Abstract
A 61-year-old male diagnosed with laryngeal squamous cell carcinoma presented with hoarseness, progressive dysphagia leading to aspiration, and dyspnea one month after definitive radiation therapy. Examination revealed a diffusely swollen glottis, paralyzed vocal cords, and post-radiation fibrosis. Several glottic biopsies yielded results negative for malignancy and favored radiation-induced changes. When presented with the option of further diagnostic testing with a positron emission tomography (PET) scan or an outright laryngectomy, the patient decided on the latter. Final histopathologic diagnosis was negative for recurrence of malignancy.
This case demonstrates treatment dilemmas for patients with laryngeal carcinoma with uncertain recurrence wherein radical surgical management may prove to be a viable option to achieve both diagnostic certainty and ultimate relief of symptoms.
Keywords: laryngeal cancer, laryngectomy, radiotherapy
INTRODUCTION
The larynx in humans has evolved to function as an organ for respiration, airway protection, and phonation. Normal anatomical configuration and coordinated movement of the individual structures must result in adequate opening and closing of the glottis or laryngeal inlet in order to ensure proper inspiration and expiration, to protect the airway from entry of foreign bodies such as food during swallowing, to expel material from the lungs through coughing, and to produce a normal-sounding voice during phonation.1 Pathologies causing structural and functional changes to the anatomy can lead to symptoms that can range from bothersome to life-threatening, depending on the severity. These disorders of the larynx can be caused by infection, inflammation, trauma, and neoplasms, among others.
Laryngeal carcinoma is one etiology that must be addressed urgently and adequately to avoid long-term sequelae. It represents about 33% of all head and neck malignancies with an incidence of around 2.76 cases per year per 100,000 with an increasing incidence and prevalence through the years.2,3 For early-stage laryngeal carcinomas such as T1 and T2, which qualify for laryngeal preservation treatment modalities, definitive radiation therapy (RT) without surgical intervention is an option.4 This non-surgical option treats the malignancy and preserves laryngeal function.
However, it is known that RT has its own set of side effects. Toxic side effects of RT are oxidative changes and vascular compromise which result in a dysfunctional larynx causing symptoms such as hoarseness, odynophagia, dysphagia, and dyspnea.5 Aside from radiation side effects, surveillance is warranted to detect recurrence which is common even among early-stage lesions. Recurrences can occur in 5-13% of T1 cases and 25-30% of T2 cases.3 Management of these dysfunctional larynges requires both determining a possible malignant recurrence and addressing complications, such as aspiration and respiratory distress.
CASE PRESENTATION
A 61-year-old man with diabetes, no history of smoking, and occasional alcohol intake presented with a 6-year history of hoarseness for which he was initially managed as a case of laryngopharyngeal reflux and given proton pump inhibitors. In the interim, there was persistent hoarseness but with no noted dyspnea, dysphagia, weight loss, or presence of palpable neck masses. Due to the persistence of hoarseness, a laryngeal videostroboscopy was done with unrecalled results. The patient was then advised to undergo direct laryngoscopy (DL) and biopsy in another institution which revealed squamous cell carcinoma. Records were unavailable for review. He was diagnosed with glottic squamous cell carcinoma St. II (cT2N0M0) and offered definitive RT. He underwent 29 sessions of RT and was given a total of 65.25 Gy to the larynx and neck.
One month after RT, the hoarseness persisted, and the patient began experiencing progressive dyspnea and dysphagia to both solids and liquids. This later led to episodes of aspiration. Flexible laryngoscopy showed bilateral vocal cord paralysis with no distinct masses while MRI imaging showed diffused swelling of the laryngeal mucosa (Figure 1). In order to address the progressive respiratory distress and aspiration, a tracheostomy was done. A repeat DL and biopsy were also done which yielded necrotic tissue with cellular atypia favoring radiation-induced changes. Episodes of aspiration and difficulty of breathing eventually resolved, and decannulation of the tracheostomy tube was done two weeks after.
Figure 1.
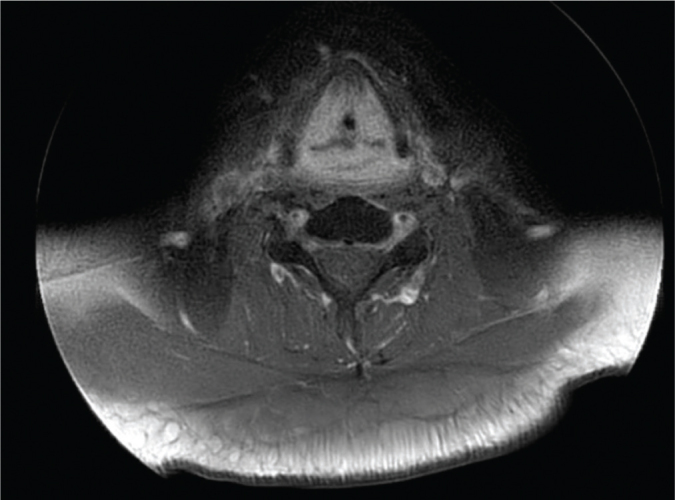
MRI of the larynx 1 month after completing radio-therapy showing diffuse swelling of the glottis.
One year after decannulation, there was recurrence of respiratory distress, dysphagia, and aspiration. He underwent a repeat tracheostomy, DL, and biopsy 14 months after RT where a hard mass on the right true and false vocal cord was noted (Figure 2). Final histopathology once again showed reactive changes with no malignant cells seen. An MRI of the larynx was repeated which showed an ill-defined enhancing soft tissue focus in the left glottic-supraglottic region and thickening on the right glottic region (Figure 3). On indirect laryngoscopy one month after, the entire laryngeal complex was markedly edematous with bilateral vocal cord paralysis (Figure 4).
Figure 2.
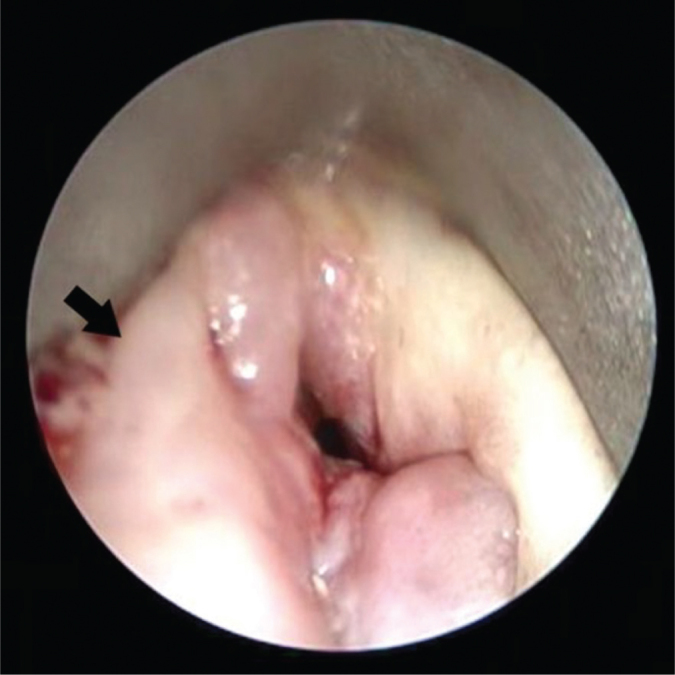
Direct laryngoscopy 14 months after RT; intraoperative finding of a hard fixed mass on the right vocal cord (arrow).
Figure 3.
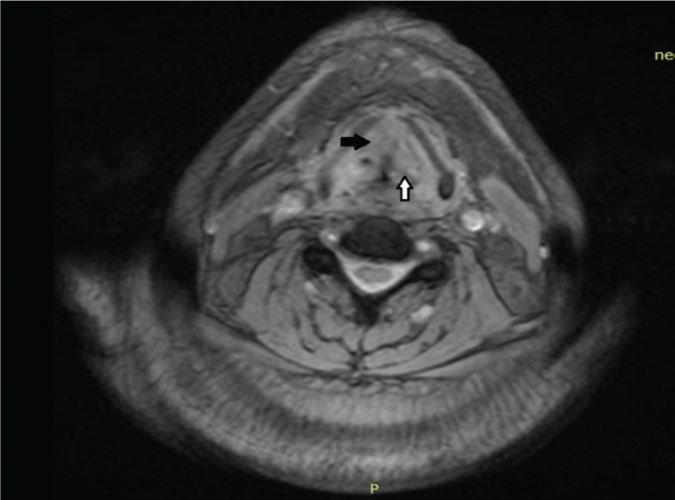
MRI of the larynx 15 months after RT; an ill-defined enhancing focus on the left vocal cord (white arrow) and thickening of the right vocal cord (black arrow).
Figure 4.
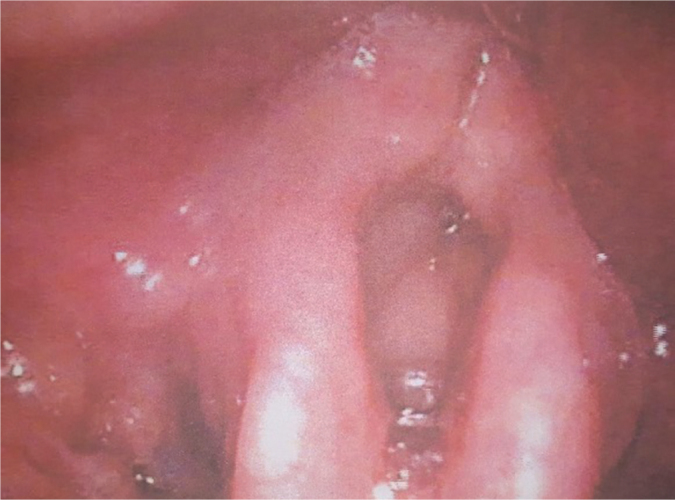
Indirect laryngoscopy 16 months after RT showing diffuse edema.
This case of a dysfunctional irradiated larynx posed a dilemma in diagnosis. Despite multiple negative biopsies, a malignant recurrence could not be completely ruled out. This case also presented a challenge in management, specifically determining the best treatment plan which would resolve the recurrent aspiration and dyspnea.
Following a proposed treatment algorithm by McGuirt for post-irradiation laryngeal carcinoma, the ideal adjunct to a biopsy to determine the presence of malignancy is a positron emission tomography computed tomography (PET-CT) scan.5 However, another treatment option to resolve the symptoms of a dysfunctional larynx is an outright total laryngectomy. Both options of a PET-CT scan and a total laryngectomy were presented to the patient. He refused the PET-CT. Risks and benefits of the surgery were explained, which the patient understood and accepted. He opted for surgery, and a total laryngectomy was done. Final histopathology showed no residual tumor as well as chronic inflammation with fibrosis, granulation tissue formation, and necrosis, and the case was signed out as a dysfunctional larynx from post-radiation changes.
Post-operatively, the patient was able to resume oral feeding with no recurrence of aspiration and was able to breathe without difficulty through the tracheostomal site. He was also referred for voice rehabilitation via esophageal speech. Since then, there has been no recurrence of dyspnea, dysphagia, or any visible mass, and on his last follow up three years after the total laryngectomy, the patient reported improvement of quality of life.
DISCUSSION
Early-stage laryngeal carcinomas (stage T1-T2) may be treated with definitive RT. The National Comprehensive Cancer Network 2022 Guidelines stated that the total radiation dose for T2 glottic cancers may range from 65.25 Gy to 70 Gy with a radiation fraction size of 2.25 Gy and 2.0 Gy, respectively.4 Yamazaki et al. compared the use of fraction sizes 2.25 Gy and 2.0 Gy and noted better local control (92% versus 77%) in the former regimen but with no significant differences between 5-year survival rates (100% versus 97%) and adverse outcomes between the two.6 The same study also did not note any severe late radiation changes in either treatment group. Other studies have also shown significantly lower incidences of late radiation changes in patients given 2.25 Gy per fraction or less.7 Although a lower rate of postradiation changes have been documented in this fractionation range, the absence of these changes cannot be ascertained.
The damage that RT causes in both malignant and healthy cells leads to several early and late radiotherapy-related changes.8-10 Early radiation-related changes occur less than 3 months after RT and are caused by direct oxidative stress leading to DNA damage and cell death.8 Late radiation-related changes are caused by irreversible damage to the vascular supply of the larynx which results in ischemia and fibrosis.8 Both early and late changes may present with edema, hoarseness, dysphagia, and odynophagia, but late changes may cause cartilage necrosis, muscle fibrosis, progressive edema, and fistula formation.8 These cause problematic and even potentially life-threatening symptoms such as aspiration from swallowing difficulties and respiratory distress. Patients who present with these severe complications are classified as having dysfunctional larynges.
Thus, the above case of complications in an irradiated larynx presented a dilemma in terms of surveillance. These dysfunctional larynges are problematic due to the need to determine whether symptoms are caused by a malignant recurrence or post-radiation changes. Some studies have reported five-year laryngectomy-free survival rates at 80–86% for T2 stage cancers after RT.11,12 Studies have reported various rates of dysfunctional larynges after radiation ranging from 0.3-21% although stratification of cancer stage was not specified in any of the studies.9,11,12 Allen et al. reviewed several studies which showed that 45%-75% of those with post-radiation changes were found to have recurrent or residual malignancy.8 It is therefore imperative for an occult malignancy to be evaluated since this would impact management.
Several studies have advocated the use of biopsy and PET-CT scans as the most sensitive combination of diagnostics to work up a possible recurrence of malignancy.5,8,13 PET-CT alone has reported high specificity (85-95%) with lower and varying degrees of sensitivity (35-85%) in detecting recurrent malignancies.14,15 Inflammation from radiation changes shows increased fluorodeoxy-D-glucose uptake;hence, scans must be done 8-12 weeks after RT to decrease false positive rates.13,14 Positive PET-CT findings warrant further investigation with a biopsy due to false positives from irradiated and inflamed tissue.13-15 Although an ideal imaging modality, relative inaccessibility and price hinder the wider use of PET-CT scan in cancer surveillance.
Another major concern was the severity of the symptoms the patient experienced. Both radiation-induced laryngeal changes and a recurrence of malignancy may present with a wide spectrum of symptoms reaching debilitating levels. These may require permanent tracheostomies, feeding tubes, and recurrent treatment for aspiration pneumonia.9 Even if the PET-CT scan was negative for signs of malignancy, this would not have relieved the patient’s laryngeal dysfunction. Surgical management would have been recommended nonetheless.
Total laryngectomy is the standard for salvage treatment of recurrent laryngeal carcinoma and a definitive option for dysfunctional irradiated larynges.10,16 Other conservation laryngeal surgical (CLS) procedures also exist such as transoral laser microsurgery, laryngofissure with cordectomy, vertical partial laryngectomy, supracricoid partial laryngectomy, and supraglottic laryngectomy. However, their use has been studied only among patients with laryngeal carcinoma needing salvage surgery for recurrence after radiation failure and not for patients with dysfunctional larynges after RT.16,17 Due to the absence of evidence showing the benefit of CLS in the latter group, a total laryngectomy was deemed the best option to resolve the aspiration, to improve feeding, and to address the respiratory problems.10 Notably, the previous negative biopsies could not completely rule out a malignancy due to the possibility of harboring an occult one. This could have only been identified with removal and examination of the entire organ. In this case, a total laryngectomy was chosen both for therapeutic and diagnostic purposes.
Studies on dysfunctional larynges after RT have shown that a total laryngectomy can resolve symptoms of dysphagia and dyspnea, restore proper feeding, and remove the need for a tracheostomy tube.9,10 Given its radical nature, it is advised to maximize diagnostic work-up before immediately performing a total laryngectomy. It remains controversial for an organ with no evidence of malignancy to be removed; however, there has been evidence that removal of a dysfunctional larynx may be practical and preferable in the background of life-threatening complications in irradiated larynges.
It is suggested for further research to be done regarding probable risk factors or predictors for patients undergoing RT for early-stage laryngeal cancers. Such findings can determine which patients may be at risk of developing late radiotherapy-induced changes and who might be candidates for salvage total laryngectomy. If such predictors are identified, these may allow physicians to better advice select patients regarding the possible risk of having to undergo a total laryngectomy even after definitive RT.
CONCLUSION
This case demonstrated treatment dilemmas for a patient with laryngeal carcinoma with uncertain recurrence wherein radical surgical management via total laryngectomy may prove to be a viable option to achieve both diagnostic certainty and ultimate relief of symptoms.
Statement of Authorship
PAUS contributed in the conceptualization of work, acquisition of data, and drafting and revising of manuscript; ACAC and ACFL contributed in the conceptualization of work, drafting and revising, and final approval of the version to be published. CVLV contributed in the drafting and revising of manuscript, and final approval of the version to be published.
Author Disclosure
All authors declared no conflicts of interest.
Funding Source
None.
REFERENCES
- 1.Woodson GE. Laryngeal and pharyngeal function. In: Flint PW, Francis HW, Haughey BH, Lesperance MM, Lund VJ, Robbins KT, et al., eds. Cummings otolaryngology head and neck surgery, 7th ed. Philadelphia: Elsevier; 2021. p. 802 [Google Scholar]
- 2.Li P, Hu W, Zhu Y, Liu J. Treatment and predictive factors in patients with recurrent laryngeal carcinoma: A retrospective study. Oncol Lett. 2015. Nov;10(5):3145-3152. doi: 10.3892/ol.2015.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nocini R, Molteni G, Mattiuzzi C, Lippi G. Updates on larynx cancer epidemiology. Chin J Cancer Res. 2020. Feb;32(1):18-25. doi: 10.21147/j.issn.1000-9604.2020.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . Head and neck cancers [Internet]. 2022. [cited 2022 Sep]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 5.McGuirt WF, Greven KM, Keyes JW Jr, Williams DW 3rd, Watson N. Laryngeal radionecrosis versus recurrent cancer: a clinical approach. Ann Otol Rhinol Laryngol. 1998. Apr;107(4):293-6. doi: 10.1177/000348949810700406. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006. Jan;64(1):77-82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Cahlon O, Lee N, Le QT, Kaplan MJ, Colevas AD. Cancer of the larynx. In: Hoppe R, Phillips T, Roach M, eds. Leibel and Phillips Textbook of Radiation Oncology, 3rd ed. Philadelphia: Elsevier Saunders; 2010. pp. 642-665. [Google Scholar]
- 8.Allen CT, Lee CJ, Merati AL. Clinical assessment and treatment of the dysfunctional larynx after radiation. Otolaryngol Head Neck Surg. 2013. Dec;149(6):830-9. doi: 10.1177/0194599813503802. [DOI] [PubMed] [Google Scholar]
- 9.Theunissen EAR, Timmermans AJ, Zuur CL, Hamming-Vrieza O, de Boer JP, Hilgers FJM, et al. Total laryngectomy for a dysfunctional larynx after (chemo)radiotherapy. Arch Otolaryngol Head Neck Surg. 2012. Jun;138(6):548-55. doi: 10.1001/archoto.2012.862. [DOI] [PubMed] [Google Scholar]
- 10.Topf MC, Magana LC, Salmon K, Hamilton J, Keane WM, Luginbuhl A, et al. Safety and efficacy of functional laryngectomy for end-stage dysphagia. Laryngoscope. 2018. Mar;128(3):597-602. doi: 10.1002/lary.26760. [DOI] [PubMed] [Google Scholar]
- 11.Anschuetz L, Shelan M, Dematte M, Schubert AD, Giger R, Elicin O. Long-term functional outcome after laryngeal cancer treatment. Radiat Oncol. 2019. Jun;14(1):101. doi: 10.1186/s13014-019-1299-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cetinayak O, Dogan E, Kuru A, Akturk N, Aydin B, Umay C, et al. Outcome of early-stage glottic laryngeal carcinoma patients treated with radical radiotherapy using different techniques. J Oncol. 2019. Nov;2019:8640549. doi: 10.1155/2019/8640549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaldi P, Leccisotti L, Bussu F, Micciche F, Rufini V. Role of (18) F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013. Feb;33(1):1-8. [PMC free article] [PubMed] [Google Scholar]
- 14.Plaxton NA, Brandon DC, Corey AS, Harrison CE, Karagulle Kendi AT, Halkar RK, et al. Characteristics and limitations of FDG PET/ CT for imaging of squamous cell carcinoma of the head and neck: A comprehensive review of anatomy, metastatic pathways, and image findings. AJR Am J Roentgenol. 2015. Nov;205(5):W519-31. doi: 10.2214/AJR.14.12828 [DOI] [PubMed] [Google Scholar]
- 15.Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011. Nov;38(11):2083-95. doi: 10.1007/s00259-011-1893-y [DOI] [PubMed] [Google Scholar]
- 16.Holsinger FC, Funk E, Roberts DB, Diaz EM Jr. Conservation laryngeal surgery versus total laryngectomy for radiation failure in laryngeal cancer. Head Neck. 2006. Sep;28(9):779-84. doi: 10.1002/hed.20415 [DOI] [PubMed] [Google Scholar]
- 17.Chen MM, Holsinger FC, Laccourreye O. Salvage conservation laryngeal surgery after radiation therapy failure. Otolaryngol Clin North Am. 2015. Aug;48(4):667-75. doi: 10.1016/j.otc.2015.04.011 [DOI] [PubMed] [Google Scholar]


