Abstract
Objective
As asthma is a chronic inflammatory disease of the airways, anti-inflammatory treatment should be positioned at the forefront of guideline-directed asthma care. However, patients tend to rely on short-acting β2-agonists (SABAs) for rapid-onset symptom relief. The impact of SABA overuse and associated clinical outcomes have been investigated extensively in Europe and North America. Limited data are available from countries in Asia, Africa, Latin America, and the Middle East. The SABA use IN Asthma (SABINA) III program, a large multicountry, observational study, was undertaken to describe the global extent of SABA use and its potential contribution to suboptimal disease control. As part of the SABINA III study, we aimed to characterize SABA prescription collection and asthma-related clinical outcomes among patients in the Philippines.
Methods
This nationwide, observational, cross-sectional, SABINA III study included patients (aged ≥12 years) with a documented asthma diagnosis recruited between May 2019 and January 2020 from 10 sites in the Philippines. Demographics, disease characteristics and prescribed asthma treatments, including SABA and inhaled corticosteroids (ICS) in the 12 months preceding study start, were recorded during a single visit, and transcribed onto an electronic case report form (eCRF). Patients were classified by investigator-defined asthma severity, guided by the 2017 Global Initiative for Asthma (GINA) report and practice type, either primary or pulmonary medicine specialist care.
Results
Of 245 patients analyzed, 63.3% were classified as having moderate-to-severe asthma (GINA steps 3−5), and most patients (63.3%) were enrolled by pulmonary medicine specialists. Overall, 33.1% (n=81) of patients had experienced ≥1 severe exacerbation in the previous 12 months and 18.4% (n=45) of patients had uncontrolled asthma. With respect to asthma treatments, a total of 6.5% (n=16), 40.4% (n=99), and 2.4% (n=6) of patients were prescribed SABA monotherapy, SABA in addition to maintenance therapy, and ICS, respectively, in the 12 months prior to their study visit. Most patients (n=156 [63.7%]) received prescriptions of fixed-dose combinations of ICS and long-acting β2-agonists. SABA over-prescription, defined as ≥3 SABA canister prescriptions per year, was observed in 10.6% (n=21) of patients. Additionally, 25.6% (n=23) of patients classified as having mild asthma were prescribed either nebulized SABA (n=17) or oral SABA (n=6). Nearly one-third of patients (n=75 [30.6%]) had purchased over-the-counter (OTC) SABA, and 46.9% (n=115) were prescribed antibiotics.
Conclusion
In this SABINA III Philippines study cohort, more than 10% of patients were over-prescribed SABA canisters. Additionally, prescriptions for oral or nebulized SABA, the purchase of non-prescription (OTC) SABA, and the high percentage of prescriptions for antibiotics warrant country-wide improvements in asthma care and management.
Keywords: asthma, bronchodilator agents, Philippines, prescriptions
INTRODUCTION
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation and defined by a history of respiratory symptoms such as wheezing, dyspnea, chest tightness, and cough, which fluctuate over time and intensity, together with variable expiratory airflow limitation.1,2 While limited data exist, results from a National Nutrition and Health Survey from 17 regions of the Philippines estimated an overall prevalence of asthma based on episodes of wheezing in the preceding 12 months as 8.7% (SE 0.4%).3,4 All patients with asthma, regardless of its severity, are at risk of exacerbations.5,6 Indeed, results from an online survey of patients with asthma from eight Asian countries/regions, including the Philippines, reported that 73% had received ≥1 course of oral corticosteroids (OCS) in the previous 12 months.7 Filipino patients with asthma also have a high probability of requiring emergency care,8 likely for the treatment of more severe exacerbations.
While asthma-related mortality is preventable, between 2011 and 2015, the age-standardized asthma mortality rate in the Philippines was 150 deaths per million persons, the second highest among low- and middle-income countries.9 Poor disease control is also widespread across the Asia-Pacific region, including the Philippines.8,10,11 Results from an online survey of 2,467 patients with asthma between December 2013 and March 2014 from eight Asian countries, including the Philippines, reported nearly half of the participants had uncontrolled asthma.7 Consequently, asthma imposes a significant disease burden on both patients and the national healthcare system.12
Evidence-based treatment guidelines represent a principal cornerstone of improved asthma outcomes. In 2019, a major shift in asthma management and prevention occurred when the Global Initiative for Asthma (GINA) updated its report to no longer recommend SABA monotherapy for symptomatic relief of mild asthma (treatment steps 1–2). Instead, low-dose inhaled corticosteroids (ICS)/formoterol is now the preferred reliever for patients across all treatment steps and for those patients with moderate-to-severe asthma (treatment steps 3–5) prescribed both maintenance and reliever therapy.13 Nevertheless, before this change, a long-held paradox of asthma management, which relied on as-needed SABA to address bronchoconstriction in patients with mild asthma,14,15 likely influenced clinician and patient behavior, fostering an over-reliance on SABAs for immediate symptom relief while disregarding the need to treat the underlying inflammatory processes of the disease with ICS. This lag in adopting contemporary, evidence-based treatment guidelines by healthcare providers (HCPs) has become a matter of particular concern as globally, SABA overuse is associated with an increased risk of asthma exacerbations, hospitalizations, and even mortality.16,17 Moreover, in the Philippines, the price of generic budesonide was three times the international reference price in 2011,18 thereby limiting the availability of optimal asthma care.
To date, there is a paucity of data on potential SABA overuse and associated clinical outcomes in the Philippines. In the absence of robust, countrywide healthcare databases, most earlier studies in this region were of a survey design8,10 and did not specifically focus on SABA use. Therefore, the SABA use IN Asthma (SABINA) multicountry (III) arm of the SABINA program19 was designed to capture clinical information, including SABA prescription data, in local healthcare settings with the use of electronic case report forms (eCRFs). Here, we report results from the Philippines cohort of the SABINA III study, which describes the prevalence of SABA prescriptions and provides real-world evidence of asthma management practices in this country.
METHODS
Study design and data source
The study design has been described previously.20 In brief, this observational, cross-sectional SABINA III Philippines study was conducted at 10 sites distributed across Luzon, Visayas, and Mindanao, with patients recruited between May 2019 and January 2020. The study was approved by the Institutional Ethics Review Board, Lung Center of the Philippines; the Research Ethics Review Committee (RERC), Batangas Medical Center; the Unified Biomedical RERC, West Visayas State University; the RERC, Western Visayas Medical Center; the Cluster RERC, Metro Davao Medical & Research Center; the Institutional Review Board, The Medical City; and the Institutional Ethics Review Committee, Davao Doctors Hospital.
The objectives of this study were to describe the demographic and clinical features of the asthma population in the Philippines stratified by asthma severity and to estimate SABA and ICS canister prescriptions per patient in the 12 months preceding study entry.
Study sites were selected with the aim of ensuring a nationally representative, purposive sample of both physicians (primary care and pulmonary medicine specialists) and patients. At each site, during a single study visit, pre-specified patient data were extracted by HCPs and transcribed onto an eCRF.
Study population
Patients aged ≥12 years with a documented physician diagnosis of asthma in their medical records, ≥3 prior consultations with the same HCP or HCP practice, and medical records containing data for ≥12 months before the study visit were included in the study. Patients with a diagnosis of chronic obstructive pulmonary disease, other chronic respiratory diseases, or an acute or chronic condition, which in the opinion of the investigator would limit the ability of the patient to participate in this study, were excluded. Signed informed consent was obtained from all patients or legal guardians for those aged <18 years.
Variables
During the cross-sectional study visit, retrospective data were obtained from existing medical records, and patient data, including an assessment of current asthma symptom control, were collected and entered into an eCRF by the investigator. SABA prescriptions were categorized as 0, 1–2, 3–5, 6–9, 10–12, and ≥13 canisters, with over-prescription of SABA defined as ≥3 SABA canisters in the 12 months prior to the study visit.19 ICS canister prescriptions were recorded and described by their average daily dose of low, medium, or high.21
Patients were classified by practice type (primary or pulmonary medicine specialist care) and investigator-defined asthma severity, as guided by GINA 2017 recommendations. Patients at GINA treatment steps 1–2 were classified as having mild asthma, and those at GINA steps 3–5 with moderate-to-severe asthma.21 In addition to patient age and sex, other demographic variables included body mass index (BMI); smoking status; healthcare insurance; level of education; and disease characteristics, such as time since asthma diagnosis, number of comorbidities, number of severe exacerbations, and level of asthma symptom control based on the 2017 GINA assessment of asthma control.21 Also, data were collected on prescribed asthma treatments including ICS; fixed-dose combinations of ICS and long-acting β2-agonists (LABAs); short courses of OCS (OCS burst); long-term OCS; antibiotics prescribed for asthma; and over-the-counter (OTC) SABA purchases, in the 12 months prior to study entry. A short course of OCS (OCS burst) was defined as a course of intravenous corticosteroids or OCS administered for 3 to 10 days or a single dose of an intra-muscular corticosteroid to treat an asthma exacerbation.
Statistical analysis
Descriptive analyses were used to characterize patients according to baseline demographics and clinical characteristics. Continuous variables were summarized by the number of non-missing values, mean [standard deviation (SD)], median, and range. Categorical variables were summarized as frequency counts and percentages.
RESULTS
Patient disposition
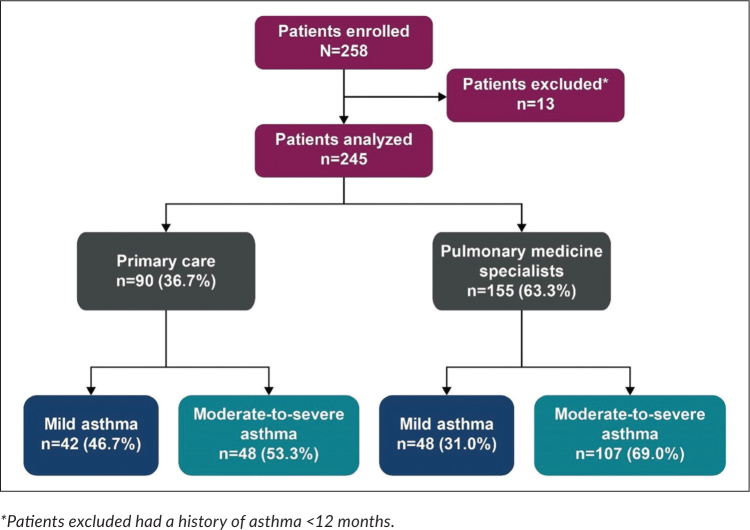
Of the 258 patients enrolled in the study, 13 were excluded from the analysis because the duration of their asthma was <12 months (Figure 1). Overall, 36.7% of patients had investigator-classified mild asthma (GINA steps 1–2) and 63.3%, moderate-to-severe asthma (GINA steps 3–5). A higher proportion of patients were enrolled by pulmonary medicine specialists (comprising pulmonologists, immunologists, pediatricians, and general medicine practitioners; n=155 [63.3%]) than by primary care physicians (n=90 [36.7%]).
Figure 1.
Patient disposition and study population by practice type and investigator-classified asthma severity in the SABINA III Philippines cohort.
Patient demographics and lifestyle characteristics
The patients who qualified for analysis were primarily female (70.6%), with a median (range) age of 35.0 (12.0–86.0) years (Table 1). Overall, 51%) of patients (n=125) were aged ≥18–54 years; nearly one-quarter of patients (n=60 [24.5%]) were adolescents aged 12–17 years, with the remaining aged 55 years and older. The mean (SD) BMI of all patients was 24.7 (5.7) kg/m2, and the majority of adolescents (63.3% [n=38]) were in the healthy BMI range (Appendix Table 1), whereas most adults (n=146 [59.6%]) were either overweight or obese. A higher percentage of patients treated in primary care were overweight and/ or obese (BMI ≥23 kg/m2) compared with those treated by pulmonary medicine specialists (64.4% vs 56.8%). The majority of patients had never smoked (n=214 [87.3%]). Most patients had received at least high school education (n=219 [89.3%]), with 42.9% receiving university and/or post-graduate education. Irrespective of prescriber type or disease severity, approximately three-quarters of all patients did not receive healthcare reimbursement.
Table 1.
Demographics and baseline clinical characteristics of the SABINA III Philippines cohort by investigator-classified asthma severity and practice type (N=245)
| All (N=245) | Primary Care (n=90) |
Pulmonary Medicine Specialists (n=155) |
|||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n=42) | Investigator-classified moderate-to-severe asthma (n=48) | All (n=90) | Investigator-classified mild asthma (n=48) | Investigator-classified moderate-to-severe asthma (n=107) | All (n=155) | ||
| Age (years) | |||||||
| Median (min, max) | 35.0 (12.0,86.0) |
18.5 (12.0,65.0) |
36.0 (20.0,82.0) |
32.0 (12.0,82.0) |
14.0 (12.0,80.0) |
52.0 (12.0,86.0) |
41.0 (12.0,86.0) |
|
| |||||||
| Age group (years), n (%) | |||||||
| 12–17 | 60 (24.5) | 17 (40.5) | 0 (0.0) | 17 (18.9) | 36 (75.0) | 7 (6.5) | 43 (27.7) |
| ≥18–54 | 125 (51.0) | 23 (54.8) | 40 (83.3) | 63 (70.0) | 9 (18.8) | 53 (49.5) | 62 (40.0) |
| ≥55 | 60 (24.5) | 2 (4.8) | 8 (16.7) | 10 (11.1) | 3 (6.2) | 47 (43.9) | 50 (32.3 |
|
| |||||||
| Sex, n (%) | |||||||
| Female | 173 (70.6) | 24 (57.1) | 38 (79.2) | 62 (68.9) | 26 (54.2) | 85 (79.4) | 111 (71.6) |
|
| |||||||
| BMI (kg/m2) | |||||||
| Mean (SD) | 24.7 (5.7) | 22.7 (3.5) | 28.0 (7.3) | 25.5 (6.4) | 21.9 (4.6) | 25.3 (5.3) | 24.3 (5.3) |
|
| |||||||
| BMI groups (kg/m2)a, n (%) | |||||||
| <18.5 | 23 (9.4) | 6 (14.3) | 1 (2.1) | 7 (7.8) | 11 (22.9) | 5 (4.7) | 16 (10.3) |
| ≥18.5 to 22.9 | 76 (31.0) | 16 (38.1) | 9 (18.8) | 25 (27.8) | 19 (39.6) | 32 (29.9) | 51 (32.9) |
| ≥23 to 24.9 | 42 (17.1) | 8 (19.0) | 10 (20.8) | 18 (20) | 6 (12.5) | 18 (16.8) | 24 (15.5) |
| ≥25 | 104 (42.4) | 12 (28.6) | 28 (58.3) | 40 (44.4) | 12 (25.0) | 52 (48.6) | 64 (41.3) |
|
| |||||||
| Education level, n (%) | |||||||
| Primary or secondary school | 26 (10.6) | 2 (4.8) | 6 (12.5) | 8 (8.9) | 8 (16.7) | 10 (9.3) | 18 (11.6) |
| High school | 114 (46.5) | 23 (54.8) | 10 (20.8) | 33 (36.7) | 35 (72.9) | 46 (43.0) | 81 (52.3) |
| University and/or post-graduate education | 105 (42.9) | 17 (40.5) | 32 (66.7) | 49 (54.4) | 5 (10.4) | 51 (47.7) | 56 (36.1) |
|
| |||||||
| Smoking status history, n (%) | |||||||
| Active smoker | 8 (3.3) | 3 (7.1) | 5 (10.4) | 8 (8.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Former smoker | 23 (9.4) | 7 (16.7) | 11 (22.9) | 18 (20.0) | 1 (2.1) | 4 (3.7) | 5 (3.2) |
| Never-smoker | 214 (87.3) | 32 (76.2) | 32 (66.7) | 64 (71.1) | 47 (97.9) | 103 (96.3) | 150 (96.8) |
|
| |||||||
| Healthcare insurance/medication funding, n (%) | |||||||
| Not reimbursed | 183 (74.7) | 32 (76.2) | 36 (75.0) | 68 (75.6) | 34 (70.8) | 81 (75.7) | 115 (74.2) |
| Partially reimbursed | 8 (3.3) | 7 (16.7) | 1 (2.1) | 8 (8.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fully reimbursed | 44 (18.0) | 2 (4.8) | 3 (6.2) | 5 (5.6) | 14 (29.2) | 25 (23.4) | 39 (25.2) |
| Not specified | 10 (4.1) | 1 (2.4) | 8 (16.7) | 9 (10.0) | 0 (0.0) | 1 (0.9) | 1 (0.6) |
BMI is categorized according to the Asia-Pacific classification22
BMI, body mass index; max, maximum; min, minimum; SD, standard deviation.
Disease characteristics
The average asthma duration was 12.4 (SD: 9.8) years, with 34.7% of patients (n=85) at GINA treatment step 1 (Table 2). More than half of all patients (n=144 [58.8%]) had no comorbidities. In the 12 months before study start, patients experienced a mean (SD) of 0.5 (1.0) severe asthma exacerbations, with 33.1% (n=81) experiencing ≥1 severe exacerbation.
Table 2.
Asthma characteristics of the SABINA III Philippines cohort according to investigator-classified asthma severity and practice type (N=245)
| All (N=245) | Primary Care (n=90) |
Pulmonary Medicine Specialists (n=155) |
|||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n=42) | Investigator-classified moderate-to-severe asthma (n=48) | All (n=90) | Investigator-classified mild asthma (n=48) | Investigator-classified moderate-to-severe asthma (n=107 | All (n=155) | ||
| Asthma duration (years) | |||||||
| Mean (SD) | 12.4 (9.8) | 15.1 (8.5) | 18.0 (12.9) | 16.7 (11.1) | 9.5 (3.8) | 10.2 (9.4) | 9.9 (8.1) |
| Median (min, max) | 10.0 (1.0, 59.0) | 13.0 (4.0, 50.0) | 15.0 (1.0, 59.0) | 13.0 (1.0, 59.0) | 10.0 (1.0, 16.0) | 9.0 (1.0, 57.0) | 9.0 (1.0, 57.0) |
|
| |||||||
| GINA classification, n (%) | |||||||
| Step 1 | 85 (34.7) | 39 (92.9) | 0 (0.0) | 39 (43.3) | 46 (95.8) | 0 (0.0) | 46 (29.7) |
| Step 2 | 5 (2.0) | 3 (7.1) | 0 (0.0) | 3 (3.3) | 2 (4.2) | 0 (0.0) | 2 (1.3) |
| Step 3 | 57 (23.3) | 0 (0.0) | 37 (77.1) | 37 (41.1) | 0 (0.0) | 20 (18.7) | 20 (12.9) |
| Step 4 | 48 (19.6) | 0 (0.0) | 9 (18.8) | 9 (10.0) | 0 (0.0) | 39 (36.4) | 39 (25.2) |
| Step 5 | 50 (20.4) | 0 (0.0) | 2 (4.2) | 2 (2.2) | 0 (0.0) | 48 (44.9) | 48 (31.0) |
|
| |||||||
| Number of comorbidities, n (%) | |||||||
| None | 144 (58.8) | 31 (73.8) | 32 (66.7) | 63 (70.0) | 24 (50.0) | 57 (53.3) | 81 (52.3) |
| 1–2 | 91 (37.1) | 11 (26.2) | 13 (27.1) | 24 (26.7) | 22 (45.8) | 45 (42.1) | 67 (43.2) |
| 3–4 | 9 (3.7) | 0 (0.0) | 2 (4.2) | 2 (2.2) | 2 (4.2) | 5 (4.7) | 7 (4.5) |
| ≥5 | 1 (0.4) | 0 (0.0) | 1 (2.1) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Number of severe asthma exacerbations in the previous 12 months before study visit | |||||||
| Mean (SD) | 0.5 (1.0) | 0.0 (0.2) | 0.5 (0.7) | 0.3 (0.6) | 0.2 (0.6) | 0.9 (1.3) | 0.7 (1.1) |
|
| |||||||
| Number of severe asthma exacerbations in the previous 12 months before study visit by group, n (%) | |||||||
| 0 | 164 (66.9) | 41 (97.6) | 32 (66.7) | 73 (81.1) | 43 (89.6) | 48 (44.9) | 91 (58.7) |
| 1 | 51 (20.8) | 1 (2.4) | 11 (22.9) | 12 (13.3) | 3 (6.2) | 36 (33.6) | 39 (25.2) |
| 2 | 17 (6.9) | 0 (0.0) | 4 (8.3) | 4 (4.4) | 1 (2.1) | 12 (11.2) | 13 (8.4) |
| 3 | 11 (4.5) | 0 (0.0) | 1 (2.1) | 1 (1.1) | 1 (2.1) | 9 (8.4) | 10 (6.5 |
| >3 | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.9) | 2 (1.3) |
|
| |||||||
| Level of asthma control, n (%) | |||||||
| Well controlled | 135 (55.1) | 32 (76.2) | 21 (43.8) | 53 (58.9) | 30 (62.5) | 52 (48.6) | 82 (52.9) |
| Partly controlled | 65 (26.5) | 4 (9.5) | 15 (31.2) | 19 (21.1) | 11 (22.9) | 35 (32.7) | 46 (29.7) |
| Uncontrolled | 45 (18.4) | 6 (14.3) | 12 (25.0) | 18 (20.0) | 7 (14.6) | 20 (18.7) | 27 (17.4) |
GINA, Global Initiative for Asthma; max, maximum; min, minimum; SD, standard deviation.
In primary care, the average number of exacerbations was greater in patients with moderate-to-severe disease compared with those with mild asthma (mean [SD]: 0.5 [0.7] vs 0.0 [0.2]). Similar results were observed among those under pulmonary medicine specialist care (mean [SD]: 0.9 [1.3] vs 0.2 [0.6]). Overall, the level of asthma symptom control was assessed as well controlled or partly controlled in 81.6% of patients; the remaining 18.4% were considered to have uncontrolled asthma. Across both treatment modalities, asthma was well controlled in a higher percentage of patients with mild asthma than in those with moderate-to-severe disease (68.9% vs 47.1%).
Asthma treatments
SABA prescription categorization
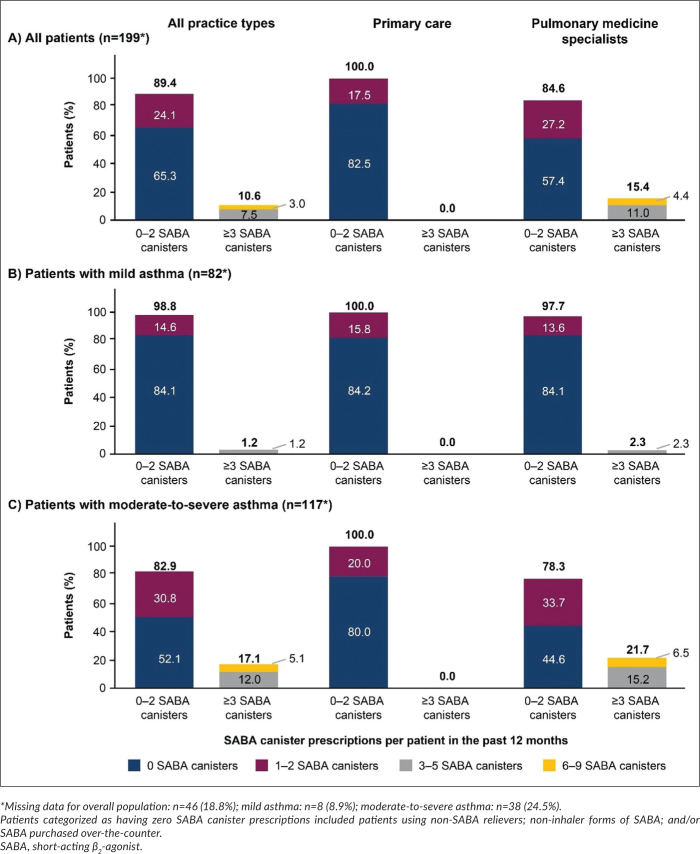
Overall, 24.1% (n=48) of patients were prescribed 1–2 SABA canisters and 10.6% (n=21), ≥3 SABA canisters in the 12 months preceding their study visit.
More than half of all patients (65.3%) had not been prescribed any SABA canisters (Figure 2). A greater percentage of patients with moderate-to-severe asthma compared with mild asthma were prescribed ≥3 SABA canisters in the 12 months prior to study start (17.1% vs 1.2%). In addition, 25.6% (n=23) of patients with mild asthma, 69.6% of whom were adolescents, were prescribed other forms of SABAs, such as nebulized (n=17) or oral (n=6) formulations.
Figure 2.
Proportion of patients (%) receiving SABA prescriptions in the 12 months before study entry according to investigator-classified asthma severity and practice type in the SABINA III Philippines cohort: (A) all patients, (B) mild asthma, (C) moderate-to-severe asthma.
SABA monotherapy and SABA in addition to maintenance therapy
Overall, 6.5% (n=16) of patients were prescribed SABA monotherapy, all of whom were classified as having mild asthma. Patients were prescribed a mean (SD) of 1.1 (0.3) SABA canisters in the 12 months prior to study start. Twice as many patients in primary care were prescribed SABA monotherapy compared with those receiving care from a pulmonary medicine specialist (10.0% vs 4.5%) (Table 3).
Table 3.
SABA prescriptions in the SABINA III Philippines cohort in the previous 12 months (N=245)
| All (N=245) | Primary Care (n=90) |
Pulmonary Medicine Specialists (n=155) |
||||||
|---|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n=42) | Investigator-classified moderate-to-severe asthma (n=48) | All (n=90) | Investigator-classified mild asthma (n=48) | Investigator-classified moderate-to-severe asthma (n=107) | All (n=155) | |||
| Patients prescribed SABA monotherapy, n (%) | ||||||||
| Yes | 16 (6.5) | 9 (21.4) | 0 (0.0) | 9 (10.0) | 7 (14.6) | 0 (0.0) | 7 (4.5) | |
| No | 229 (93.5) | 33 (78.6) | 48 (100.0) | 81 (90.0) | 41 (85.4) | 107 (100.0) | 148 (95.5) | |
|
| ||||||||
| Number of canisters or inhalers per patient prescribed in the previous 12 months before study visit | ||||||||
| Number of patients | 12 | 6 | 0 | 6 | 6 | 0 | 6 | |
| Mean (SD) | 1.1 (0.3) | 1.2 (0.4) | 0 (0.0) | 1.2 (0.4) | 1.0 (0.0) | 0 (0.0) | 1.0 (0.0) | |
| High dose | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0 (0, 0 | 1.0 (1.0, 2.0) | 1.0 (1.0, 1.0) | 0 (0, 0) | 1.0 (1.0, 1.0) | |
|
| ||||||||
| Number of canisters or inhalers (as categories) per patient prescribed in the previous 12 months before study visit, n (%) | ||||||||
| 0–2 | 12 (100.0) | 6 (100.0) | 0 (0.0) | 6 (100.0) | 6 (100.0) | 0 (0.0) | 6 (100.0) | |
| ≥3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Missing data | 4 | 3 | 0 | 3 | 1 | 0 | 1 | |
| Total | 12 | 6 | 0 | 6 | 6 | 0 | 6 | |
|
| ||||||||
| Patients prescribed SABA reliever in addition to maintenance therapy, n (%) | ||||||||
| Yes | 99 (40.4) | 1 (2.4) | 28 (58.3) | 29 (32.2) | 4 (8.3) | 66 (61.7) | 70 (45.2) | |
| No | 146 (59.6) | 41 (97.6) | 20 (41.7) | 61 (67.8) | 44 (91.7) | 41 (38.3) | 85 (54.8) | |
|
| ||||||||
| Number of canisters or inhalers per patient prescribed in the previous 12 months before study visit | ||||||||
| Number of canisters | 57 | 0 | 5 | 5 | 1 | 51 | 52 | |
| Mean (SD) | 2.5 (1.6) | 0 (0.0) | 1.2 (0.4) | 1.2 (0.4) | 4.0 (NA) | 2.6 (1.7) | 2.6 (1.6) | |
| Median (min, max) | 2.0 (1.0, 6.0) | 0 (0.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 4.0 (4.0, 4.0) | 2.0 (1.0, 6.0) | 2.0 (1.0, 6.0) | |
| Missing data | 42 | 1 | 23 | 24 | 3 | 15 | 18 | |
|
| ||||||||
| Number of canisters or inhalers (as categories) per patient prescribed in the previous 12 months before study visit, n (%) | ||||||||
| 0–2 | 36 (63.2) | 0 (0.0) | 5 (100.0) | 5 (100.0) | 0 (0.0) | 31 (60.8) | 31 (59.6) | |
| 3–5 | 15 (26.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 14 (27.5) | 15 (28.8) | |
| 6–9 | 6 (10.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (11.8) | 6 (11.5) | |
| >9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Missing data | 42 | 1 | 23 | 24 | 3 | 15 | 18 | |
| Total | 57 | 0 | 5 | 5 | 1 | 51 | 52 | |
SABA, short-acting β2-agonist; max, maximum; min, minimum; SD, standard deviation.
A total of 40.4% (n=99) of patients were prescribed SABA reliever therapy in addition to maintenance therapy. Nearly all of these patients were classified as having moderate-to-severe asthma (n=94 [94.9%]), with 45.2% under the care of a pulmonary medicine specialist (vs 32.2% in primary care). Patients in primary and pulmonary medicine specialist care were prescribed a mean (SD) of 1.2 (0.4) and 2.6 (1.6) canisters, respectively. In total, 36.8% were prescribed ≥3 canisters in the previous 12 months.
SABA purchase
Of the 30.6% (n=75) of patients who purchased OTC SABA, 24.0% (n=18) collected 1–2 SABA canisters and 4.0% (n=3) purchased ≥3 SABA canisters in the 12 months prior to their study visit. Of these patients, 72% (n=54) provided no information on the number of SABA canisters purchased without a prescription, although it is likely that they purchased non-canister forms of SABA, e.g., oral or nebulized formulations (Appendix Table 2). Patients treated in primary care purchased more OTC SABA canisters than those treated by pulmonary medicine specialists (38.9% vs 25.8%). In primary care, more patients with moderate-to-severe asthma purchased SABA without a prescription than did those with mild asthma (47.9% vs 28.6%). Conversely, a greater percentage of patients with mild asthma and under pulmonary medicine specialist care purchased OTC SABA than did their counterparts with moderate-to-severe disease (37.5% vs 20.6%).
Maintenance medication
An ICS was prescribed to 2.4% (n=6) of patients, all of whom were classified with moderate-to-severe asthma and were treated by pulmonary medicine specialists (Table 4). However, ICS/LABA fixed-dose combinations were prescribed to 63.7% (n=156) of patients, almost all of whom were classified as having moderate-to-severe disease. Of these patients, 43.2% (n=67), 20.6% (n=32), and 36.1% (n=56) were prescribed low-, medium-, and high-dose ICS, respectively.
Table 4.
Other asthma treatments prescribed to patients in the SABINA III Philippines cohort in the previous 12 months (N=245)
| All (N=245) | Primary Care (n=90) |
Pulmonary Medicine Specialists (n=155) |
|||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n=42) | Investigator-classified moderate-to-severe asthma (n=48) | All (n=90) | Investigator-classified mild asthma (n=48) | Investigator-classified moderate-to-severe asthma (n=107) | All (n=155) | ||
| Patients prescribed ICS, n (%) | |||||||
| Yes | 6 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (5.6) | 6 (3.9) |
| No | 239 (97.6) | 42 (100.0) | 48 (100.0) | 90 (100.0) | 48 (100.0) | 101 (94.4) | 149 (96.1) |
|
| |||||||
| Total daily ICS dose prescribed, n (%) | |||||||
| Low dose | 4 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (66.7) | 4 (66.7) |
| Medium dose | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| High dose | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Total | 6 | 0 | 0 | 0 | 0 | 6 | 6 |
|
| |||||||
| Number of canisters or inhalers per patient prescribed in the previous 12 months before study visit | |||||||
| Number of patients | 6 | 0 | 0 | 0 | 0 | 6 | 6 |
| Mean (SD) | 1.0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0 (0.0) | 1.0 (0.0) |
| Median (min, max) | 1.0 (1.0, 1.0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 1.0 (1.0, 1.0) | 1.0 (1.0,1.0) |
|
| |||||||
| Patients prescribed ICS/LABA (fixed-dose combination), n (%) | |||||||
| Yes | 156 (63.7) | 2 (4.8) | 48 (100.0) | 50 (55.6) | 1 (2.1) | 105 (98.1) | 106 (68.4) |
| No | 89 (36.3) | 40 (95.2) | 0 (0.0) | 40 (44.4) | 47 (97.9) | 2 (1.9) | 49 (31.6) |
|
| |||||||
| Total daily ICS dose prescribed, n (%) | |||||||
| Low dose | 67 (43.2) | 2 (100.0) | 30 (63.8) | 32 (65.3) | 1 (100.0) | 34 (32.4) | 35 (33.0) |
| Medium dose | 32 (20.6) | 0 (0.0) | 11 (23.4) | 11 (22.4) | 0 (0.0) | 21 (20.0) | 21 (19.8) |
| High dose | 56 (36.1) | 0 (0.0) | 6 (12.8) | 6 (12.2) | 0 (0.0) | 50 (47.6) | 50 (47.2) |
| Missing data | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Total | 155 | 2 | 47 | 49 | 1 | 105 | 106 |
|
| |||||||
| Patients prescribed OCS burst/short-course OCS treatment, n (%) | |||||||
| Yes | 85 (34.7) | 14 (33.3) | 9 (18.8) | 23 (25.6) | 7 (14.6) | 55 (51.4) | 62 (40.0) |
| No | 160 (65.3) | 28 (66.7) | 39 (81.2) | 67 (74.4) | 41 (85.4) | 52 (48.6) | 93 (60.0) |
|
| |||||||
| Patients prescribed OCS long-term/OCS maintenance treatment, n (%) | |||||||
| Yes | 16 (6.5) | 0 (0.0) | 1 (2.1) | 1 (1.1) | 0 (0.0) | 15 (14.0) | 15 (9.7) |
| No | 229 (93.5) | 42 (100.0) | 47 (97.9) | 89 (98.9) | 48 (100.0) | 92 (86.0) | 140 (90.3) |
ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; max, maximum; min, minimum; OCS, oral corticosteroids; SD, standard deviation
Oral corticosteroids
A short course of OCS (OCS burst) was prescribed to 34.7% (n=85) of patients in the 12 months prior to their study visit (Table 4). The percentage of patients prescribed an OCS burst by a pulmonary medicine specialist was higher than those treated by a primary care provider (40.0% vs 25.6%). In primary care, a greater percentage of patients classified as having mild asthma compared with moderate-to-severe asthma (33.3% vs 18.8%) were prescribed OCS burst treatment. Conversely, more patients under the care of a pulmonary medicine specialist and classified as having moderate-to-severe asthma than mild asthma were prescribed an OCS burst (51.4% vs 14.6%).
Antibiotics
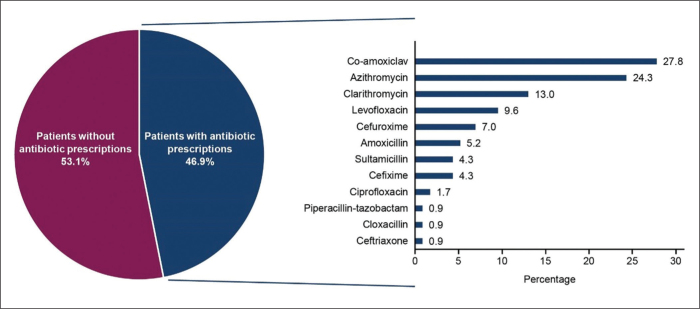
Overall, 46.9% (n=115) of patients were prescribed antibiotics, with co-amoxiclav (n=29 [27.8%]), azithromycin (n=28 [24.3%]), and clarithromycin (n=15 [13.0%]) being the most commonly prescribed (Figure 3). A higher percentage of patients under the care of a pulmonary medicine specialist were prescribed antibiotics compared with patients treated in primary care (52.3% vs 37.8%). Primary care physicians prescribed marginally more antibiotics to patients classified as having mild asthma than to those with moderate-to-severe asthma (40.5% vs 35.4%). Conversely, pulmonary medicine specialists prescribed more antibiotics to patients with moderate-to-severe asthma than to those classified with mild disease (54.2% vs 47.9%).
Figure 3.
Antibiotics prescribed to patients with asthma in the 12 months prior to the study visit.
Asthma treatments and exacerbations
When stratified by treatments prescribed in the previous 12 months, most patients prescribed short-courses of OCS experienced ≥1 severe exacerbation (68.2%, n=85), followed by those prescribed SABA add-on therapy (53.5%, n=99), ICS/LABA fixed-dose combination (48.7%, n=156), and antibiotics (44.3%, n=115).
DISCUSSION
This nationwide cross-sectional study was conducted as part of the global SABINA studies to characterize the asthma patient population and describe the extent of SABA prescriptions in the Philippines. Despite most patients (63.3%) in this study being classified with moderate-to-severe asthma, our findings indicated that in 81.6% of all patients, the disease was considered well controlled or partly controlled, irrespective of practice type. Thus, patients experienced a relatively low asthma exacerbation burden in the 12 months before their study visit. Overall, ~11% of all patients were prescribed ≥3 SABA canisters in the previous 12 months, with patients classified as having moderate-to-severe asthma treated by pulmonary medicine specialists reporting a higher rate of SABA over-prescriptions compared with their counterparts classified as having mild disease, as well as those treated by primary care providers. Taken together, these data suggest that over-prescription of SABA canisters may not be a widespread practice in the Philippines and that the management and treatment of patients with asthma in this country may have improved since earlier reports.8,11 However, patients may continue to rely on other SABA formulations, as evident in the 2020 Asthma Patients’ and Physicians’ Perspectives on the Burden and Management of Asthma (APPaRENT) study conducted in four countries, including the Philippines, where 33.7% of Filipino patients exclusively used rescue/reliever inhalers for rapid alleviation of symptoms.23
Recent assessments of patients with asthma in the Philippines are lacking, thereby challenging cross-study comparisons. However, data from the 2000 Asthma Insights and Reality in Asia-Pacific (AIRIAP) study, which surveyed 3,207 adults, more than one-quarter of whom were diagnosed with moderate-to-severe disease, indicated that quick-relief medications were used by >90% of Filipino patients with asthma, whereas anti-inflammatory medications were used by <10%.8 The most common reasons for not using asthma medication as directed were: treatment not considered important, fear of adverse effects, lack of an immediate therapeutic effect, and/or the cost-of-drug. However, the AIRIAP survey was conducted at a single site in the Philippines, implying that results may not be generalizable to the entire asthma population in that country.8 In contrast, results from this SABINA cohort study demonstrate considerable improvements in asthma care during the 20 years since the AIRIAP survey, as anti-inflammatory medication in the form of fixed-dose ICS/LABA was prescribed to ~64% of all patients with asthma and, as expected, nearly all classified as having moderate-to-severe disease.
Although our findings indicated a relatively low percentage of SABA canister over-prescriptions and thus, potentially less overuse, these data should be interpreted with the caveat that only data on canister or inhaler forms of SABA were collected and that SABA prescription data represent a surrogate for actual medication usage. Across all 24 countries included in the SABINA III study, the Philippines reported the greatest number of patients using non-canister forms of SABAs, predominantly nebulized with a lower proportion of oral formulations. This may be attributed to local clinical practice guidelines,4 which recommend nebulization for patients who experience severe respiratory distress or who require mechanical ventilation. The use of an alternative, oral SABA or short-acting theophylline is discouraged, given their corresponding slower onsets of action and higher risk of adverse effects.4 However, it is unknown how many patients in the SABINA Philippines cohort who were prescribed nebulized SABA, in reality, were experiencing severe respiratory distress. Notably, almost 70% of patients using other forms of SABA were adolescents.
SABA purchase data gave additional evidence of the potential underestimation of its usage. Overall, ~30% of patients had purchased OTC SABA, with the potential majority obtained in non-canister form. The impact of non-prescription SABA availability on asthma clinical outcomes in the Philippines has not been studied, but evidence from other countries has underscored the reasons for concern. While asthma control in patients who purchase OTC SABA relievers has not been reported to depart significantly from that in patients using prescription rescue medication, a subset of patients with asthma who purchase OTC relievers have been shown to neglect regular controller medication.24,25 Further, such patients are also more likely to avoid physician visits for follow-up asthma reviews.24 While there are merits to OTC availability of relievers,26 healthcare policies focused on patient and pharmacist education, as well as regulations to restrict dispensing of more SABA canisters than required, may help to limit SABA dependence among patients with asthma across all disease severities.
Antibiotic prescriptions for asthma were common in the Philippines, occurring in 47% of patients, a practice observed more frequently under pulmonary medicine specialist care than primary care. While antibiotics may have been prescribed to treat asthma exacerbations provoked by underlying or superimposed bacterial infections,4 occurrence of such exacerbations is considered rare.27 This was evidenced by our findings, which indicated that the number of patients who received antibiotics exceeded those with asthma exacerbations, suggesting that not all who received a prescription for antibiotics may have exhibited signs and/ or symptoms of a bacterial infection. Furthermore, the role of antibiotics and their added benefit to asthma treatment remain inconclusive.28 Indeed, the 2019 Philippine Consensus Report on Asthma Diagnosis and Management opposes the routine use of antibiotics in asthma management, recognizing their potential clinical benefit only in cases where there is clear evidence of an exacerbation induced by a bacterial infection.4 Reasons for such unrestrained antibiotic use in patients with a chronic respiratory disease may be manifold including cost and availability compared with more expensive anti-inflammatory medication.11
Our data also show that a substantial number of patients in the SABINA Philippines cohort were prescribed a short course of OCS. While typically, this treatment is reserved for acute asthmatic symptoms and exacerbations,21 we observed that 31.8% of patients prescribed an OCS burst had not experienced any asthma exacerbations in the 12 months before their study visit. This departure from guideline-directed asthma management might be attributed to prevailing treatment practices in the Philippines in which OCS may be prescribed for non-exacerbation asthmatic events. In addition, evidence is mounting that consistent use of OCS is associated with adverse systemic consequences, including loss of bone mineral density, hypertension, and gastrointestinal disorders.29 Moreover, while OCS medications are relatively inexpensive, the long-term economic impact of treating OCS-induced adverse events among patients with asthma is concerning.30 Therefore, strategies to reduce the inappropriate use of OCS should lower the burden of disease on both patients with asthma and the healthcare system as a whole.
Taken together, government, physician, and community initiatives that focus on broader communication with patients and parents of children with asthma to prevent the dissemination of misinformation on asthma treatments, including OTC SABA, antibiotics, and OCS, are critical components of the effort to optimize asthma care in the Philippines. Overall, asthma treatment practices in the Philippines were generally consistent with evidence-based guidelines in use at the time of the study (May 2019 – January 2020),20 and our findings suggest that despite more than one-third of the SABINA Philippines cohort being classified at GINA treatment step 1 (36.7% [mild asthma]), only 6.5% were prescribed SABA monotherapy, although the use of non-canister SABA forms by these patients cannot be discounted. No patients with mild asthma were prescribed low-dose ICS, although a few received prescriptions for ICS/LABA. Such outcomes also may reflect local practice patterns and realities, such as the availability of ICS/LABA fixed-dose combinations as opposed to ICS inhalers.
With the updated GINA recommendations31 and the 2019 Philippine Consensus Report on Asthma Diagnosis and Management4 heralding a major paradigm shift in asthma care, it is even more crucial to translate these modifications into clinical practice to optimize sustained asthma control. In parallel with initiatives focused on improving patient, physician, and pharmacist education on updated treatment guidelines, policy changes aimed at expanding healthcare accessibility, appreciable improvements in affordability of appropriate treatment, and curtailing potentially harmful prescribing habits may contribute to further advances in asthma management and prevention in the Philippines. Previous government initiatives have focused specifically on optimizing asthma management in the Philippines. Notably, the Asthma Awareness, Education and Treatment Act of 200532 launched disease awareness programs and epidemiologic surveillance activities and provided funds to support the management and prevention of respiratory conditions in low-income families. In addition, the Universal Health Care Act (RA 11223)33 of 2018 aims to regulate prescriptions at all levels of care supported by cost-effectiveness studies and updates to clinical practice guidelines. The mandatory accreditation of service delivery networks for payments through capitation is expected to ensure adherence to the law and to limit the unwarranted prescribing of medications, such as SABA. Furthermore, the availability of affordable anti-inflammatory drugs under universal healthcare mandates is likely to improve adherence to guideline-preferred maintenance medication (ICS/ LABA), thereby reducing SABA prescriptions. Thus, growing awareness among both patients and HCPs, in combination with the implementation of these recent national initiatives, may have contributed to our findings, which suggest that asthma was relatively less severe and better controlled in the Philippines cohort compared with the overall SABINA III asthma population. Indeed, country-aggregated data from all 24 countries of SABINA III demonstrated poorer asthma outcomes and a considerably greater prevalence of SABA over-prescriptions at 38%.20
That said, we acknowledge some limitations to this study. Since data transcribed onto the eCRF relied on investigator assessments, our findings may have been impacted by misinterpretation of instructions or incorrect patient classification. In addition, SABA prescription was used as a proxy for actual SABA usage, as it is possible that patients did not use all the SABA canisters prescribed. Further, this study focused on SABA canister prescriptions, and therefore, the data did not fully capture potential overuse of oral (tablets) and nebulized forms of SABA. Additionally, the large volume of missing data on SABA prescriptions in the 12 months preceding the date of the study visit precluded a thorough assessment of the extent of SABA use in the Philippines. This study was restricted to adults and adolescents, enrolled a relatively small, analyzable sample size (N=245), and did not include pediatric patients aged <12 years; therefore, the study population may not be truly representative of the overall asthma patient population or reflect the way asthma is currently being managed in the Philippines, meaning that results should be generalized with caution. In addition, due to the low patient numbers, it was not feasible to examine the association between SABA prescriptions and asthma-related outcomes. Finally, this study did not examine the impact of a range of factors, including comorbidities, exposure to allergens, adherence to treatment regimens, inhaler technique, patient education, patient-physician communication, and patient’s psychological dependence on rescue medication, on asthma control.
Overall, this is the first study to investigate the current status of SABA prescriptions and clinical outcomes of patients with asthma in the Philippines. The use of a standardized threshold for defining SABA over-prescription enabled a comparison of potential SABA use not only across regions but also globally across countries, thereby adding to the data generated by other SABINA studies describing the combined global magnitude and significance of SABA over-prescription and ICS under-prescription.
CONCLUSION
While SABA over-prescription was relatively low in the Philippines, the availability of non-inhaler forms of SABA and the high percentage of OTC SABA purchases suggest that actual SABA usage may be greater than documented. Notably, almost 50% of patients classified as having mild or moderate-to-severe asthma were prescribed antibiotics. These findings may necessitate the implementation of policy changes in the Philippines that target non-prescription SABA purchases and antibiotic prescriptions to improve overall asthma care and management.
Acknowledgments
Medical writing support was provided by Niraj Babu, PhD, and Michelle Rebello, PhD, of Cactus Life Sciences (part of Cactus Communications) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and funded by AstraZeneca.
APPENDICES
Appendix Table 1.
BMI of adolescent patients with asthma.
| BMI groupsa, n (%) | All (n=60) |
|---|---|
| Underweight, less than the 5th percentile | 2 (3.3) |
| Healthy weight, 5th percentile up to the 85th percentile | 38 (63.3) |
| Overweight, 85th to less than the 95th percentile | 10 (16.7) |
| Obese, equal to or greater than the 95th percentile | 10 (16.7) |
BMI is categorized according to the WHO1 and CDC2 guidelines
BMI, body mass index; CDC, Centers for Disease Control and Prevention; WHO, World Health Organization.
World Health Organization. Growth reference data for 5-19 years [Internet]. 2007 [cited 2021 Nov]. Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years.
Centers for Disease Control and Prevention. BMI percentile calculator for child and teen [Internet]. [cited 2021 Nov]. Available from: https://www.cdc.gov/healthyweight/bmi/calculator.html.
Appendix Table 2.
SABA canisters purchased OTC without a prescription in the previous 12 months by patients in the SABINA III Philippines cohort (N=245).
| All (N=245) | Primary Care (n=90 |
Pulmonary Medicine Specialists (n=155) |
|||||
|---|---|---|---|---|---|---|---|
| Investigator-classified mild asthma (n=42) | Investigator-classified moderate-to-severe asthma (n=48) | All (n=90) | Investigator-classified mild asthma (n=48) | Investigator-classified moderate-to-severe asthma (n=107) | All (n=155) | ||
| Number of patients who received SABA without a prescription in the previous 12 months before study visit | |||||||
| Yes | 75 (30.6) | 12 (28.6) | 23 (47.9) | 35 (38.9) | 18 (37.5) | 22 (20.6) | 40 (25.8) |
| No | 170 (69.4) | 30 (71.4) | 25 (52.1) | 55 (61.1) | 30 (62.5) | 85 (79.4) | 115 (74.2) |
|
| |||||||
| Number of canisters or inhalers (as categories) per patient obtained without a prescription (OTC) | |||||||
| 1–2 | 18 (24.0) | 1 (8.3) | 0 (0.0) | 1 (2.9) | 3 (16.7) | 14 (63.6) | 17 (42.5) |
| 3–5 | 3 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 1 (4.5) | 3 (7.5) |
| >5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not applicable* | 54 (72.0) | 11 (91.7) | 23 (100.0) | 34 (97.1) | 13 (72.2) | 7 (31.8) | 20 (50.0) |
Data are presented as n (%) unless otherwise specified.
“Not applicable” could be selected in the eCRF when patients purchased non-canister forms of SABA (e.g., oral or nebulized SABA) without a prescription.
eCRF, electronic case report form; OTC, over-the-counter; SABA, short-acting β2-agonist
Data availability
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Statement of Authorship
DVD was involved in the conduct of the study and contributed to the collection and analysis of the data. LAN, JFO, and RSM were involved in conceptualization, and contributed to the collection and analysis of the data. ELPS, BKBJ, and RZS contributed to data collection. MEVC contributed to data collection and analysis. MHM contributed to the collection and analysis of the pediatric/ adolescent data. EFHG contributed to the data analysis. JCB collected and reviewed the adolescent data. MJHIB designed the study, and was involved in conceptualization and data analysis. All authors reviewed, finalized and approved the submitted manuscript.
Author Disclosure
DVD received funding and honorarium from Astra-Zeneca as principal investigator for the present study. BKBJ received funding from AstraZeneca for the present study, and for serving as a moderator during an AstraZeneca webcast. JCB received funding, including a study assistant fee, from AstraZeneca for the present study. EFHG and MJHIB were employees of AstraZeneca at the time this study was conducted. LAN, ELPS, MEVC, RZS, JFO, MHM, RSM, and JCB report no conflicts of interest.
REFERENCES
- 1.World Health Organization . Asthma [Internet]. [cited 2021 Nov]. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma.
- 2.Reddel HK, Levy ML. The GINA asthma strategy report: what’s new for primary care? NPJ Prim Care Respir Med. 2015. Jul; 25:15050. doi: 10.1038/npjpcrm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varona LL, Alava HDA, Abong JM, Castor MAR, De Leon JC, Kwong SL. Prevalence of asthma among Filipino adults based on the National Nutrition and Health Survey (NNHeS). Philipp J Intern Med. 2014. Oct; 52(4):182-188. [Google Scholar]
- 4.Philippine College of Chest Physicians . Philippine Consensus Report on Asthma Diagnosis and Management [Internet]. 2019. [cited 2021 Apr]. Available from: http://philchest.org/xp/publications/clinical-practice-guidelines/.
- 5.Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014. Jun; 24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017. Apr; 17(1):74. doi: 10.1186/s12890-017-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy. 2015. Sep; 8:93-103. doi: 10.2147/JAA.S82633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zainudin BMZ, Lai CKW, Soriano JB, Jia-Horng W, De Guia TS, Asthma Insights and Reality in Asia-Pacific (AIRIAP) Steering Committee . Asthma control in adults in Asia-Pacific. Respirology. 2005. Nov; 10(5):579-86. doi: 10.1111/j.1440-1843.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 9.Global Asthma Network . The global asthma report [Internet]. 2018. [cited 2021 Nov]. Available from: http://www.globalasthmareport.org/.
- 10.Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, et al. Asthma in Asia: Physician perspectives on control, inhaler use and patient communications. J Asthma. 2016. Sep; 53(7):761-9. doi: 10.3109/02770903.2016.1141951. [DOI] [PubMed] [Google Scholar]
- 11.Lalloo UG, Walters RD, Adachi M, De Guia T, Emelyanov A, Fritscher CC, et al. Asthma programmes in diverse regions of the world: challenges, successes and lessons learnt. Int J Tuberc Lung Dis. 2011. Dec; 15(12):1574-87. doi: 10.5588/ijtld.11.0289. [DOI] [PubMed] [Google Scholar]
- 12.Lai CKW, Kim YY, Kuo SH, Spencer M, Williams AE. Cost of asthma in the Asia-Pacific region. Eur Respir Rev. 2006. Jun; 15(98):10-6. doi: 10.1183/09059180.06.00009802 [DOI] [Google Scholar]
- 13.Global Initiative for Asthma . Global strategy for asthma prevention and management [Internet]. 2019. [cited 2021 Nov]. Available from: https://ginasthma.org/reports/2019-gina-report-global-strategy-for-asthma-management-and-prevention/.
- 14.Beasley R, Bird G, Harper J, Weatherall M. The further paradoxes of asthma management: time for a new approach across the spectrum of asthma severity. Eur Respir J. 2018. Nov; 52(5):1800694. doi: 10.1183/13993003.00694-2018. [DOI] [PubMed] [Google Scholar]
- 15.O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017. Sep; 50(3):1701103. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 16.FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017. Oct; 131:135-40. doi: 10.1016/j.rmed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Stanford RH, Shah MB, D’Souza AO, Dhamane AD, Schatz M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012. Dec; 109(6):403-7. doi: 10.1016/j.anai.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Babar ZU, Lessing C, Mace C, Bissell K. The availability, pricing and affordability of three essential asthma medicines in 52 low- and middle-income countries. Pharmacoeconomics. 2013. Nov; 31(11):1063-82. doi: 10.1007/s40273-013-0095-9. [DOI] [PubMed] [Google Scholar]
- 19.Cabrera CS, Nan C, Lindarck N, Beekman MJHI, Arnetorp S, van der Valk RJP. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J. 2020. Feb; 55(2):1901858. doi: 10.1183/13993003.01858-2019. [DOI] [PubMed] [Google Scholar]
- 20.Bateman ED, Price DB, Wang HC, Khattab A, Schonffeldt P, Catanzariti A, et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022. May; 59(5):2101402. doi: 10.1183/13993003.01402-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global Initiative for Asthma . Global strategy for asthma prevention and management [Internet]. 2017. [cited 2021 Nov]. Available from: https://ginasthma.org/2017-gina/
- 22.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. Jan; 363(9403):157-63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Chapman KR, An L, Bosnic-Anticevich S, Campomanes CM, Espinosa J, Jain P, et al. Asthma patients’ and physicians’ perspectives on the burden and management of asthma. Respir Med. 2021. Sep; 186:106524. doi: 10.1016/j.rmed.2021.106524. [DOI] [PubMed] [Google Scholar]
- 24.Reddel HK, Ampon RD, Sawyer SM, Peters MJ. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open. 2017. Sep; 7(9):e016688. doi: 10.1136/bmjopen-2017-016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglass JA, Goeman DP, McCarthy EA, Sawyer SM, Aroni RA, Stewart K, et al. Over-the-counter beta2-agonist purchase versus script: a cross-sectional study. Respir Med. 2012. Feb; 106(2):223-29. doi: 10.1016/j.rmed.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Gerald JK, Wechsler ME, Martinez FD. Reply: safety of short-acting β-agonists not a barrier to making asthma rescue and controller medications available over the counter. Ann Am Thorac Soc. 2014. Sep; 11(7):1162-63. doi: 10.1513/AnnalsATS.201407-318LE. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations--a GA2LEN-DARE systematic review. Allergy. 2011. Apr; 66(4):458-68. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque M. Antimicrobial use, prescribing, and resistance in selected ten selected developing countries: a brief overview. Asian J Pharm Clin Res. 2017. Aug; 10(8):37-45. doi: 10.22159/ajpcr.2017.v10i8.19468 [DOI] [Google Scholar]
- 29.Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020. Apr; 29(155):190151. doi: 10.1183/16000617.0151-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017. Jun; 18(1):129. doi: 10.1186/s12931-017-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Global Initiative for Asthma . Global strategy for asthma prevention and management [Internet]. 2022. [cited 2022 August]. Available from: https://ginasthma.org/gina-reports/
- 32.Senate Office of the Secretary, Republic of the Philippines . Asthma Awareness, Education and Treatment Act of 2005 [nternet]. 2005. [cited 2021 Nov]. Available from: http://legacy.senate.gov.ph/lisdata/33362218!.pdf.
- 33.Department of Health, Republic of the Philippines , Republic Act No. 11223, An act instituting universal health care for all Filipinos, prescribing reforms in the health care system, and appropriating funds therefor [Internet]. 2018. [cited 2021 Nov]. Available from: https://doh.gov.ph/RA11223.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.