideline on the management of HDV infection7. In contrast, the current version of the guidelines on the treatment of HBV infection of the American Association for the Study of Liver Diseases (AASLD) recommends HDV screening only in patients at risk for coinfection8. A recent study from Spain has demonstrated that the implementation of reflex testing for anti-HDV in hepatitis B surface antigen (HBsAg)-positive patients recruited both primary and secondary/tertiary care quintupled the number of identified anti-HDV positive patients, corresponding to a seropositivity rate of 8.2% in the reflex testing cohort9. Importantly, many anti-HDV positive patients did not show any known risk factors for HDV9. Thus, these data support the recommendation for universal/systematic screening for HDV in HBsAg-positive patients. However, the aforementioned study did not provide information on risk factors for HDV in patients who did not show anti-HDV9.
Therefore, we investigated risk factors and testing patterns for HDV among patients testing positive for HBsAg at a large academic hospital in Central Europe.
Methods
Study cohort and design
We retrospectively included all individual patients who tested positive for HBsAg at the Medical University of Vienna during a 5-year period spanning from 2018 to 2022. The patients were identified by an automated query of the databases of the Department of Clinical Virology of the Medical University of Vienna. Patients were excluded if they had a recorded positive HBsAg test before 2018, or if they were less than 18 years old.
All available medical records were manually reviewed to assess general patient characteristics and medical history of the included patients.
The final study cohort comprised of two subcohorts: the ‘reflex testing cohort’ included patients who were tested for HBsAg at our hepatitis outpatient clinic, where anti-HDV reflex testing had been implemented, whereas the ‘standard testing cohort’ included patients who were tested outside our hepatitis outpatient clinic, where anti-HDV screening was conducted only upon active order at the discretion of the treating physician. HDV-RNA PCR needed to be ordered separately from anti-HDV in both cohorts at the discretion of the treating physician.
Risk factors for HDV that were evaluated in this study were defined in accordance with the most recent AASLD guidelines and included the origin from an HDV endemic country, alanine aminotransferase (ALT) above the upper limit of normal (ULN) despite HBV-DNA < 2000 IU/mL, coinfection with hepatitis C virus or human immunodeficiency virus, a history of sexually transmittable diseases, men having sex with men, and intravenous drug use8.
Assessment of routine laboratory and virological parameters
Routine laboratory tests were performed by the ISO-certified Department of Laboratory Medicine of the Medical University of Vienna. The ULN for ALT was 40 U/L for both genders, in accordance with EASL recommendations6. Fibrosis-4 Index (FIB-4) was calculated according to the published formula10.
Commercially available chemiluminescent immunoassays were applied to determine qualitative HBsAg and HBeAg status. A real-time PCR assay (COBAS® AmpliPrep/COBAS® TaqMan® version 2, Roche Diagnostics, Mannheim, Germany) with a lower limit of linear quantification of 20 IU/mL, a limit of detection of 10 IU/mL, and an upper limit of linear quantification of 1.7 × 108 IU/mL was used to quantify HBV-DNA. The Abbott ARCHITECT® assay (Abbott Diagnostics, Abbot Park, IL) with a lower limit of linear quantification and detection of 0.05 IU/mL and an upper limit of linear quantification of 250 IU/mL was applied to quantify HBsAg levels. The qualitative detection of hepatitis D antibodies was conducted using the LIAISON® XL murex Anti-HDV chemiluminescence immunoassay (DiaSorin S.p.A., Saluggia [VC], Italy) on a fully automated DiaSorin Liaison XL platform, following the manufacturer’s instructions. HDV-RNA was quantified by PCR, applying a highly sensitive assay with a lower limit of linear quantification and detection of 100 copies/mL developed in-house by the Center for Virology of the Medical University of Vienna, i.e., the Austrian reference center for HDV diagnostics11.
Transient elastography
Liver stiffness measurement (LSM) was conducted applying transient elastography, i.e., the FibroScan® system (Echosens, Paris, France), as previously described10. Significant fibrosis (≥ F2) was diagnosed if LSM > 7.1 kPa, and cirrhosis (F4) was diagnosed if LSM > 12.5 kPa.
Statistical analysis
Statistical analysis was conducted using R 4.0.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, https://cran.r-project.org/bin/windows/base/old/4.0.2/) and GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA, http://www.graphpad.com/). Group comparisons of categorial variables were performed using χ2 test or Fisher’s exact test, as applicable. For unpaired comparisons of continuous variables, Mann–Whitney U test was applied. Categorial variables are presented as number (percentage) of patients with a certain characteristic, while continuous variables are presented as median (25th—75th percentile).
A two-sided p-value ≤ 0.05 was considered statistically significant.
Ethics
The study was approved by the ethics committee of the Medical University of Vienna (Vote No. 1515/2020). It was performed in conformity with the current version of the Helsinki Declaration. The requirement of informed consent was waived by the ethic committee due to the retrospective nature of the study.
Results
Patient cohort characteristics
During the study period, HBsAg could be detected in 1408 individual patients. We excluded 831 patients who had previously been diagnosed with HBV infection. Another 17 patients were excluded because they were younger than 18 years. Finally, the study cohort comprised 560 patients.
The median age of the included patients was 47 (35–60) years, and the majority (58.4%) were male. HBeAg was negative in 86.6% of patients. Overall, 49.1% of patients had at least one risk factor for HDV. The most prevalent risk factors were origin from an HDV endemic country (31.6%), followed by ALT > ULN despite low-level HBV-DNA (21.6%). Detailed information on patient characteristics is given in Table 1.
Table 1.
Baseline characteristics of the overall patient cohort and comparison of baseline characteristics between patients who were diagnosed with hepatitis B surface antigen (HBsAg) at the hepatitis outpatient clinic (reflex testing cohort) or outside the hepatitis outpatient clinic of the Medical University of Vienna (standard testing cohort).
| Parameter | Overall cohort n = 560 | Standard testing cohort n = 407 | Reflex testing cohort n = 153 | P-value |
|---|---|---|---|---|
| Age, years (IQR) | 47 (35–60) | 50 (36–62) | 40 (31–53) | < 0.001 |
| Sex, male (%) | 327 (58.4) | 241 (59.2) | 86 (56.2) | 0.585 |
| Ethnicity | 0.025 | |||
| Caucasian, n (%) | 497 (88.8) | 368 (90.4) | 129 (84.3) | 0.051 |
| Asian, n (%) | 45 (8.0) | 25 (6.1) | 20 (13.1) | 0.013 |
| African, n (%) | 18 (3.2) | 14 (3.4) | 4 (2.6) | 0.791 |
| Any risk factor, n (%) | 275 (49.1) | 202 (49.6) | 73 (47.7) | 0.757 |
| HDV endemic country, n (%) | 177 (31.6) | 128 (31.4) | 49 (32.0) | 0.923 |
|
ALT > ULN & HBV-DNA < 2000 IU/mL, n (%) * |
121 (21.6) | 89 (21.9) | 32 (20.9) | 0.910 |
| HIV/HCV coinfection, n (%) | 33 (5.9) | 24 (5.9) | 9 (5.9) | 1.000 |
| History of STD, n (%) | 19 (3.4) | 17 (4.2) | 2 (1.3) | 0.120 |
| MSM, n (%) | 16 (2.9) | 9 (2.2) | 7 (4.6) | 0.163 |
| PWID, n (%) | 12 (2.1) | 10 (2.5) | 2 (1.3) | 0.527 |
| HDV screening, n (%) | 415 (74.0) | 265 (65.1) | 150 (98.0) | < 0.001 |
| Anti-HDV positive among screened, n (%) | 28 (6.7%) | 14 (5.3) | 14 (9.3) | 0.169 |
| HBeAg negative, n (%) † | 485 (86.6) | 354 (87.0) | 131 (85.6) | 0.273 |
| Quant. HBsAg, log10 IU/mL (IQR) ‡ | 3.42 (2.58–4.2) | 3.45 (2.53–4.18) | 3.42 (2.72–4.16) | 0.770 |
| HBV-DNA * | < 0.001 | |||
| Undetectable, n (%) | 85 (18.7) | 74 (24.2) | 11 (7.4) | 0.004 |
| < Lower limit of linear quantification, n (%) | 67 (14.8) | 48 (15.7) | 19 (12.8) | 0.885 |
| > Lower limit of quantification, n (%) | 302 (66.5) | 184 (60.1) | 118 (79.7) | < 0.001 |
| FIB-4 §, points (IQR) | 1.15 (0.71–1.88) | 1.16 (0.71–1.88) | 1.09 (0.70–1.81) | 0.692 |
| PLT §, G/L (IQR) | 222 (171–268) | 225 (171–269) | 216 (171–258) | 0.131 |
| ALT §, U/L (IQR) | 29 (19–50) | 28 (18–46) | 32 (22–79) | 0.009 |
| ALT > ULN, n (%) | 169 (30.2) | 116 (28.5) | 53 (34.6) | 0.191 |
| AST §, G/L (IQR) | 28 (21–42) | 27 (20–39) | 30 (22–61) | 0.013 |
HDV hepatitis D virus, ALT alanine aminotransferase, HIV human immunodeficiency virus, HCV hepatitis C virus, STD sexually transmittable diseae, MSM men having sex with men, PWID person who injects drugs, HBeAg hepatitis B e antigen, FIB-4 Fibrosis-4 Index, PLT platetel count, AST aspartate aminotransferase.
Significant values are in bold.
*HBV-DNA missing in n = 106.
†HBeAg missing in n = 15.
‡Quant. HBsAg missing in n = 332.
§FIB-4, PLT, ALT and AST missing in n = 47, n = 31, n = 40, and n = 47, respectively.
The standard testing cohort and the reflex testing subcohorts comprised 407 (72.3%) and 153 (27.7%) patients, respectively. Patients who were evaluated at our hepatitis outpatient clinic (reflex testing cohort) tended to be younger (40 [31.53] vs. 50 [36–62] years, p < 0.001), had detectable HBV-DNA levels above the lower limit of quantification more frequently (79.8% vs. 60.1%, p < 0.001), and had higher ALT (32 [22–79] vs. 28 [18–46] U/L, p = 0.009) and AST (30 [22–61] vs. 27 [20–39] U/L, p = 0.013) in comparison to patients from outside our outpatient clinic (standard testing cohort).
HDV screening patterns and prevalence
While 265 (65.1%) of patients included in the standard testing cohort were screened for anti-HDV, 142 (34.9%) were not screened. As shown in Supplementary Table 1, the only significant difference between these patient groups was the higher proportion HBV-DNA undetectability (42.7% vs. 14.8%; p < 0.001) among patients who were not screened.
Among patients who were included in the reflex testing cohort, 150 (98.0%) were screened for anti-HDV. In three patients, the test could not be conducted owing to a lack of (additional) material.
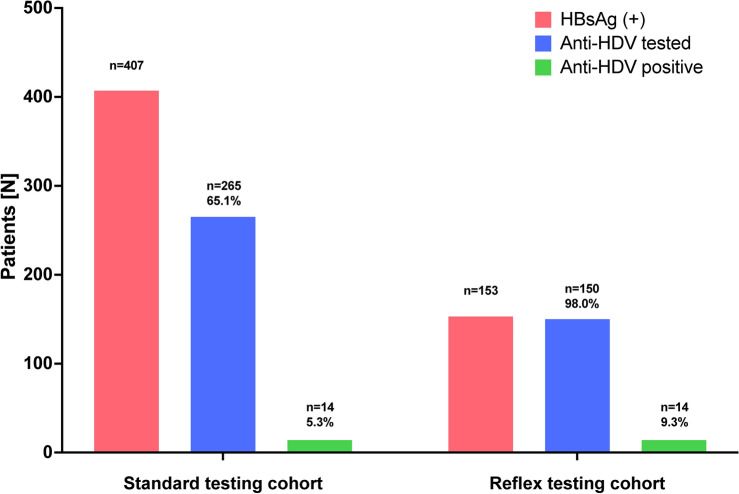
Anti-HDV could be detected in 14/265 (5.3%) and 14/150 (9.3%) of screened patients in the standard and reflex testing cohort, respectively, as shown in Fig. 1. The overall prevalence of anti-HDV among screened patients was 6.7%.
Fig. 1.
Prevalence of antibodies against hepatitis D virus (anti-HDV) among patients who were included in the standard testing cohort or and reflex testing cohort.
Characteristics of anti-HDV positive patients
In comparison to anti-HDV negative patients, a higher proportion of anti-HDV positive patients showed risk factors for HDV (89.3% vs. 46.5%; p < 0.001). In addition, more anti-HDV positive patients had undetectable or unquantifiable HBV-DNA (61.5% vs. 33.2%; p < 0.001) and elevated ALT (64.3% vs. 28.7%; p < 0.001) levels. Furthermore, anti-HDV positive patients showed higher FIB-4 levels (1.37 [1.08–2.70] vs. 1.13 [0.71–1.96]; p = 0.048). A comprehensive comparison of baseline characteristics between anti-HDV positive and negative patients is given in Table 2.
Table 2.
Comparison of baseline characteristics between patients with or without antibodies against hepatitis D virus (anti-HDV).
| Parameter | Anti-HDV (-) n = 387 | Anti-HDV ( +) n = 28 | P-value |
|---|---|---|---|
| Age, years (IQR) | 46 (35–60) | 40 (33–53) | 0.200 |
| Sex, male (%) | 223 (57.6) | 18 (63.3) | 0.623 |
| Ethnicity | 0.214 | ||
| Caucasian, n (%) | 336 (86.8) | 23 (82.1) | 0.885 |
| Asian, n (%) | 36 (9.3) | 12 (17.9) | 0.203 |
| African, n (%) | 15 (3.9) | 0 (0.0) | 0.612 |
| Any risk factor, n (%) | 180 (46.5) | 25 (89.3) | < 0.001 |
| HDV Endemic country, n (%) | 114 (29.5) | 18 (64.3) | 0.018 |
|
ALT > 40 IU/mL & HBV-DNA < 2000 IU/mL, n (%) * |
71 (18.3) | 16 (57.1) | 0.001 |
| HIV/HCV coinfection, n (%) | 19 (4.9) | 7 (25.0) | 0.002 |
| History of STD, n (%) | 12 (3.1) | 1 (3.6) | 0.603 |
| MSM, n (%) | 12 (3.1) | 2 (7.1) | 0.255 |
| PWID, n (%) | 8 (2.1) | 1 (3.6) | 0.474 |
| HBeAg negative, n (%) † | 337 (87.1) | 21 (75.0) | 0.064 |
| Quant. HBsAg, log10 IU/mL (IQR) ‡ | 3.76 (1.91–4.31) | 3.40 (2.69–4.16) | 0.987 |
| HBV-DNA * | < 0.001 | ||
| Undetectable, n (%) | 35 (10.8) | 6 (23.1) | 0.108 |
| < Lower limit of linear quantification, n (%) | 40 (12.4) | 10 (38.5) | 0.003 |
| > Lower limit of quantification, n (%) | 248 (76.8) | 10 (38.5) | 0.125 |
| FIB-4 §, points (IQR) | 1.13 (0.71–1.96) | 1.37 (1.08–2.70) | 0.048 |
| PLT §, G/L (IQR) | 221 (175–262) | 164 (134–239) | 0.016 |
| ALT §, U/L (IQR) | 29 (19–49) | 54 (33–83) | 0.005 |
| ALT > ULN, n (%) | 111 (28.7) | 18 (64.3) | < 0.001 |
| AST §, G/L (IQR) | 27 (21–42) | 45 (35–90) | < 0.001 |
HDV hepatitis D virus, ALT alanine aminotransferase, HIV human immunodeficiency virus, HCV hepatitis C virus, STD sexually transmittable diseae, MSM men having sex with men, PWID person who injects drugs, HBeAg hepatitis B e antigen, FIB-4 Fibrosis-4 Index, PLT platetel count, AST aspartate aminotransferase.
Significant values are in bold.
*HBV-DNA missing in n = 66.
†HBeAg missing in n = 7.
‡Quant. HBsAg missing in n = 210.
§FIB-4, PLT, ALT and AST missing in n = 29, n = 17, n = 25, and n = 29, respectively.
Prevalence of detectable HDV-RNA
HDV-RNA PCR was ordered in 21/28 (75.0%) of anti-HDV positive patients, and HDV-RNA could be detected in 16/21 (76.2%) patients. As shown in Table 3, viremic CHD patients were significantly older than HDV-RNA negative patients (49 [39–58] vs. 32 [30–46] years; p = 0.018), and more often had undetectable or unquantifiable HBV-DNA levels (75.0% vs. 0.0%; p = 0.013). In addition, viremic CHD patients showed higher LSM (9.2 [7.4–33.2] vs. 4.0 [3.9–4.0] kPa; p < 0.002), and whilst none of the patients without detectable HDV-RNA had significant fibrosis or cirrhosis, 12 (75.0%) and 6 (37.5%) of viremic patients showed LSM values indicating ≥ F2 or F4 fibrosis, respectively.
Table 3.
Comparison of baseline characteristics between HDV-RNA positive and negative patients in whom HDV-RNA PCR was ordered (n = 21).
| Parameter | HDV-RNA (-) n = 5 | HDV-RNA ( +) n = 16 | P-value |
|---|---|---|---|
| Age, years (IQR) | 32 (30–36) | 49 (39–58) | 0.018 |
| Sex, male (%) | 2 (40.0) | 11 (68.8) | 0.530 |
| HBeAg negative , n (%) † | 2 (40.0) | 4 (25.0) | 0.935 |
| Quant. HbsAg, log10 IU/mL (IQR) | 3.6 (2.8–4.5) | 4.2 (4.0–4.5) | 0.671 |
| HBV-DNA levels | 0.013 | ||
| Undetectable, n (%) | 0 (0.0) | 5 (31.2) | 0.545 |
| < Lower limit of linear quantification, n (%) | 0 (0.0) | 7 (43.8) | 0.289 |
| > Lower limit of quantification, n (%) | 5 (100.0) | 4 (25.0) | 0.115 |
| FIB-4, points (IQR) | 0.59 (0.52–0.63) | 1.67 (1.19–5.69) | 0.001 |
| PLT, G/L (IQR) | 251 (195–314) | 143 (97–225) | 0.069 |
| ALT, U/L (IQR) | 28 (15–46) | 60 (45–89) | 0.148 |
| ALT > ULN, n (%) | 2 (40.0) | 13 (81.2) | 0.224 |
| AST, G/L (IQR) | 30 (13–33) | 54 (40–104) | 0.023 |
| Liver stiffness, kPa (IQR) | 4.0 (3.9–4.0) | 9.2 (7.4–33.2) | 0.002 |
| ≥ F2 | 0 (0.0) | 12 (75.0) | 0.015 |
| F4 | 0 (0.0) | 6 (37.5) | 0.292 |
HBeAg hepatitis B e antigen, HBsAg hepatitis B surface antigen, FIB-4 Fibrosis-4 Index, PLT platelet count, ALT alanine aminotransferase, AST aspartate aminotransferase.
Significant values are in bold.
Discussion
CHD has gained significant attention during recent years owing to the advent of novel treatment options1. However, the true prevalence of CHD is still unknown owing to a lack of high-quality epidemiological data from many (endemic) regions worldwide3. To date, the global prevalence of CHD is estimated at approximately 12 million people, however, seropositivity rates among HBsAg-positive patients differ significantly depending on the geographical region and setting from which data has been generated3.
A recent report from Austria has estimated the anti-HDV seroprevalence among HBsAg-positive patients at approximately 1%5. However, this figure likely underestimates the true prevalence of CHD due to a lack of universal screening for anti-HDV12, especially outside specialized hepatitis clinics, because anti-HDV screening is currently not reimbursed by Austrian social insurance in primary care.
In our study that comprising 560 HBsAg-positive patients that have not had previously diagnosed at the Medical University of Vienna, we found an overall anti-HDV prevalence of 6.7% among the 74% of patients who were screened. Notably, our study comprised two subcohorts, i.e., one group of patients who were diagnosed at our hepatitis outpatient clinic, where reflex testing for anti-HDV had already been implemented, and one group of patients who were diagnosed outside our hepatitis outpatient clinic, in whom anti-HDV tests needed to be manually ordered. The prevalence of anti-HDV was 9.3% in the reflex testing cohort, and 5.3% among the 65% of patients who were screened as part of the standard testing cohort. The overall prevalence of anti-HDV observed in our cohort is in line with a recent report from Spain, in which anti-HDV reflex testing yielded a seropositivity rate of 8.1%9. Importantly, one third of patients of the standard testing cohort was not screened, rendering the estimation of the true seroprevalence among these patients impossible. However, risk factors for HDV were similar among screened and unscreened patients, making it likely that the seroprevalence can be extrapolated to these patients. In contrast to the Spanish study, in which risk factors for HDV were unknown in most patients9, approximately 90% of anti-HDV positive patients identified in our study showed risk factors for HDV. This could be explained by the fact that our study considered all risk factors proposed by the AASLD8, including biochemical/virological parameters, whereas the Spanish study only considered the risk factors ‘blood-borne’ risk factors, and ‘HDV endemic country’9. Additionally, the high proportion of migrants from HDV endemic countries in our cohort (64.3%) may have contributed to this difference, which is also reflected by the overall prevalence of risk factors for HDV of almost 50%. However, comparing the included population from our study with an Italian study analyzing anti-HDV reflex testing in HBsAg positive subjects showed similar results regarding their included study population with a relatively high proportions of non-native Italians13.
In turn, one out of ten patients who were ultimately diagnosed with anti-HDV did not show any of the previously proposed risk factors. One crucial limitation of risk factor based screening is that risk factors must be reported, identified, and acknowledged to link patients to HDV screening. This is reflected by the high prevalence of (identified) risk factors of approximately 50% even among patients who were not screened for anti-HDV in our cohort. Taken together, these points render risk factor based screening quite unpractical.
It has been recently shown that reflex anti-HDV screening seems manageable for healthcare systems in low-endemic settings, whereas cost-effectiveness needs further investigation in areas with a high prevalence of HBV but a low prevalence of HDV infection14. In our study, almost half of the cohort had one or more risk factors for HDV. In absolute numbers, the required number of anti-HDV tests required for centralized reflex testing of all incident HBV infections in our large Central European hospital would have doubled from 275 to only 560—over a five-year period. This clearly imposes an unsignificant burden on the healthcare system of any high-income country. While it could be argued that risk factors for HDV might be scarcer among HBsAg-positive patients outside specialized clinics/hospitals, increasing the proportion of tests needed for persons without known risk factors, we would still strongly argue for a nationwide reflex testing owing to the outlined, obvious limitations of risk factor based screening, and the low incidence of HBV and HDV infection in Austria5,12.
Along the same lines, we would suggest the implementation of reflex testing for HDV-RNA in patients who are diagnosed with anti-HDV. In our cohort, HDV-RNA PCR was ordered in three out of four anti-HDV positive patients, and approximately 75% had detectable HDV-RNA levels. These patients tended to be older than HDV-RNA negative patients and showed higher levels of ALT/AST. Moreover, HDV-RNA positive patients showed lower levels of HBV-DNA, supporting previous observations9. Importantly, three out of four viremic patients showed LSM values indicating advanced fibrosis, and one out of three had already progressed to cirrhosis, while none of the HDV-RNA negative patients showed advanced liver disease, highlighting the importance of viremia for the natural history of CHD5. Again, the number of additionally required HDV-RNA PCR assays for the implementation of double-reflex testing seems negligible in our low-endemic country. Moreover, the availability of novel antiviral drugs that are highly effective and well tolerated warrants the investigation of viremia in all anti-HDV positive patients to link patients to therapy.
The most important limitation of our study is its retrospective design, which does not allow for the identification of reasons why one out of three patients among the standard testing cohort were not screened for anti-HDV. Accordingly, the anti-HDV seroprevalence among patients included in the standard testing cohort might be slightly under- or overestimated. In turn, reflex testing allowed for a representative estimation of anti-HDV in HBsAg-positive patients treated at our specialized hepatitis outpatient clinic. The high prevalence of risk factors for HDV, mostly owing to a high proportion of migrants from high-endemic countries in our cohort, must also be considered when extrapolating our results to other settings and regions. On the other hand, some risk factors, most importantly relying on serological markers such as HBV-DNA, which were not universally available, might have been underrecognized. This might also have influenced between-group comparison of risk factors between anti-HDV positive and negative patients. The reasons for the order of HBV-DNA PCR and other markers, including quantitative HBsAg levels, were not captured in our study. Lastly, we did not conduct a formal cost-effectiveness analysis for our proposed centralized double-reflex testing approach, however, it has been recently suggested by an international consortium that such a strategy will be paralleled by little burden on the healthcare system of high-income, low-endemic countries, whilst removing important barriers in linking patients to adequate care and treatment14.
In conclusion, in a large cohort of HBsAg-positive patients diagnosed at a large hospital in Central Europe, we found a considerable seroprevalence of anti-HDV. Risk factors for HDV were found in many, but not all anti-HDV positive patients. Double-reflex testing for anti-HDV in HBsAg positive patients and HDV-RNA PCR in anti-HDV positive patients will increase the diagnostic yield in high-income, low-endemic countries with little economic burden on healthcare systems and allow for early identification and treatment of a patient population who is at particular risk for adverse hepatic outcomes.
Supplementary Information
Abbreviations
- Anti-HDV
Antibodies against hepatitis D virus
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AASLD
American Association for the Study of Liver Disease
- CHD
Chronic hepatitis D
- EASL
European Association for the Study of the Liver
- FIB-4
Fibrosis-4 index
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HDV
Hepatitis D virus
- IQR
Interquartile range
- LSM
Liver stiffness measurement
- PLT
Platelet count
- ULN
Upper limit of normal
Author contributions
All authors contributed to conceptualization (J.B. and M.J.), data curation (all authors), formal analysis and visualization (J.B. and M.J.), writing of the original draft (J.B. and M.J.), reviewing and editing (all authors), or supervision (M.J. and T.R.).
Funding
This project has been supported by an unrestricted research grant from Gilead Sciences Ges.m.b.H. to the MedicalUniversity of Vienna (PI: Mathias Jachs).
Data availability
Data will be made available upon reasonable request to the corresponding authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Reiberger, Email: thomas.reiberger@meduniwien.ac.at.
Mathias Jachs, Email: mathias.jachs@meduniwien.ac.at.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77737-4.
References
- 1.Asselah, T. & Rizzetto, M. Hepatitis D Virus Infection. N. Engl. J. Med.389, 58–70 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Miao, Z. et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J. Infect. Dis.221, 1677–1687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockdale, A. J. et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol.73, 523–532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamal, H. et al. Long-Term Study of Hepatitis Delta Virus Infection at Secondary Care Centers: The Impact of Viremia on Liver-Related Outcomes. Hepatology72, 1177–1190 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Jachs, M. et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United Eur. Gastroenterol. J.9, 1119–1127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol.2017(67), 370–398 (2017). [DOI] [PubMed] [Google Scholar]
- 7.EASL Clinical Practice Guidelines on hepatitis delta virus. J. Hepatol.79, 433–460 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology67, 1560–1599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palom, A. et al. Implementation of anti-HDV reflex testing among HBsAg-positive individuals increases testing for hepatitis D. JHEP Rep.4, 100547 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659–689 (2021). [DOI] [PubMed]
- 11.Jachs, M. et al. Response-guided long-term treatment of chronic hepatitis D patients with bulevirtide-results of a “real world” study. Aliment Pharmacol. Ther.56, 144–154 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jachs, M. et al. Eligibility for antiviral therapy and treatment uptake in chronic hepatitis B patients referred to a European tertiary care center. United Eur. Gastroenterol. J.11, 293–304 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossiga, V. et al. Anti-HDV reflex testing in HBsAg-positive subjects: An efficacious strategy to identify HDV infection. Liver Int.44, 148–154 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Razavi, H. A. et al. Hepatitis D double reflex testing of all hepatitis B carriers in low-HBV- and high-HBV/HDV-prevalence countries. J. Hepatol.79, 576–580 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request to the corresponding authors.