Abstract
Posttraumatic stress disorder (PTSD) is associated with glutamatergic neuron hyperactivation in the basolateral amygdala (BLA) brain area, while GABAergic interneurons in the BLA modulate glutamatergic neuron excitability. Studies have shown that propofol exerts its effects through potentiation of the inhibitory neurotransmitter γ-aminobutyric acid. The neuronal mechanism by which propofol anesthesia modulates fear memory is currently unknown. Here, we used optogenetics and chemogenetics to suppress glutamatergic neurons or activate GABAergic interneurons in the BLA to assess alterations in neuronal excitation-inhibition balance and investigate fear memory. The excitability of glutamatergic neurons in the BLA was significantly reduced by the suppression of glutamatergic neurons or activation of GABAergic interneurons, while propofol-mediated enhancement of fear memory was attenuated. We suggest that propofol anesthesia could reduce the excitability of GABAergic neurons through activation of GABAA receptors, subsequently increasing the excitability of glutamatergic neurons in the mice BLA; the effect of propofol on enhancing mice fear memory might be mediated by strengthening glutamatergic neuronal excitability and decreasing the excitability of GABAergic neurons in the BLA; neuronal excitation-inhibition imbalance in the BLA might be important in mediating the enhancement of fear memory induced by propofol.
Subject terms: Trauma, Fear conditioning
Propofol mediates enhancement of fear memory in mice by decreasing excitability of GABAergic neurons and enhancing excitability of glutamatergic neurons in BLA.
Introduction
Posttraumatic stress disorder (PTSD) is a sustained mental disorder that emerges with a delay following an experience of an exceptionally threatening, catastrophic event1, and symptoms include hyperreactivity to and avoidance of trauma-related stimuli or situations; prolonged negative cognitions and emotions; and recurrent, distressingly compulsive recollections of the traumatic experience2,3. PTSD is strongly associated with depression, substance abuse and suicidal behavior4,5, and the lifetime prevalence of PTSD is 2–5%6. Approximately more than 70% of the population worldwide has experienced traumatic life events at least once7,8. Even with exposure therapy, up to 40% of PTSD patients exhibit poor outcomes or treatment ineffectiveness9,10. It is estimated that up to 23% of PTSD patients with concomitant somatic injuries exhibit symptoms of PTSD one year after discharge from the hospital11. These patients with a trauma and ensuing PTSD may receive propofol while hospitalized for surgery, and clinical studies have shown that propofol may facilitate the formation of traumatic memories and induce long-term changes in cognitive and emotional processes, thereby increasing the likelihood of developing stress-related disorders12,13. Studies in animals have also shown that intraperitoneal injection of propofol immediately after footshock enhanced the consolidation of memory for suppressive avoidance training in rats14; propofol anesthesia enhanced 48 h memory retention, induced enduring traumatic memory enhancement, and anxiogenic effects15.
Only the amygdala showed activation in response to harmful stimuli in rats under deep anesthesia with propofol16; in addition, PTSD patients showed markedly elevated amygdala excitatory activity during traumatic memory-related events17. We therefore believe that the impact of propofol anesthesia on memory is mediated by the amygdala. Through projections to the hippocampus and prefrontal cortex, the amygdala executes fear memory learning and formation18–21, and the long-term synaptic plasticity of its inputs underpins fear memory acquisition and storage22. The amygdala consists of four clusters: basolateral, superficial, medial and central cluster23,24. The basolateral amygdala (BLA) encodes stimulus information and plays a key role in the formation of associations between information and fear memory25–27, and the projection of stimulus information from the BLA to the central amygdala (CeA) mediates the expression of emotional responses (fear)28. The BLA contains 80% glutamatergic neurons and 20% GABAergic neurons (mostly local circuit neurons)29. The convergence of injurious stimulus information acting on BLA neurons during fear memory formation causes the depolarization of glutamatergic neurons, releasing large amounts of glutamate and predisposing long-term potentiation (LTP)30. Maintenance of excitability in glutamatergic neurons is crucial for fear memory formation, whose excitatory activity is precisely regulated by GABAergic interneurons31. γ-Aminobutyric acid type-A (GABAA) receptors are the principal inhibitory receptors in the central nervous system. Propofol exerts its effects through potentiation of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) at the GABAA receptors thus prolonging the inhibitory postsynaptic GABAergic currents32,33.
In this study, we used the Pavlovian fear conditioning protocol to establish a PTSD mouse model34 to induce a PTSD-like condition, modulated the excitability of glutamatergic or GABAergic neurons in the BLA by optogenetics and chemogenetics, and examined the association between the altered excitability of glutamatergic or GABAergic neurons and the enhancement of fear memory in mice after propofol anesthesia.
Results
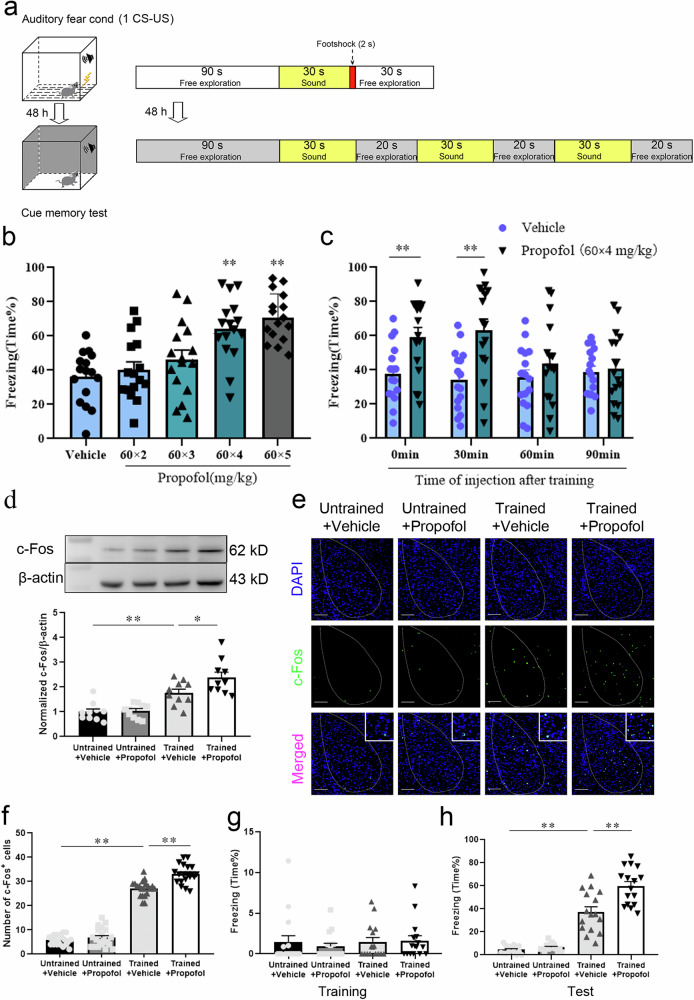
Fear memory was enhanced by propofol injection after FC training in mice
To determine the dose of propofol that enhanced fear memory in mice, we immediately injected vehicle or various doses of propofol (60 × 2, 60 × 3, 60 × 4, and 60 × 5 mg/kg) intraperitoneally after the completion of fear conditioning (FC) training in WT mice, and then auditory fear conditioning test (FCT) was performed to evaluate the fear memory of mice 48 h later. We found that compared with the vehicle group, the fear memory of mice in the 60 × 2 and 60 × 3 mg/kg propofol group was not enhanced, while the fear memory of mice in the 60 × 4 mg/kg and 60 × 5 mg/kg propofol groups was evidentially enhanced; no apparent difference was observed between the 60 × 4 mg/kg and 60 × 5 mg/kg propofol groups (Fig. 1b). This result indicated that intraperitoneal injection of propofol greater than a dose of 60 × 4 mg/kg could significantly enhance fear memory in mice. To determine the time points at which propofol injection enhanced fear memory in mice, we administered propofol (60 × 4 mg/kg) to mice immediately or 30, 60, and 90 min after training. The results revealed that fear memory was greatly enhanced in mice that were injected intraperitoneally with 60 × 4 mg/kg propofol 0 and 30 min after training compared with the vehicle group, but there was no alteration in mice injected intraperitoneally with 60 × 4 mg/kg propofol at 60 and 90 min after training (Fig. 1c). These findings showed that intraperitoneal injection of 60 × 4 mg/kg propofol within 30 min after FC training could effectively strengthen fear memory in mice.
Fig. 1. Fear memory was enhanced by propofol after FC training in mice.
a Diagram of fear conditioning (FC) training and fear conditioning test (FCT) patterns. b Freezing in response to the conditioned tone after the infusion of different doses of propofol. Propofol enhanced freezing at doses of 60 × 4 and 60 × 5 mg/kg but not 60 × 2 and 60 × 3 mg/kg (n = 16/group, one-way ANOVA, F (4, 75) = 11.66, p < 0.001, **p < 0.01). c Freezing in response to the conditioned tone after the infusion of vehicle or propofol at different time points after the conditioning. Propofol enhanced freezing at 0 and 30 min but not at 60 min or 90 min (n = 16/group, two-tailed unpaired t test, **p < 0.01). d Representative images of Western blots and quantification of c-Fos expression among the 4 groups (n = 10, one-way ANOVA, F (3, 36) = 18.82, p < 0.0001, *p = 0.030, **p < 0.01). e Representative images of c-Fos/DAPI immunofluorescence in BLA neurons after vehicle or propofol treatment; scale bar, 100 µm. f The number of c-Fos+ cells in the BLA in mice that were administered propofol after FC training was increased compared to that in trained mice administered the vehicle (n = 20/group, one-way ANOVA, F (3, 76) = 349.8, p < 0.0001, **p < 0.01). g Freezing was comparable across all groups during FC training (n = 16/group, Kruskal-Wallis test, p = 0.523). h Trained mice administered propofol showed enhanced fear freezing compared to those administered the vehicle (n = 16/group, one-way ANOVA, F (3, 60) = 75.48, p < 0.001, **p < 0.01). All data are presented as the mean ± SEM.
To further investigate the effect of propofol anesthesia on fear memory, we separately analyzed the variation in fear memory in mice with/without FC training that were treated with propofol or vehicle. No obvious discrepancy was detected in the expression level and number of c-Fos+ cells in the BLA of WT mice without FC training that received propofol (Untrained+Propofol group) or vehicle (Untrained+Vehicle group). In contrast, the expression level and number of c-Fos+ cells in the BLA were markedly higher in WT mice administered propofol after FC training (Trained+Propofol group) than in mice administered vehicle (Trained+Vehicle group) (Fig. 1d–f). Freezing was comparable across all groups during FC training (Fig. 1g). There was no statistical distinction in fear memory between untrained mice administered propofol or vehicle, but fear memory was dramatically enhanced in propofol-administered mice after FC training compared to vehicle-administered mice (Fig. 1h). These results demonstrated that intraperitoneal injection of propofol reinforced fear memory in mice receiving FC training.
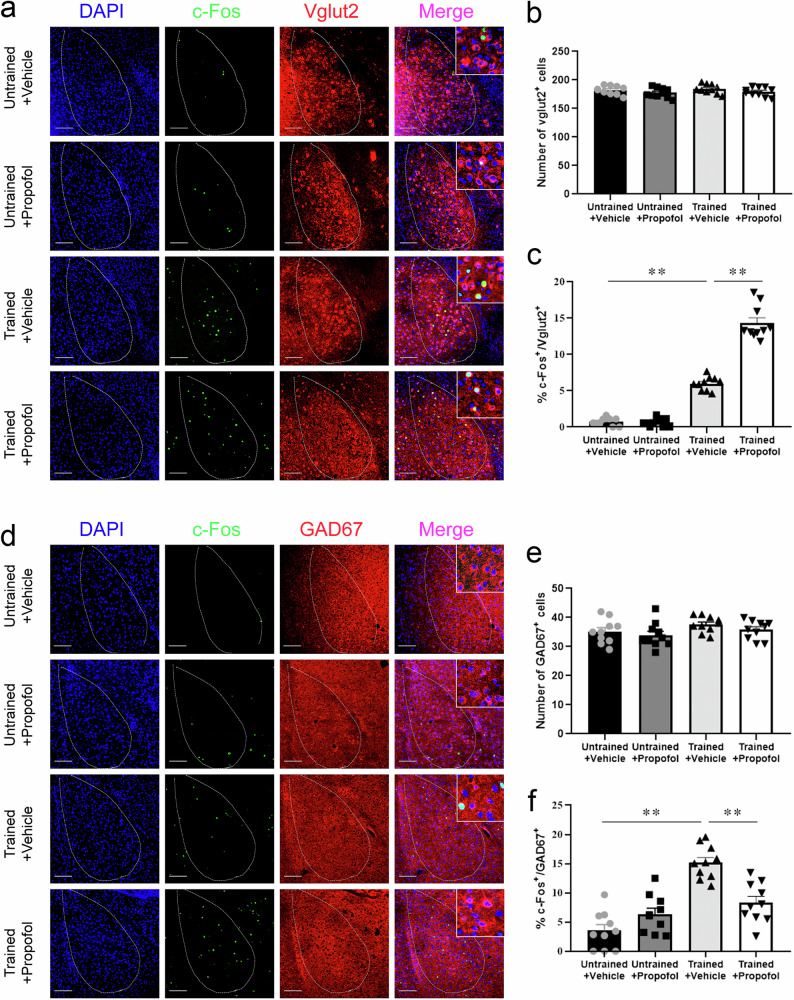
The excitability of glutamatergic and GABAergic neurons in the BLA was enhanced or attenuated, respectively, by propofol anesthesia after FC training in mice
To investigate the impact of propofol anesthesia on different types of neurons in the BLA, we investigated the coexpression of c-Fos in the mouse BLA with Vglut2 or GAD67 by immunofluorescence. Statistically, there was no difference in the number of Vglut2+ cells in the BLA in the Untrained+Vehicle, Untrained+Propofol, Trained+Vehicle and Trained+Propofol groups, whereas the proportion of c-Fos+ & Vglut2+ cells in the BLA in the Trained+Propofol group was substantially higher than that in the BLA of the Trained+Vehicle group (Fig. 2a–c). There was no significant difference in the number of GAD67+ cells in the BLA between the four groups, whereas the proportion of c-Fos+ & GAD67+ cells in the BLA in the Trained+Propofol group was notably lower than that in the Trained+Vehicle group (Fig. 2d–f).
Fig. 2. The excitability of glutamatergic and GABAergic neurons in the BLA was enhanced or attenuated, respectively, by propofol anesthesia after FC training in mice.
a Representative images of c-Fos/DAPI/Vglut2 immunofluorescence in BLA neurons after vehicle or propofol treatment; scale bar, 100 µm. b No significant difference was detected in the percentage of Vglut2+ cells between mice administered vehicle or propofol (n = 10/group, two-tailed unpaired t test). c The percentage of c-Fos+ & Vglut2+ cells in the BLA in trained mice administered propofol was higher than that in trained mice administered vehicle (n = 10 mice/group, Mann–Whitney U test, **p < 0.01). d Representative images of c-Fos/DAPI/GAD67 immunofluorescence in BLA neurons after vehicle or propofol treatment; scale bar, 100 µm. e No significant difference was detected in the percentage of GAD67+ cells between mice administered vehicle or propofol (n = 10/group, two-tailed unpaired t test). f The percentage of c-Fos+& GAD67+ cells in the BLA in trained mice administered propofol was decreased compared to that in trained mice administered vehicle (n = 10 mice/group, two-tailed unpaired t test, **p < 0.01). All data are presented as the mean ± SEM.
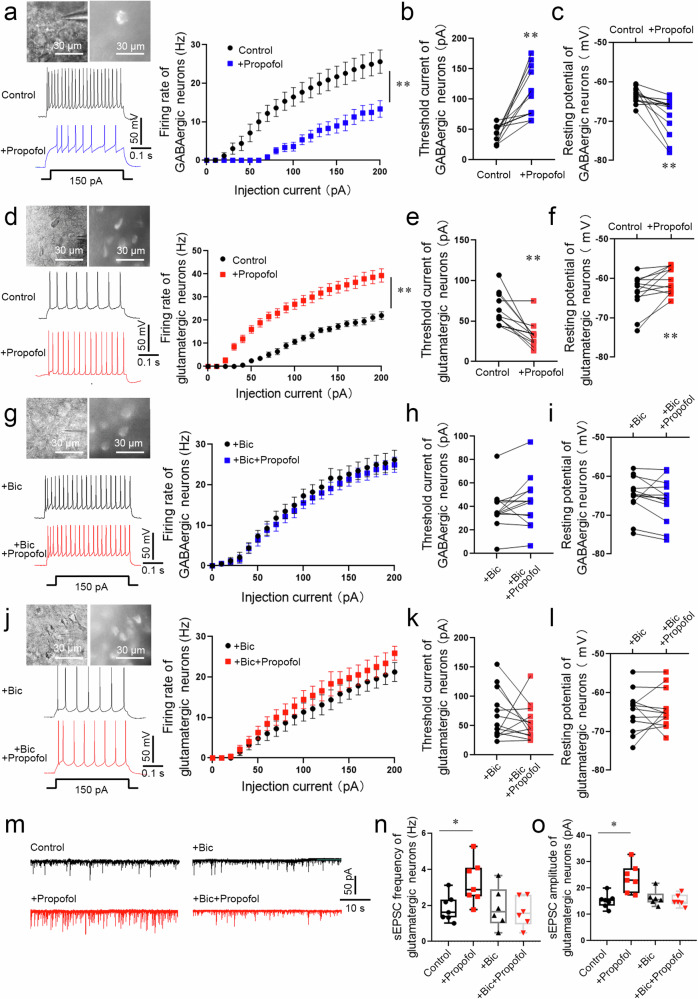
To investigate the effects of propofol on excitability of GABAergic and glutamatergic neurons in the BLA, inward current (0–200 pA) was injected to depolarize the membrane potential to evoke tonic firing. After recording baseline for 5 min, propofol (5 µM) was perfused for 60 s and followed with whole-cell recordings of acute sections from Vgat-cre mice. GABAergic neurons showed increased threshold current and decreased firing rate and resting potential in BLA after propofol application (Fig. 3a–c). Meanwhile, glutamatergic neurons showed decreased threshold current and increased firing rate in BLA of Vglut2-cre mice after propofol application, with the resting potential significantly elevated (Fig. 3d–f).
Fig. 3. The excitability of glutamatergic and GABAergic neurons in the BLA was enhanced or attenuated, respectively, by propofol anesthesia after FC training in mice.
a The electrophysiology of GABAergic (mCherry+) neurons in the BLA of Vgat-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 2.936, p < 0.0001, **p < 0.01 vs. Control group). Threshold currents that evoked the first action potential (b) and comparison of resting membrane potential (c), (n = 13 neurons from 6 mice, two-tailed paired t test and two-tailed Wilcoxon matched-pairs signed rank test, **p < 0.01). d The electrophysiology of glutamatergic neurons (mCherry+) in the BLA of Vglut2-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 502) = 5.073, p < 0.0001, **p < 0.01). e, f Threshold currents that evoked the first action potential and comparison of resting membrane potential, (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, **p < 0.01). g The electrophysiology of GABAergic neurons (mCherry+) perfused bicuculline (30 µM) in the BLA of Vgat-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 0.076, p > 0.999). h Threshold currents that evoked the first action potential, values were presented (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, p > 0.05). i Comparison of resting membrane potential (n = 13 neurons from 6 mice, two-tailed paired t test, p > 0.05). j The electrophysiology of glutamatergic neurons (mCherry+) perfused bicuculline (30 µM) in the BLA of Vglut2-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 0.212, p > 0.999). k Threshold currents that evoked the first action potential, values were presented (n = 13 neurons from 6 mice, two-tailed paired t test, p > 0.05). l Comparison of resting membrane potential (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, p > 0.05). m Representative traces of sEPSC from glutamatergic neurons (mCherry+) in the BLA of Vglut2-cre mice. Scale bars = 50 pA, 10 s. n The sEPSC frequency in glutamatergic neurons was significantly increased in brain slices perfused propofol (5 µM) compared with those from control (n = 7 neurons from 6 mice, two-tailed paired t test, *p = 0.032). While The sEPSC frequency showed no statistical difference in brain slices perfused bicuculline + propofol and slices perfused bicuculline (n = 6 neurons from 6 mice, two-tailed paired t test, p > 0.05). o The sEPSC amplitude in glutamatergic neurons was significantly increased in brain slices perfused propofol (5 µM) compared with those from control (n = 7 neurons from 6 mice, two-tailed paired t test, *p = 0.013). While The sEPSC amplitude showed no statistical difference in brain slices perfused bicuculline + propofol and slices perfused bicuculline (n = 6 neurons from 6 mice, two-tailed paired t test, p > 0.05). All data are presented as the mean ± SEM.
To determine whether the altered excitability of glutamatergic neurons induced by propofol is mediated through activation of GABAA receptors, we preperfused brain slices ex vivo with bicuculline (Bic, 30 µM) to competitively antagonize GABAA receptors prior to propofol perfusion, and GABAergic neurons showed no statistical changes in threshold current, firing rate and resting potential in BLA after propofol application (Fig. 3g–i). Glutamatergic neurons also did not show statistical changes in threshold current, discharge rate and resting potentials in the BLA after application of propofol (Fig. 3j–l). Further testing of spontaneous excitatory postsynaptic current (sEPSC) in glutamatergic neurons indicated the identical findings. Both frequency and amplitude of glutamatergic neurons were considerably increased by perfusion of propofol only, but preperfusion of bicuculline abolished the efficacy of propofol in altering frequency and amplitude (Fig. 3m–o).
These findings revealed that during fear conditioning, propofol anesthesia could dramatically reduce the excitability of GABAergic neurons through activation of GABAA receptors, subsequently increasing the excitability of glutamatergic neurons in the mice BLA.
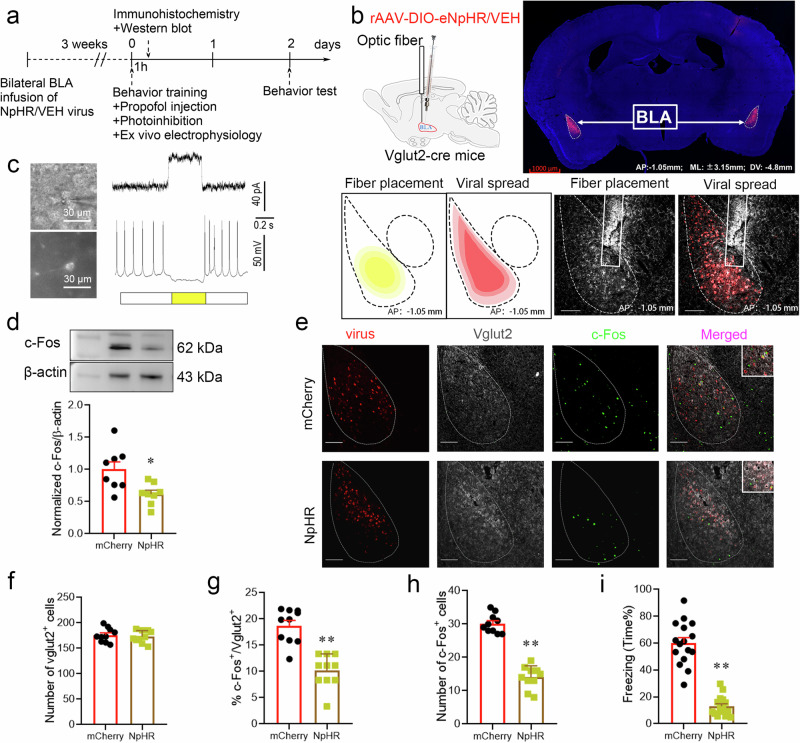
Inhibiting glutamatergic neuron activity in the BLA by optogenetic regulation attenuated the enhanced effect of propofol on fear memory
To validate the impact of propofol anesthesia on the excitability of glutamatergic and GABAergic neurons in the BLA and its association with fear memory, we first inhibited glutamatergic neurons with optogenetics. Vglut2-cre mice BLA glutamatergic neurons expressed mCherry fluorescent protein 21 days after virus (rAAV-EF1-DIO-eNpHR3.0-mCherry or rAAV-EF1α-DIO-mCherry) injection (Fig. 4b). Neurons expressing mCherry fluorescence were visualized in the BLA when ex vivo brain slices were placed under a microscope (Fig. 4c). The BLA was illuminated using a laser at a wavelength of 589 nm constant for 50 min immediately after the FC training to specifically inhibit glutamatergic neurons, and viral expression was authenticated with electrophysiological recordings. Electrophysiological recordings were performed during laser illumination in the BLA. If both stimulation and recording were performed in the same cell, light stimulation of 0.5 s was selected (Figs. 4c and 5d). If GABAergic neurons were stimulated while the excitability was recorded in glutamatergic neurons, light stimulation of 10 s was selected to ensure that the released GABA neurotransmitter would have enough time to modulate the activity of the glutamatergic neurons (Fig. 5f). Outward currents were recorded in voltage-clamp mode, and suppressed action potentials (30 pA) were registered in current-clamp mode during glutamatergic neuron photoinhibition (Fig. 4c). Following photoinhibition of BLA glutamatergic neurons, no significant difference was found in the number of BLA Vglut2+ cells between the two groups. Compared with the mCherry group, the percentage of c-Fos+ & Vglut2+ cells in the BLA and the total quantity of c-Fos+ cells were obviously diminished in the NpHR group (Fig. 4e–h); the c-Fos expression level was reduced appreciably (Fig. 4d); and, the effect of propofol on enhancing fear memory in mice was consequently weakened (Fig. 4i). BLA glutamatergic neuron photoinhibition attenuated the effect of propofol on enhancing fear memory, suggesting that the propofol-induced enhancement of fear memory in mice might be mediated by strengthening the excitability of BLA glutamatergic neurons.
Fig. 4. Inhibiting glutamatergic neurons in the BLA by optogenetic regulation attenuated the enhanced effect of propofol on fear memory.
a Experimental time course for surgery, FC training, propofol injection, photoinhibition, and FCT. b Schematic of 2/9rAAV-DIO-eNpHR-mCherry or 2/9rAAV-DIO-mCherry injection and optic fiber implantation into the BLA of Vglut2-cre mice; representative immunohistochemical staining (scale bar, 1000 µm, AP: -1.05 mm, ML: ±3.15 mm, DV: 4.8 mm); schematic of fiber placement and viral spread in the BLA (scale bar, 100 µm, AP: -1.05 mm). c Neurons expressing mCherry fluorescence were observed in the BLA when ex vivo brain slices were exposed under a microscope. The pulse train evokes outward currents and abolishes action potentials (30 pA) in glutamatergic neurons. d Representative Western blot image and quantification of c-Fos expression. The expression of c-Fos was reduced after the photoinhibition of glutamatergic neurons in the BLA (n = 8, two-tailed unpaired t test, *p = 0.012). e Representative images of mCherry/Vglut2/c-Fos immunofluorescence in BLA neurons after virus treatment and photoinhibition; scale bar, 100 µm. f–h The ratio of c-Fos+ & Vglut2+ cells and the total number of c-Fos+ cells in the BLA after the photoinhibition of glutamatergic neurons were reduced (n = 10/group, two-tailed unpaired t test, **p < 0.01). i Reduction in freezing in mice after the photoinhibition of glutamatergic neurons in the BLA (n = 16/group, two-tailed unpaired t test, **p < 0.01). All data are presented as the mean ± SEM.
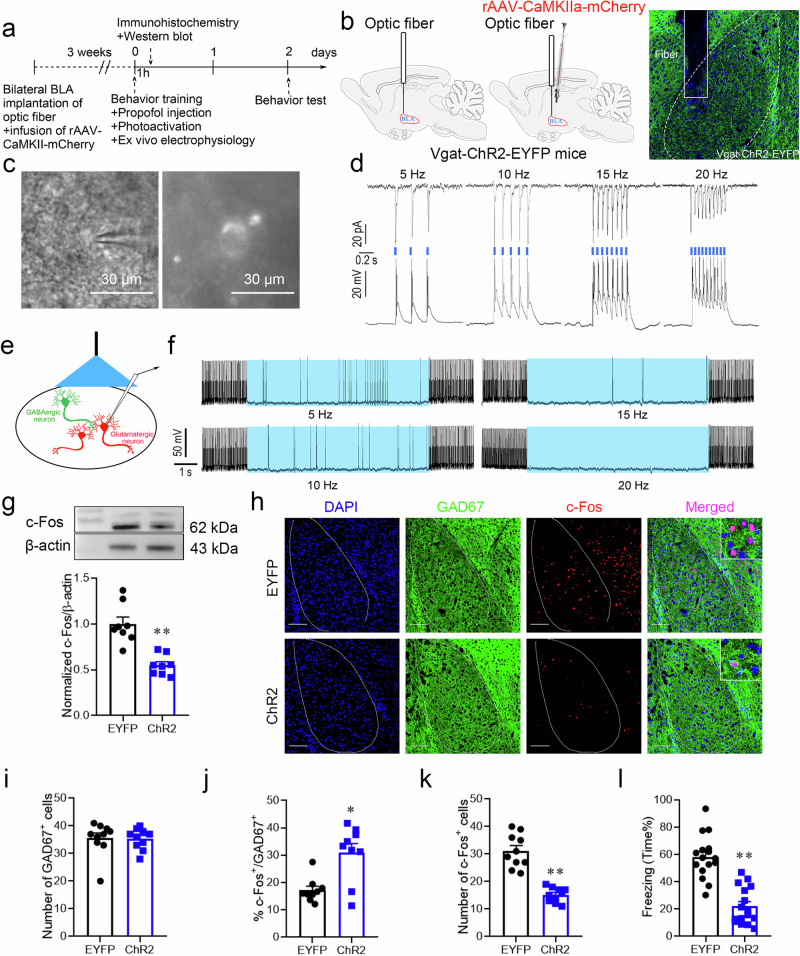
Fig. 5. Activating GABAergic interneurons in the BLA by optogenetic regulation attenuated the enhanced effect of propofol on fear memory.
a Experimental time course for surgery, FC training, propofol injection, photoactivation, and FCT. b Schematic of fiber optic implantation and rAAv-CaMKIIa-mCherry injection into the BLA of Vgat-ChR2-EYFP mice. Schematic of fiber optic implantation into the BLA of Vgat-ChR2-EYFP mice. c Neurons expressing EYFP fluorescence were observed in the BLA when ex vivo brain slices were exposed under a microscope. d Trains of pulses (pulses at 5, 10, 15, and 20 Hz) evoke reproducible currents (top) and action potentials (bottom) in a GABAergic interneuron. e Light illumination was applied to the BLA slice, and the activity of glutamatergic neurons was recorded. f Photoactivation (20 Hz) of GABAergic interneurons reduced firing in glutamatergic neurons, and firing recovered after the light illumination was terminated. g Representative Western blot image and quantification of c-Fos expression. The expression of c-Fos was reduced after photoactivation of GABAergic interneurons in the BLA (n = 8, two-tailed unpaired t test, **p < 0.01). h Representative images of DAPI/GAD67/c-Fos immunofluorescence in BLA neurons after photoactivation; scale bar, 100 µm. The ratio of c-Fos+ & GAD67+ cells in the BLA was increased after photoactivation of GABAergic interneurons, while the total number of c-Fos+ cells was decreased (n = 10/group), Mann–Whitney U test (i, j), two-tailed unpaired t test (k), *p = 0.01, **p < 0.01. l Reduction in freezing in mice after photoactivation of GABAergic interneurons in the BLA (n = 16/group, two-tailed unpaired t test, **p < 0.01). All data are presented as the mean ± SEM.
Activating GABAergic interneurons in the BLA by optogenetic regulation attenuated the enhanced effect of propofol on fear memory
Optogenetically activated GABAergic interneurons expressing EYFP fluorescence could be observed microscopically in live brain sections of the BLA (Fig. 5c). The BLA was illuminated using a laser at a wavelength of 470 nm and 20 Hz for 50 min immediately after the FC training to specifically activate GABAergic neurons. Light stimulation induced rapid, reliable, and continuous currents and action potentials (Fig. 5d). Therefore, ChR2 can be used to tightly control the activation of GABAergic interneurons in the BLA. Next, we tested whether GABAergic interneurons regulated the activity of glutamatergic neurons. Visualization of glutamatergic neurons in the BLA 21 days after rAAv-CaMKIIa-mCherry injection revealed that photoactivation of GABAergic interneurons inhibited current-evoked firing in glutamatergic neurons (Fig. 5e, f). Immunofluorescence analysis showed that in response to light stimulation of GABAergic neurons, there was no significant distinction in the number of GAD67+ cells in the BLA between the two groups. The ChR2 group had an increased ratio of c-Fos+ & GAD67+ cells in the BLA, while the total quantity of c-Fos+ cells was markedly decreased (Fig. 5h–k); the overall c-Fos expression level was clearly decreased (Fig. 5g), and the effect of propofol on enhancing fear memory in mice was blunted compared with that of the EYFP group (Fig. 5l). GABAergic neuron photoactivation in the BLA attenuated the effect of propofol on enhancing fear memory. It was suggested that the effect of propofol on enhancing fear memory in mice might be accomplished by lowering the excitability of GABAergic interneurons in the BLA.
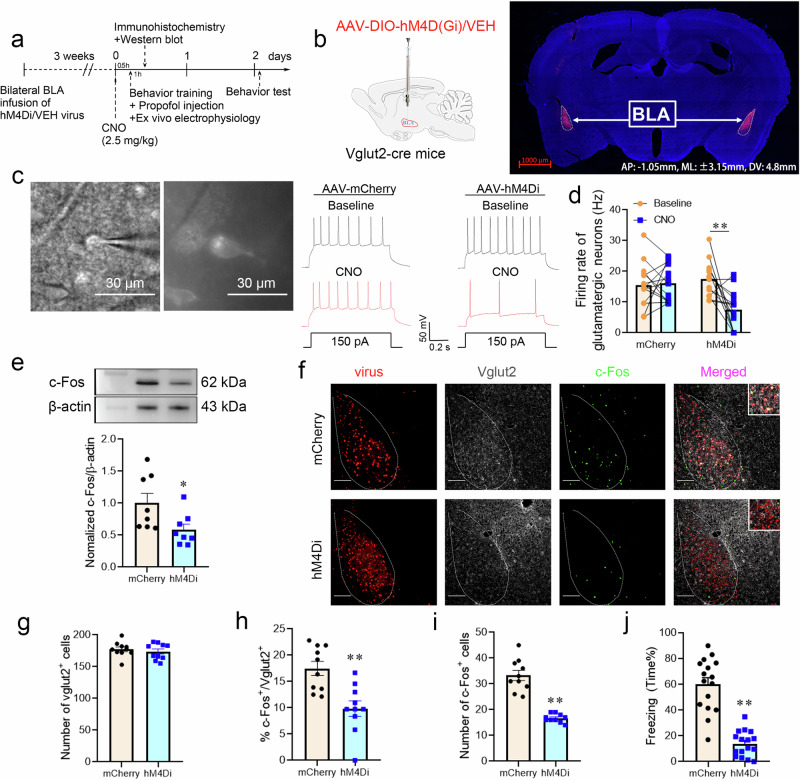
Inhibiting glutamatergic neuron activity in the BLA by chemogenetic regulation attenuated the enhanced effect of propofol on fear memory
The BLA is a long and narrow brain area, it is difficult to uniformly regulate all the targeting neurons in BLA by the laser illumination during the optogenetic manipulation, whereas chemogenetics can compensate for this deficiency well. To further examine the effect of propofol anesthesia on the excitability of glutamatergic and GABAergic interneurons in the BLA and its correlation with fear memory, we suppressed BLA glutamatergic neurons using chemogenetics. Living brain slices of the BLA could be viewed under a microscope, and glutamatergic neurons expressed mCherry fluorescence in the hM4Di and mCherry groups. The frequency of cellular action potential delivery was captured in a whole-cell patch on mCherry-expressing fluorescent neurons using a current clamp, and representative recordings displayed the action potentials (150 pA) evoked in Vglut2-cre mice injected with AAV-mCherry and AAV-hM4Di before and after the application of CNO (10 µM) (Fig. 6c). We found that the action potential firing rate declined after CNO activation of hM4Di receptors. By tallying the action potential firing rate, it was confirmed that CNO activation of hM4Di receptors suppressed the action potential firing rate (Hz) of glutamatergic neurons in the BLA (Fig. 6d). Immunofluorescence analysis showed that chemogenetic inhibition of BLA glutamatergic neurons did not elicit a discrepancy in the number of BLA Vglut2+ cells between the two groups. The proportion of BLA c-Fos+ & Vglut2+ cells and the aggregate quantity of c-Fos+ cells were visibly reduced in the hM4Di group (Fig. 6f–i); the level of c-Fos expression in the BLA was decreased (Fig. 6e), while the effect of propofol on enhancing fear memory in mice was attenuated in comparison to the mCherry group (Fig. 6j). Chemogenetic suppression of BLA glutamatergic neuronal excitability impaired the impact of propofol on enhancing fear memory in mice. The findings of the chemogenetic assay suggested that the effect of propofol on enhancing mice fear memory might be mediated by strengthening glutamatergic neuronal excitability in the BLA.
Fig. 6. Inhibiting glutamatergic neurons in the BLA by chemogenetic regulation attenuated the effect of propofol on enhancing fear memory.
a Experimental time course for surgery, CNO injection, FC training, propofol injection, and FCT. b Schematic of 2/9AAV-DIO-hM4D(Gi)-mCherry or 2/9AAV-DIO-mCherry injection into the BLA of Vglut2-cre mice. Representative immunohistochemical staining (scale bar, 1000 µm, AP: -1.05 mm, ML: ±3.15 mm, DV: 4.8 mm). c Neurons expressing mCherry fluorescence were observed in the BLA when ex vivo brain slices were exposed under a microscope. Bath application of CNO (10 µM) decreased the firing rate in hM4Di-positive BLA neurons in vitro. d Quantification of the firing rate (n = 14 cells from 6 mice injected with AAV-VEH and n = 14 cells from 6 mice injected with AAV-hM4Di, two-tailed paired t test, **p < 0.01). e Representative Western blot image and quantification of c-Fos expression. The expression of c-Fos was reduced after chemogenetic inhibition of glutamatergic neurons in the BLA (n = 8, two-tailed unpaired t test, *p = 0.033). f Representative images of mCherry/Vglut2/c-Fos immunofluorescence in BLA neurons after virus treatment and chemogenetic manipulation; scale bar, 100 µm. g–i The percentage of c-Fos+ & Vglut2+ cells and the total number of c-Fos+ cells were reduced after chemogenetic inhibition of glutamatergic neurons in the BLA (n = 10/group), two-tailed unpaired t test, **p < 0.01. j Reduction in freezing in mice after chemogenetic inhibition of glutamatergic neurons in the BLA (n = 16/group, two-tailed unpaired t test, **p < 0.01). All data are presented as the mean ± SEM.
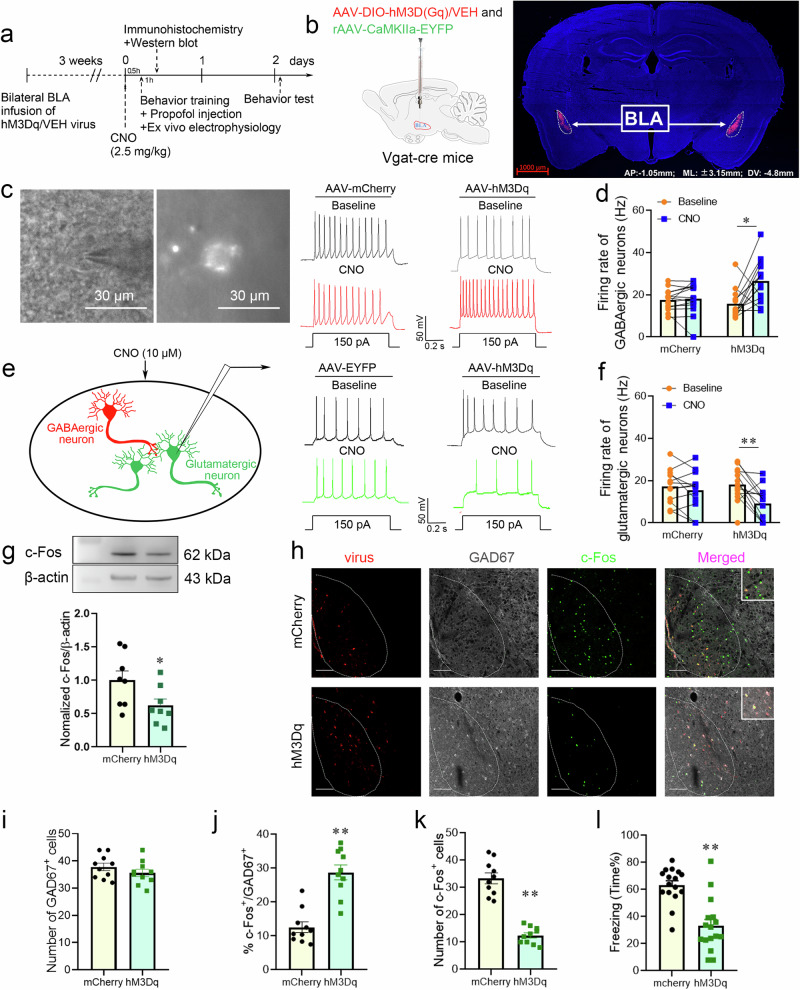
Activating GABAergic neurons in the BLA by chemogenetic regulation attenuated the enhanced effect of propofol on fear memory
Selective activation of BLA GABAergic interneurons by chemogenetics and ex vivo brain slices subjected to microscopy revealed neurons expressing mCherry fluorescence in the BLA. Representative recordings of action potentials (150 pA) evoked in Vgat-cre mice administered AAV-mCherry and AAV-hM3Dq before and after instillation of CNO (10 µM) are shown (Fig. 7c). The cellular action potential firing rate recorded in a whole-cell patch on mCherry-expressing fluorescent neurons using current clamp established that CNO activation of hM3Dq receptors enhanced the action potential firing rate (Hz) of GABAergic interneurons in the BLA (Fig. 7d). Meanwhile, the firing rate of glutamatergic neurons was markedly decreased after chemogenetic activation of GABAergic neurons (Fig. 7e, f). Immunofluorescence analysis revealed no significant variance in the number of GAD67+ cells in either group after chemogenetic activation of GABAergic neurons in the BLA. The hM3Dq group had an elevated proportions of c-Fos+& GAD67+ cells in BLA, whereas an overall reduction in the number of c-Fos+ cells was noted (Fig. 7h–k); the expression levels of c-Fos protein were reduced (Fig. 7g), and fear memory was attenuated (Fig. 7l). Chemogenetic activation of BLA GABAergic interneurons attenuated propofol-enhanced fear memory. This chemogenetic study further showed that the impact of propofol on enhancing fear memory was mediated by decreasing the excitability of GABAergic neurons in the BLA.
Fig. 7. Activating GABAergic interneurons in the BLA by chemogenetic regulation attenuated the effect of propofol on enhancing fear memory.
a Experimental time course for surgery, CNO injection, FC training, propofol injection, and FCT. b Schematic of 2/9AAV-DIO-hM3D(Gq)-mCherry or 2/9AAV-DIO-mCherry and rAAV-CaMKIIa-EYFP injection into the BLA of Vgat-cre mice. Representative immunohistochemical staining (scale bar, 1000 µm, AP: -1.05 mm, ML: ±3.15 mm, DV: 4.8 mm). c Neurons expressing mCherry fluorescence were observed in the BLA when in vitro brain slices were exposed under a microscope. Bath application of CNO (10 µM) increased the firing rate in hM3Dq-positive BLA neurons in vitro. d Quantification of the firing rate (n = 14 cells from 6 mice injected with AAV-VEH and n = 14 cells from 6 mice injected with AAV-hM3Dq, two-tailed Wilcoxon matched-pairs signed rank test, *p = 0.013). e Chemogenetic activation was applied to the BLA slice, and the activity of glutamatergic neurons was recorded. Bath application of CNO (10 µM) decreased the firing rate in BLA glutamatergic neurons in vitro. f Quantification of the firing rate (n = 12 cells from 6 mice injected with AAV-VEH+ rAAV-CaMKIIa-EYFP and n = 13 cells from 6 mice injected with AAV-hM3Dq+ rAAV-CaMKIIa-EYFP, two-tailed paired t test, **p < 0.01). g Representative Western blot image and quantification of c-Fos expression. The expression of c-Fos was reduced after chemogenetic activation of GABAergic interneurons in the BLA (n = 8, two-tailed unpaired t test, *p = 0.045). h Representative images of mCherry/GAD67/c-Fos immunofluorescence in BLA neurons after virus treatment and chemogenetic manipulation; scale bar, 100 µm. I–k The ratio of c-Fos+ & GAD67+ cells in the BLA was increased after chemogenetic activation of GABAergic interneurons, while the total number of c-Fos+ cells was decreased (n = 10/group, two-tailed unpaired t test, **p < 0.01). l Reduction in freezing in mice after chemogenetic activation of GABAergic interneurons in the BLA (n = 16/group, two-tailed unpaired t test, **p < 0.01). All data are presented as the mean ± SEM.
Discussion
Propofol is widely used in clinical practice as an anesthetic induction and maintenance drug with rapid onset, short duration of action, and few side effects33,35. Studies have suggested that propofol anesthesia impairs hippocampus-dependent learning memory and affects the consolidation of spatial memory36,37, but few studies have explored the role of propofol anesthesia in the consolidation of amygdala-related fear memory. Our findings revealed that during fear conditioning, propofol anesthesia could dramatically reduce the excitability of GABAergic neurons through activation of GABAA receptors, subsequently increasing the excitability of glutamatergic neurons in BLA of mice.
In our study, we used footshock to create fear memory to establish a PTSD animal model, and the consolidation of cued fear memory in mice was assessed by the percentage of freezing time38. The impact of propofol anesthesia on mouse fear memory retrieval was measured by alterations in fear memory and c-Fos expression levels in the BLA. We found that intraperitoneal administration of 60 × 4 mg/kg propofol within 30 min after the termination of FC training reinforced the retrieve of fear memory in mice. This is in accordance with the study of Hauer et al. (2011) which showed that propofol given in anesthetic doses immediately after training enhanced the consolidation of memory for suppressive avoidance training in rats14. Our findings are also consistent with Morena et al.‘s (2017) study suggested that propofol and ketamine may promote the formation of traumatic memories and induce long-term modifications in cognitive and emotional processes, thereby increasing the likelihood of developing stress-related disorders15. Brachman et al.‘s (2008) studies showed that propofol and ketamine may protect against stress-related disorders39–43. These conflicting results may also be related to variations in the dose and timing of propofol and ketamine administration or in the models used.
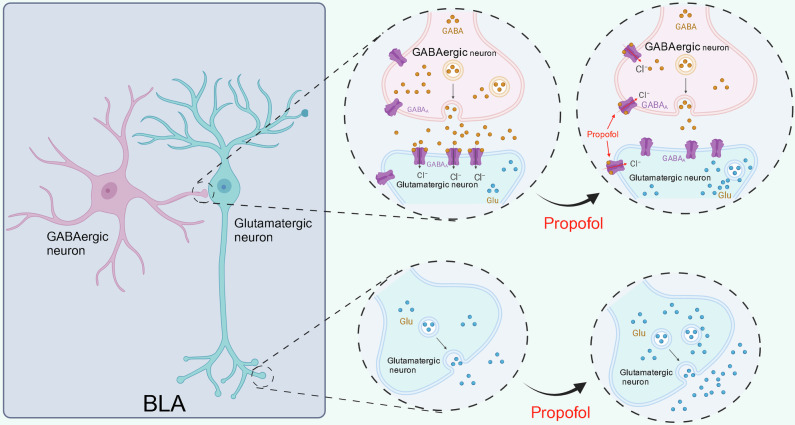
The GABAA receptor is a GABA-gated anion channel responsible for the fastest inhibitory synaptic transmission in the central nervous system of vertebrates. In mature neurons, GABAA receptors are permeable to HCO3- and Cl- ions; HCO3- migration out of the cell causing mild depolarization and Cl- entry into the cell overcomes this mild depolarization leading to a strong inhibitory hyperpolarization44. Propofol exerts its effects through potentiation of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) at the GABAA receptors thus prolonging the inhibitory postsynaptic GABAergic currents32,33. In vitro electrophysiological studies showed that GABAergic neurons in the BLA were inhibited following propofol infusion, in contrast to glutamatergic activation. Since GABAA receptors are located on both GABAergic and glutamatergic neurons, in order to investigate the reason for the paradoxical activation of glutamatergic neurons, we used bicuculline to block GABAA receptors on both GABAergic and glutamatergic neurons. It was found that the inhibitory effect of propofol on GABAergic neurons in the BLA was reversed, while the activating effect of glutamatergic neurons vanished. Accordingly, we concluded that propofol potentiates GABA efficacy on GABAA receptors in both GABAergic and glutamatergic neurons in the BLA. Glutamatergic neurons are under the regulation of GABAergic neurons in the physiological condition. When propofol acts on both GABAergic and glutamatergic neurons, as GABA released from GABAergic neurons is greatly decreased by inhibition of GABAergic neurons, GABAA receptors on glutamatergic neurons, although potentiated by propofol, are unable to function due to the lack of GABA neurotransmitter (Fig. 8). Therefore, the disinhibitory effect of GABAergic neurons on glutamatergic neurons is stronger than the potentiation of GABAA receptors on glutamatergic neurons by propofol itself, leading to the manifestation of activating effects on glutamatergic neurons.
Fig. 8. The disinhibitory effect of GABAergic neurons on glutamatergic neurons is stronger than the potentiation of GABAA receptors on glutamatergic neurons by propofol itself, leading to the manifestation of activating effects on glutamatergic neurons.
Created in BioRender. Ning, W. (2023) BioRender.com/k92f213.
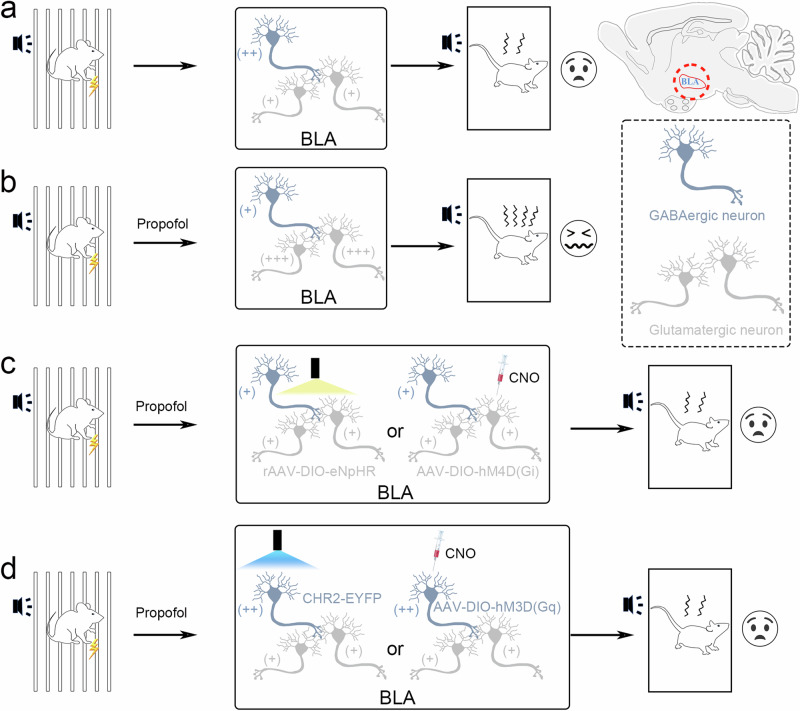
As our previous studies have revealed that propofol activates glutamatergic neurons and inhibits GABAergic neurons in the BLA, to further validate the role of GABAergic neurons modulating glutamatergic neurons after propofol anesthesia, we specifically inhibited/activated BLA glutamatergic/GABAergic neurons by optogenetics and chemogenetics. We found that concomitant application of propofol after FC training reversed the fear memory enhancing efficacy of propofol after successful inhibition of BLA glutamatergic neurons using optogenetics or chemogenetics, and that successful activation of BLA GABAergic neurons inhibited the glutamatergic neurons originally activated by propofol, simultaneously reversing the fear memory enhancing efficacy of propofol (Fig. 9). This finding indicated that GABAergic neurons have a powerful modulatory effect on glutamatergic neurons, and that propofol may reinforce fear memory in mice by altering neuronal excitability in the BLA. Previous studies have reported that glutamatergic neurons in the BLA elicit fear responses by projecting excitatory signals to the central amygdala while receiving local circuit modulation from GABAergic neurons in the BLA31, and our experimental results support this perspective.
Fig. 9. Inhibiting glutamatergic neurons or activating GABAergic interneurons in the BLA attenuated the effect of propofol on enhancing fear memory.
a, b Fear memory was enhanced by propofol after FC training in mice. c Inhibiting glutamatergic neuron activity in the BLA attenuated the effect of propofol on enhancing fear memory. d Activating GABAergic interneurons in the BLA attenuated the effect of propofol on enhancing fear memory.
It has been previously demonstrated that propofol anesthesia impairs learning and spatial memory by facilitating the distribution of GABA37,45, eliminating the formation of explicit memory involving the superior temporal gyrus46. Some studies showed that propofol anesthesia in neonatal or developmental animals induces apoptosis in hippocampal neurons, decreases the presence of dendritic spines in neonatal rats and causes spatial learning and memorization deficits in adulthood47, as well as impairment of short- and long-term learning memory48,49. The administration of propofol anesthesia to rats during early pregnancy resulted in learning and memory disturbances in the offspring50. These studies all suggested that propofol anesthesia can weaken hippocampus-dependent learning and remembering capacity. This finding appears to be at odds with our finding that propofol enhanced fear memory in mice. The main explanation for this may be attributed to the fact that the former is a spatial learning memory process mediated by the hippocampus, whereas the present study focused on a fear memory process closely affiliated with the amygdala, and there are fundamental differences between the two in terms of animal model establishment and drug administration. Research has shown that propofol anesthesia impairs spatial learning memory-related cerebral regions mainly in the hippocampus and superior temporal gyrus, but enhances memory consolidation of inhibitory avoidance training in rats following footshock training14; enhances fear memory retention for 48 h and induces long-lasting traumatic memory enhancement and anxiety effects15. And the present study primarily explored functional shifts in the amygdala that were closely correlated with fear memory, which may explain the inconsistency between our conclusion that propofol can strengthen amygdala-related fear memory and the finding that propofol anesthesia attenuates hippocampus-dependent spatial learning memory capability. The objective of our study was to explore the effect of propofol anesthesia on the reinforcement of fear memory in mice undergoing fear conditioning; therefore, propofol anesthesia was given to mice that completed FC training to examine whether propofol could strengthen and consolidate their fear memory. The design of this study effectively simulates the clinical condition of patients suffering from malignant trauma and adverse stress events requiring propofol anesthesia treatment, which has important theoretical guidance and clinical reference value to the scientific selection of general anesthetics for patients who need surgical operation after trauma.
Based on prior research, it is widely assumed that propofol anesthesia enhances the central system suppressive neuronal efficacy of GABA and evokes general anesthetic effects45, whereas our findings showed that propofol anesthesia leads to heightened excitability of glutamatergic neurons in the BLA, thereby reinforcing fear memory in mice. This occurrence could be attributed to the suppression of GABAergic interneuron excitability in the BLA by propofol, whereby BLA glutamatergic neurons are modulated by the local GABAergic interneuron microcircuit, leading to decreased inhibitory modulation of glutamatergic neurons as GABAergic interneuron excitability decreases. This disinhibitory effect of GABAergic interneurons on glutamatergic neurons led to hyperexcitability in glutamatergic neurons, inducing consolidation and reinforcement of fear memory in mice.
Although our study provided preliminary evidence that propofol anesthesia enhances fear memory in mice by modifying the excitability of neurons in the BLA, our current study has limitations. Initially, to contain variables on our assay, we used adult male and female mice as our subjects, and it is unclear whether propofol exerts an analogous influence on mice of various ages. Then, our present study examined BLA-mediated fear memory alone, and the upstream and downstream brain regions of BLA and the neural circuits they established in propofol-enhanced fear memory in mice remain unknown. In the present study, the fear memory model of mice was established by one-footshock paradigm and it was found that propofol significantly enhanced fear memory. However, the effect of propofol on fear memory induced by more or stronger footshocks remains to be further explored. Finally, the memory of mice was only evaluated 48 h after training by the fear conditioning test. The long-term memory of mice after training remains to be investigated in the future studies to further elucidate the mechanisms by which propofol affects fear memory.
In summary, our study preliminarily showed that fear memory in mice was reinforced after propofol anesthesia, while the excitability of GABAergic interneurons in the BLA was inhibited and glutamatergic neurons were activated. Activation of GABAergic interneurons or suppression of glutamatergic neurons in the BLA using optogenetics or chemogenetics notably attenuated the effect of propofol on enhancing fear memory in mice. Propofol may trigger excitation-inhibition imbalance by altering the excitability of neurons in the BLA, thus enhancing fear memory. Our study provides a classical approach and robust laboratory evidence for better understanding the mechanism of PTSD and identifying drugs for the prevention and treatment of PTSD. It also provides significant theoretical guidance for the selection of suitable general anesthetic drugs for clinical malignant trauma patients requiring general anesthesia. For malignant trauma patients requiring emergency surgery, we may optionally minimize the dosage or duration of propofol and if available, alternative agents or approaches of anesthesia may be chosen to meet the surgical needs.
Methods
Animals
We have complied with all relevant ethical regulations for animal use. WT C57BL/6J mice were obtained from the Experimental Animal Center of Xuzhou Medical University. Vglut2/Vgat-cre and Vgat-ChR2-EYFP mice were originally sourced from Jackson Laboratory (USA) and bred at Xuzhou Medical University Laboratory Animal Center. Under a 12-h light/dark cycle (22–25 °C), all mice were fed and watered ad libitum and housed in groups of no more than 5 animals per cage. The mice were randomly grouped, and experiments were performed in an unbiased double-blind manner during the daytime. Both male and female mice (6 to 8 week-old) were used in the experiments (each group included an equal number of male and female mice). Experiments were approved by the Animal Care and Ethics Committee of Xuzhou Medical University and conformed to the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Behavioral procedures
We measured fear memory in mice with the fear conditioning test (FCT). Within the fear conditioning (FC) training chamber, the mice were free to explore for 90 s, followed by 30 s of conditioned (30 s, 70 dB tone conditional stimulus)-unconditioned (2 s, 0.80 mA footshock) stimulation. For mice in the untrained group, we allowed them to explore the same FC chamber freely for 150 s, without conditioned or unconditioned stimulation. Following the performance of each mouse, the training box was wiped with 75% ethanol to remove any odor previously left in the training box. The cue-memory test with altered context was performed 48 h after training using transparent boxes in different rooms with completely altered background environments from the training phase14. The mice were free to explore the box for 90 s without any stimulation, followed by three rounds of 30 s of 70 dB tone conditional stimuli (same as the conditioned stimulus during the training phase) given at 20 s intervals. The test chamber was wiped with acetic acid after each mouse test to remove any residual odor from the previous animal (Fig. 1a). Throughout the test, the FCS system was used to capture the activities of the mice in the stimulation box, and the Med Associates Animal Behavior Analysis System was used to automatically analyze freezing behavior and record the percentage of time the mice spent in the three acoustic stimulation periods, which was recorded as the freezing time in percent (%). The freezing time (%) during the training period = [the freezing time of 118 s before the footshock]/118 s ×;100%. The freezing time (%) during the test period = [the freezing time of 90 s during the acoustic stimulation periods]/90 s × 100%.
Western blotting analysis
Western blotting was performed 1 h after FC training. BLA tissue samples were obtained from the brain by adding 6-fold net tissue weight of RIPA lysate (Beyotime, P0013B, Shanghai, China) and PMSF (Beyotime, ST506, Shanghai, China), an enzyme inhibitor at a concentration of 1% RAPI volume; the samples were homogenized and centrifuged at 12,000 rpm × 15 min to extract the supernatant. Sample concentrations were calculated by measuring the absorbances with bicinchoninic acid (BCA) kits (Beyotime, P0010, Shanghai, China), and the samples were normalized to the same concentration. Electrophoresis and a trans-blot electrophoresis transfer system were used to transfer the proteins to PVDF membranes (Merck Millipore, ISEQ00010, USA). The protein strips were then incubated overnight with rabbit anti-c-Fos (1:1500, 2250, Cell Signaling Technology, USA) at 4 °C. Signals were finally detected with the enhanced ECL system after incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:1000, Beyotime) for 2 h. Image analysis was performed with ImageJ software.
Immunohistochemistry
The mice were perfused with 4% paraformaldehyde (PFA) at 4 °C under 5% isoflurane anesthesia 1 h after FC training, brain tissue was removed and fixed with 4% PFA for 6 h and then switched to 30% sucrose for 3 days of postfixation. Coronal slices (30 μm) of BLA brain regions were prepared with a freezing microtome (CM1900, Leica, Germany). Brain slices were blocked with 10% goat serum for two hours at 37 °C, followed by incubation with anti-c-Fos (1:1000, 2250, Cell Signaling Technology, USA), anti-GAD67 (1:300, ab26116, Abcam) or anti-Vglut2 (1:300, ab79157, Abcam) antibodies overnight at 4 °C. Then, the brain slices were washed three times with phosphate-buffered saline (PBS) and incubated with discrepant colors of Alexa Fluor-labeled goat anti-rabbit/mouse antibodies (1:500, Abcam) for 2 h at room temperature. For double immunofluorescent labeling, brain slices were incubated in a mixture of two primary or secondary antibodies. The fluorescence signal was detected and photographed by confocal laser scanning microscopy (FV1000, Olympus, Japan), and the staining of cells in the BLA in the 20x field was counted manually using Olympus Fluoview Ver 4.2a software. The average number of positive cells per mouse was counted in three to four brain slices at the front, center, and rear of the BLA (AP: −0.9 mm, −1.25 mm and −1.6 mm). The counting procedure was double-blinded, and the analysis parameters of the image analysis software were all consistent. The (c-Fos+ & GAD67+)/GAD67+ (%) = [the number of c-Fos+ & GAD67+ cells]/[the number of GAD67+ cells] × 100%. The (c-Fos+ & Vglut2+)/Vglut2+ (%) = [the number of c-Fos+& Vglut2+ cells]/[the number of Vglut2+ cells] × 100%.
Surgery
Throughout the procedure, the mice were anesthetized with isoflurane (5% for induction and 1.5–2.0% for maintenance) in a stereotaxic frame (RWD Life Science, China) on a heating pad to maintain body temperature. To investigate the effects of propofol on excitability of glutamatergic and GABAergic neurons in the BLA, rAAV-EF1α-DIO-mCherry (BrainVTA, China) expressing only fluorescent proteins were injected into the BLA (AP: −1.05 mm. ML: ±3.15 mm, DV: −4.80 mm; 150 nl per side, 100 nl/min) of Vglut2/Vgat-cre mice (Fig. 3a, d, g, j). For optogenetic modulation of BLA glutamatergic neurons, virus rAAV-EF1-DIO-eNpHR3.0-mCherry (BrainVTA, China) expressing an inhibitory photosensitive protein or control virus rAAV-EF1α-DIO-mCherry (BrainVTA, China) were injected into the bilateral BLA of Vglut2-cre mice. After viral injection, the fiber (125 mm outer diameter [OD] 0.37 numerical aperture [NA], Newdoon, Shanghai, China) was inserted into the target area and fixed to the skull using dental adhesive (Fig. 4b). For optogenetic modulation of GABAergic neurons in the BLA, optical fibers were inserted directly into the BLA region of Vgat-ChR2-EYFP mice (Fig. 5b). To examine the synaptic connections between GABAergic interneurons and glutamatergic neurons, the glutamatergic neuron visualization virus rAAv-CaMKIIa-mCherry (BrainVTA, China) was injected into the bilateral BLA of Vgat-ChR2-EYFP mice (Fig. 5b). For chemogenetic modulation of glutamatergic or GABAergic neurons in the BLA, AV9-EF1α-DIO-hM4D(Gi)-mCherry or AAV9-EF1α-DIO-hM3D(Gq)-mCherry (PackGene Biotech, China) virus and control virus AAV9-EF1α-DIO-mCherry (PackGene Biotech, China) was injected into the BLA of Vglut2/Vgat-cre mice (Figs. 6b and 7b). To verify the impact of GABAergic interneurons on glutamatergic neurons, glutamatergic neurons were visualized using rAAv-CaMKIIa-EYFP (BrainVTA, China) virus (Fig. 7b). Experiments such as behavioral and ex vitro electrophysiology were performed after 3 weeks, which allowed for the mice to fully recover.
Ex vivo electrophysiology
Approximately 3 weeks after optogenetic, chemogenetic or VEH virus injection, the mice were anesthetized with 5% isoflurane and perfused with ice-cold modified sucrose-based artificial cerebral spinal fluid (sACSF) solution containing (in mM) 80 NaCl, 3.5 KCl, 4.5 MgSO4, 0.5 CaCl2, 1.25 H2PO4, 25 NaHCO3, 10 glucose, and 90 sucrose equilibrated with 95% O2/5% CO2 (pH 7.25–7.4, 310–330 mOsm). Brain tissue was coronally sliced (300 μm) with a vibratome (VT1200; Leica, Germany) and incubated for 1 h at a constant temperature of 32 °C with the same solution. Recording was started upon incubation, and room temperature was maintained at approximately 25 °C during the recording period. The recording chamber was superfused at 2 ml/min with ACSF equilibrated with 95% O2/5% CO2 and containing (in mM) 119 NaCl, 2.5 KCl, 1 MgSO4, 2.5 CaCl2, 1.25 H2PO4, 26 NaHCO3 and 10 glucose (pH 7.25–7.4, 310–330 mOsm). ACSF containing propofol (5 µM) or bicuculline (Bic, 30 µM, S7071, Selleck, USA) was used to observe the impact of propofol on GABAergic and glutamatergic neurons51,52. A pulled (P-97, Sutter Instrument, USA) pipette (6–8 MΩ) filled with (in mM) 135 K gluconate, 0.2 EGTA, 5 KCl 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.1 GTP (pH 7.25 ~ 7.4, 290 Osm) was used. Brain slices were observed with an upright microscope equipped with an X4 lens and X40 water immersion lens (Olympus). The signal recording was amplified with a multiclamp 700B amplifier (molecular device). Digitized analog signals at 10 kHz were obtained with Digidata 1550B and pClamp10 software (Molecular Devices).
Drug administration
To explore the effect of different doses of propofol on fear memory, mice received different number of injections of the same single dose of propofol. Multiple intraperitoneal injections of vehicle (vehicle×5) and different number of injections of 60 mg/kg propofol (propofol × 2 + vehicle × 3, propofol × 3 + vehicle × 2, propofol × 4 + vehicle × 1, propofol × 5, Libang Pharmaceutical Co., China) with 30 min intervals were administered at the end of FC training to maintain a desirable state of anesthesia. To explore the effect of propofol administration at different time points on fear memory in mice, 60 mg/kg × 4 propofol was injected intraperitoneally at 0, 30, 60, and 90 min after the completion of FC training. Propofol was administered intraperitoneally at a dose of 60 mg/kg × 4 following FC training in the optogenetic and chemogenetic manipulation of BLA neurons experiments. For chemogenetic manipulation of neurons in the BLA, a single intraperitoneal injection of clozapine-N-oxide (CNO, 2.5 mg/kg, BrainVTA, China) was administered 30 min prior to FC training .Dilution of CNO was performed as described before53.
Optogenetic manipulations
The mice were injected with propofol (60 × 4 mg/kg) immediately at the completion of FC training, followed by Vgat-ChR2-EYFP mice receiving blue light (2–4 mW, 10 ms pulses at 20 Hz) from a 473 nm laser emitter (Newdoon, China) via optical fiber; Vglut2-cre mice received yellow light (2–4 mW, constant) from a 589 nm laser emitter. Prior to the experiment, the power intensity of the laser was tested with a power meter (PM20, THORLABS, USA). Blue or yellow light was used to irradiate ex vivo brain slices of Vgat-ChR2-EYFP or Vglut2-cre mice, respectively, during the ex vivo electrophysiological recording experiments. Western blotting and immunofluorescence staining were performed 1 h after the completion of FC training, and behavioral tests were performed 48 h after FC training.
Chemogenetic manipulations
A single intraperitoneal injection of CNO was performed 30 min prior to FC training, and propofol (60 × 4 mg/kg) was given to each mouse at the end of training. Western blotting and immunofluorescence staining were performed 1 h after the end of FC training, and behavioral tests were performed 48 h afterward. Signals were measured before and after the application of CNO (10 µM) to ex vivo brain slices of Vgat-cre/Vglut2-cre mice in the ex vivo electrophysiological experiments.
Statistics and reproducibility
Graph Pad Prism 8.0 (GraphPad Software, USA) was used for statistical analysis. Statistical details of all experiments are available in the figure legends. All data are presented as the mean ± SEM. Sample sizes and statistical methods were used based on analogous experiments with chemogenetic and optogenetic methods54–57. Prior to analysis, all data were subjected to the Shapiro‒Wilk normality test to establish if parametric or nonparametric tests should be used. Paired or unpaired two-tailed t tests were selected to perform comparisons between two groups. Nonparametric Mann–Whitney test and Wilcoxon matched-pairs signed rank test were used for unpaired and paired abnormally distributed samples, respectively. Multiple comparisons were conducted by one-way or two-way ANOVA and Tukey’s post hoc test for multiple comparisons. p < 0.05 was considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Funding support for this work was provided by the National Natural Science Foundation of China (82371211, 82171191) and Key Subject of Colleges and Universities Natural Science Foundation of Jiangsu Province (23KJA320009).
Author contributions
Under the guidance and supervision of Y.W., C.Z., and H.Z. C.C. performed experiment design, propofol anesthesia and electrophysiology recording, and chemogenetic and optogenetic regulation. Y.Z. and S.L. collected the experimental data and conducted the data analysis. W.W. and Y.Q. took charge of the reproduction and feeding of mice. J.L., W.D., and J.W. performed part of the behavioral tests. Q.L. and H.H. performed the statistical analysis. The manuscript was prepared by C.C. and C.Z. All the authors have critically read the manuscript and approved the final version.
Peer review
Peer review information
Communications Biology thanks Stephanie Grella and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jasmine Pan. A peer review file is available.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary data 1). Uncropped and unedited blot images are provided in supplementary information 1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chen Chen, Shuai Li, Yue Zhou.
Contributor Information
Hui Zheng, Email: zhenghui@cicams.ac.cn.
Yu-Qing Wu, Email: xzmcyqwu@163.com.
Cheng-Hua Zhou, Email: xzhmuchzhou@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07105-5.
References
- 1.Harnett, N., Goodman, A. & Knight, D. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp. Neurol.330, 113331 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Brewin, C. et al. A review of current evidence regarding the ICD-11 proposals for diagnosing PTSD and complex PTSD. Clin. Psychol. Rev.58, 1–15 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Bisson, J., Cosgrove, S., Lewis, C. & Robert, N. Post-traumatic stress disorder. BMJ351, h6161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rytwinski, N., Scur, M., Feeny, N. & Youngstrom, E. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J. Trauma. stress26, 299–309 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Cougle, J., Resnick, H. & Kilpatrick, D. PTSD, depression, and their comorbidity in relation to suicidality: cross-sectional and prospective analyses of a national probability sample of women. Depression Anxiety26, 1151–1157 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Koenen, K. et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med.47, 2260–2274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjet, C. et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med.46, 327–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie, M., SaadAdeen, S. & Battat, M. Anxiety disorders and PTSD in Palestine: a literature review. BMC Psychiatry20, 509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams, T., Phillips, N., Stein, D. & Ipser, J. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst. Rev.3, CD002795 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grubaugh, A., Zinzow, H., Paul, L., Egede, L. & Frueh, B. Trauma exposure and posttraumatic stress disorder in adults with severe mental illness: a critical review. Clin. Psychol. Rev.31, 883–899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatzick, D. et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol. Med.37, 1469–1480 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Usuki, M. et al. Potential impact of propofol immediately after motor vehicle accident on later symptoms of posttraumatic stress disorder at 6-month follow up: a retrospective cohort study. Critic. Care16, R196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong, J. et al. Effects of Sevoflurane and Propofol on Posttraumatic stress disorder after emergency trauma: a double-blind randomized controlled trial. Front. Psychiatry13, 853795 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauer, D. et al. Propofol enhances memory formation via an interaction with the endocannabinoid system. Anesthesiology114, 1380–1388 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Morena, M. et al. Effects of ketamine, dexmedetomidine and propofol anesthesia on emotional memory consolidation in rats: Consequences for the development of post-traumatic stress disorder. Behav. Brain Res.329, 215–220 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Yu, O. et al. Texture analysis of brain MRI evidences the amygdala activation by nociceptive stimuli under deep anesthesia in the propofol-formalin rat model. Magn. Reson. imaging25, 144–146 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Patel, R., Girard, T., Pukay-Martin, N. & Monson, C. Preferential recruitment of the basolateral amygdala during memory encoding of negative scenes in posttraumatic stress disorder. Neurobiol. Learn. Mem.130, 170–176 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Haritha, A., Wood, K., Ver Hoef, L. & Knight, D. Human trace fear conditioning: right-lateralized cortical activity supports trace-interval processes. Cogn., Affect. Behav. Neurosci.13, 225–237 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Harnett, N. et al. Neural mechanisms of human temporal fear conditioning. Neurobiol. Learn. Mem.136, 97–104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight, D., Waters, N. & Bandettini, P. Neural substrates of explicit and implicit fear memory. NeuroImage45, 208–214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, S. & McNally, G. The conditions that promote fear learning: prediction error and Pavlovian fear conditioning. Neurobiol. Learn. Mem.108, 14–21 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci.16, 332–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sah, P., Faber, E., Lopez De Armentia, M. & Power, J. The amygdaloid complex: anatomy and physiology. Physiol. Rev.83, 803–834 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Beyeler, A. et al. Divergent routing of positive and negative information from the Amygdala during memory retrieval. Neuron90, 348–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campeau, S. & Davis, M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear- potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci.15, 2312–2327 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanselow, M. & Kim, J. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav. Neurosci.108, 210–212 (1994). [DOI] [PubMed] [Google Scholar]
- 27.LeDoux, J., Cicchetti, P., Xagoraris, A. & Romanski, L. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci.10, 1062–1069 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery, S. et al. BNST neurocircuitry in humans. NeuroImage91, 311–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spampanato, J., Polepalli, J. & Sah, P. Interneurons in the basolateral amygdala. Neuropharmacology60, 765–773 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Li, C. & Rainnie, D. Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. J. Physiol.592, 4329–4351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich, I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron62, 757–771 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Yuan, X., Zhang, D., Mao, S. & Wang, Q. Filling the Gap in Understanding the Mechanism of GABAAR and Propofol Using Computational Approaches. J. Chem. Inf. Model.61, 1889–1901 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Sahinovic, M. M., Struys, M. & Absalom, A. R. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin. Pharmacokinet.57, 1539–1558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunsmoor, J. E., Cisler, J. M., Fonzo, G. A., Creech, S. K. & Nemeroff, C. B. Laboratory models of post-traumatic stress disorder: the elusive bridge to translation. Neuron110, 1754–1776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh, C. T. Propofol: Milk of Amnesia. Cell175, 10–13 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Zhang, J., Zhang, X. & Jiang, W. Propofol impairs spatial memory consolidation and prevents learning-induced increase in hippocampal matrix metalloproteinase-9 levels in rat. Neuroreport24, 831–836 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Xu, Z. et al. Effects of ginsenosides on memory impairment in propofol-anesthetized rats. Bioengineered13, 617–623 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahan, A. L. & Ressler, K. J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci.35, 24–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brachman, R. A. et al. Ketamine as a Prophylactic against stress-induced depressive-like behavior. Biol. Psychiatry79, 776–786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGhee, L. L., Maani, C. V., Garza, T. H., Gaylord, K. M. & Black, I. H. The correlation between ketamine and posttraumatic stress disorder in burned service members. J. Trauma64, S195–S198 (2008). [DOI] [PubMed] [Google Scholar]
- 41.McGhee, L. L. et al. The intraoperative administration of ketamine to burned U.S. service members does not increase the incidence of post-traumatic stress disorder. Mil. Med.179, 41–46 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Li, S., Zhou, W., Li, P. & Lin, R. Effects of ketamine and esketamine on preventing postpartum depression after cesarean delivery: a meta-analysis. J. Affect Disord.351, 720–728 (2024). [DOI] [PubMed] [Google Scholar]
- 43.Niu, W., Duan, Y., Kang, Y., Cao, X. & Xue, Q. Propofol improves learning and memory in post-traumatic stress disorder (PTSD) mice via recovering hippocampus synaptic plasticity. Life Sci.293, 120349 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Goetz, T., Arslan, A., Wisden, W. & Wulff, P. GABA(A) receptors: structure and function in the basal ganglia. Prog. Brain Res.160, 21–41 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang, Y. et al. Different effects of etomidate and propofol on memory in immature rats. Int. J. Neurosci.125, 66–69 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Quan, X. et al. Propofol and memory: a study using a process dissociation procedure and functional magnetic resonance imaging. Anaesthesia68, 391–399 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Zhong, Y. et al. PKA-CREB-BDNF signaling pathway mediates propofol-induced long-term learning and memory impairment in hippocampus of rats. Brain Res.1691, 64–74 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Zhang, S., Liang, Z., Sun, W. & Pei, L. Repeated propofol anesthesia induced downregulation of hippocampal miR-132 and learning and memory impairment of rats. Brain Res.1670, 156–164 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Sun, M., Yuan, R., Liu, H., Zhang, J. & Tu, S. The effects of repeated propofol anesthesia on spatial memory and long-term potentiation in infant rats under hypoxic conditions. Genes Dis.7, 245–252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin, J. et al. Propofol exposure during early gestation impairs learning and memory in rat offspring by inhibiting the acetylation of histone. J. Cell Mol. Med.22, 2600–2611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forte, N., Medrihan, L., Cappetti, B., Baldelli, P. & Benfenati, F. 2‐Deoxy‐d‐glucose enhances tonic inhibition through the neurosteroid‐mediated activation of extrasynaptic GABAA receptors. Epilepsia57, 1987–2000 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Nagata, I. et al. Subanesthetic dose of propofol activates the reward system in rats. Anesthesia Analgesia135, 414–426 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Wu, W.-F. et al. Impaired synaptic plasticity and decreased glutamatergic neuron excitability induced by SIRT1/BDNF downregulation in the hippocampal CA1 region are involved in postoperative cognitive dysfunction. Cell. Mol. Biol. Lett.29, 10.1186/s11658-024-00595-5 (2024). [DOI] [PMC free article] [PubMed]
- 54.Hartley, N. D. et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat. Neurosci.22, 2000–2012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Domingo-Rodriguez, L. et al. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat. Commun.11, 782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao, W. W. et al. Nucleus accumbens neurons expressing dopamine D1 receptors modulate states of consciousness in sevoflurane anesthesia. Curr. Biol.31, 1893–1902.e1895 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Fogaca, M. V. et al. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol. Psychiatry26, 3277–3291 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary data 1). Uncropped and unedited blot images are provided in supplementary information 1.