Abstract
The field of digital therapeutics (DTx), software programs that prevent, manage, and treat medical conditions, continues to grow. DTx offers new treatment options and has the potential to close gaps in care caused by unmet patient needs, provider shortages, or socioeconomic or geographical disparities. However, the field of DTx has not seen steady adoption owing to barriers, particularly related to coverage, payer acceptance of the category, provider use, and integration within existing health care delivery tools. One challenge for payers to effectively evaluate and cover DTx products is ensuring that consistent data elements are listed for these products in traditional drug compendia databases. Managed care organizations will need similar information about DTx product features as are available for traditional medications to inform coverage and reimbursement decisions. The Academy of Managed Care Pharmacy DTx Advisory Group developed and distributed a request for information to the 5 top drug compendia companies to assess how compendia products incorporate DTx and prescription DTx. This article summarizes how DTx are listed within different compendia products and offers insights on future data needs to adequately inform payers. As the DTx sector grows and consumer demand rises, compendia listing services will need to evolve to accommodate these new therapies and treatment modalities and facilitate patient access and efficient claims processing. Recommendations for how compendia companies can support managed care in these efforts are outlined.
Plain language summary
The field of digital therapeutics (DTx), software programs that prevent, manage, and treat medical conditions, continues to offer new treatment options. These therapies can close gaps in care caused by unmet patient needs, provider shortages, or economic or geographical disparities. However, for DTx to provide optimal care to patients, information must be in an accessible format recognized by clinical and operational processes within the health care delivery system.
Implications for managed care pharmacy
The growing field of DTx can improve the quality of care for patients through innovative and personalized technologies. Managed care organizations rely on compendia data to inform critical business functions. Compendia companies have incorporated DTx into their databases, although listings remain inconsistent, with potentially critical adjudication information not included. In a system familiar with pills, injectables, biologics, and other specialty medications, information regarding DTx must be accessible in similar formats, processes, and delivery systems.
In 1995, Dr Joseph Kvedar led a program to learn about the development and application of technology for delivering care outside the traditional setup of a hospital or a doctor’s office, thus opening the door to the digital health or digital therapeutics (DTx) domain. It would take more than 15 years before the terms DTx and “e-patient” worked their way into the vernacular.1
DTx is a new category of medicine and delivers medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of diseases and disorders.2 The digital health landscape is multifaceted, including digital health, digital medicine, and DTx. Prescription DTxs (PDTs) are a subset of DTx and are software-based therapies that deliver a clinical benefit to patients, either alone or in combination with other treatments, and require a prescription from a provider.3
This article provides an overview of the DTx landscape to guide PDT developers in ensuring that their products have complete listings within the national compendia and for compendia companies to adapt their traditional processes to adequately encompass DTx products.
The distinctions and the nuances within the DTx category have been outlined by the DTx Alliance and are shown in Figure 1.
FIGURE 1.
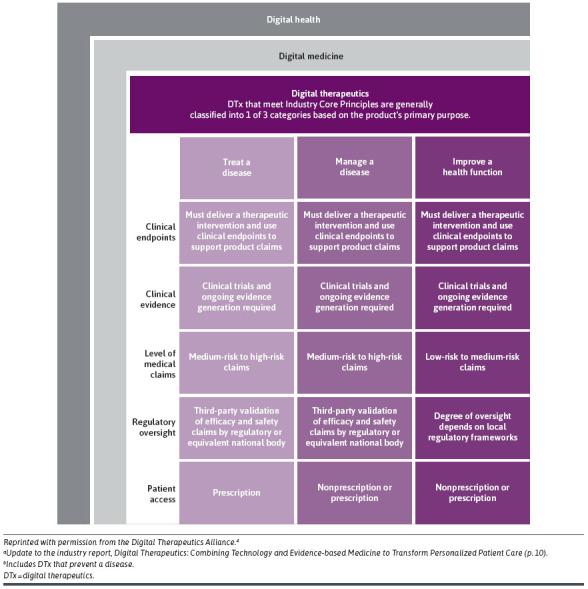
DTx Product Categoriesa
Despite the opportunities for DTx to offer new treatment options and close gaps in care caused by unmet patient needs, provider shortages, or economic or geographical disparities, the field of DTx has not seen steady payer adoption.5,6
Within the digital health and DTx domains, these technologies vary in their application, approach to care, and clinical category. For example, unlike digital apps that may track symptoms, DTx is built on science supported by technically complex software programs like artificial intelligence and blockchain. These products seek to prevent, manage, or treat a medical disorder or disease and may be used either alone or in combination with other standard-of-care treatments.5,7 They also are available to treat various conditions, such as diabetes, depression, anxiety, insomnia, irritable bowel syndrome, substance use disorders, and cancer.
For DTx products, the rigor of evidence available or required can differ. Some products receive authorization or clearance from the US Food and Drug Administration (FDA), thus allowing efficacy or safety claims. PDTs require different levels of evidence depending upon whether the product is reviewed under FDA’s de novo classification, 510(k) clearance, or premarket approval pathway. Regardless of the review process, there is debate among some stakeholders on whether regulatory review for these products is a necessity. Some developers and patient advocacy groups do not see value in clinical trials for these products, perceiving that real-world outcomes are sufficient. Other multistakeholder organizations, such as the DTx Alliance and Academy of Managed Care Pharmacy (AMCP), note that DTxs should be supported by clinical trials and real-world outcomes.5,8 These organizations further suggest that policymakers and payers should prioritize products that have undergone sufficient clinical evaluation to demonstrate product safety and efficacy in peer-reviewed journals.5,8
Over the last 5 years, the FDA has cleared more than 35 DTx products across several therapeutic categories, with hundreds of additional products in development. Eleven PDTs are available, with another 90 in development.9,10 Despite growing availability, these products continue to face barriers, particularly related to coverage, payer acceptance, and provider use, that have prevented more widespread adoption. Many payers are defining approaches to evaluate and cover DTx and PDTs. For example, some major commercial payers have created digital formularies; certain commercial payers and state Medicaid plans have announced one-off contracts, and others have declined to cover PDTs.11-13 Legislative efforts have been introduced to add PDTs to the list of services and products eligible for coverage under Medicare and Medicaid. They would also direct the Centers for Medicare and Medicaid Services to establish payment methodologies and product-specific Healthcare Common Procedure Coding System (HCPCS) billing codes, making it easier for commercial payers to process medical-benefit payments for PDTs.3,14
There are other challenges for payers to evaluate and cover PDTs, including available information in traditional drug compendia databases.5 Drug compendia are referential sources or databases that contain information about drugs and other therapeutic products. They are used by health care professionals, payers, electronic health record (EHR) systems, pharmacy dispensing software systems, drug wholesalers, pharmacy benefit managers, and others to make informed decisions about the safe and effective use of these products and play an important role in the reimbursement process. These stakeholders purchase subscriptions to 1 or more drug compendia. Payers, such as health plans and pharmacy benefit managers, use drug compendia to collect information on which treatments to cover and at what cost. For some payers, listing in compendia and the corresponding reported pricing is a critical step in the process of evaluating a product for coverage.
Only 1 company maintains a separate device database and initially listed DTx products within that database. Market pressure forced them to change their process to include DTxs in the drug database. Within managed care, approved DTx products are prescribed, dispensed, and paid for through the same infrastructure as prescription medications, which is why accurate and timely listing in the pricing compendia are important. Drug databases, not devise databases, typically feed into EHRs.
Although compendium listings for traditional medications vary in content and formatting, whichever compendia is used is relied on to provide health care professionals with important product descriptions and information about dosage and administration, approved indications, potential side effects, and potential drug interactions. They can provide critical information needed for product evaluations, to identify clinical comparators, and to guide appropriate product use criteria (eg, prior authorization, step therapy, or other utilization management strategies).
The growing field of DTx can improve the quality of care for patients through innovative and personalized technologies. As both DTx and PDTs become more prevalent and used as treatment options, managed care will need similar information about these product characteristics as is currently available for traditional medications to inform coverage and reimbursement decisions. We sought to understand how DTx are currently listed within different compendia products and offer insights for compendia to evolve and adequately address payer information needs.
Methods
To assess how compendia products incorporate DTx and PDTs, a request for information (RFI) was developed. The RFI was developed with input by the AMCP DTx Advisory Group (DTxAG) Information Technology and Database subcommittee composed of representatives from payers, health technology companies, consultancies, and DTx and biopharmaceutical manufacturers. The RFI was developed and distributed via email to 5 drug compendia companies used by virtually all managed care companies for pricing and clinical information: Cerner Multum, First Databank (FDB), Gold Standard, Medi-Span, and Red Book/Merative in September 2022. IRB approval was not required for this nonhuman research. The RFI consisted of 16 questions about the scope of compendia products and editorial policies and procedures for DTx and PDTs. Outreach via email and phone to obtain feedback was conducted with each company up to 5 times.
Details regarding the listing of DTx were provided by 4 of the 5 major drug compendia listing services used by managed care companies. Red Book/Merative did not respond to the RFI. One compendia company provided written responses to the RFI, and executives from the other 3 companies engaged in a virtual structured interview process. Qualitative, 1-hour teleconference interviews were conducted using the developed discussion guide between October and December 2022. Teleconference interviews were recorded to ensure the accuracy of the information, and the results from all 4 compendia were synthesized.
Key Findings
Drug compendia companies include DTx in their listings, but more consistency in how information is listed across the various listing services is needed. Although compendia are often thought of as drug files, in addition to a drug database, each company provides a host of different products and files for purchase. An overview of compendia company characteristics is detailed in Table 1.
Table 1.
Drug Compendia Characteristics
| Characteristic | Cerner Multum | First Databank | Gold Standard | Medi-Span |
|---|---|---|---|---|
| Parent company of compendia | Oracle | Hearst | Elsevier | Wolters Kluwer |
| Is the compendia considered a pricing data source? | Yes | Yes | Yes | Yes |
| Clinical information included in the dataset |
|
|
|
|
| Database product(s) | Cerner Multum includes prescription drugs, DTx, over-the-counter drug products, herbal products, and nutraceutical products | MedKnowledge includes FDA-approved prescription drugs, over-the-counter medications, and limited collections of disposable or reusable medication devices commonly dispensed by pharmacies. Prizm: includes medical devices. | Gold Standard Drug Database includes prescription drugs, over-the-counter drug products, herbal products, vitamin and nutritional products, medical devices, and diagnostic kits | Medi-Span includes prescription and over-the-counter drug product data, herbal products, vitamin and nutritional products, medical devices, and diagnostic tests and kits |
| Inclusion of DTx in the compendia | Yes | Yes: Prizm | Yes | Yes |
| Inclusion of PDTs in the compendia | Yes | Yes: MedKnowledge and Prizm | Yes | Yes |
DTx = digital therapeutics; FDA = US Food and Drug Administration; PDT = prescription digital therapeutics.
Once information is submitted to the compendia, DTx companies may ask what fields will be published for their product and how this information will be used. One common misconception in the industry is that drug compendia dictate how their data fields will specifically be used by those purchasing compendia subscriptions. Compendia collect and display singular pieces of information for a product, such as the product name, identifier, or price. Although compendia customers may use the data to create their own guidelines or use-case algorithms (eg, how a product appears in an EHR or dispensing software), the compendia companies themselves do not publish this level of detail for DTxs.
A summary of the information provided by drug compendia companies through the RFI process is provided in Table 2.
Table 2.
Drug Compendia Characteristics
| Category | Cerner Multum | FDB | Gold Standard | Medi-Span |
|---|---|---|---|---|
| Criteria for inclusion | Product identification information, FDA approval, and labeling | Product identification information, including a product label, FDA approval clinical use, and listing status within FDA-maintained databases | FDA approval letter and labeling. Pricing information preferred | Product identification number, description, product label, pricing information, and general information about the software use (eg, indication) |
| Information for submission process | Standardized process for manufacturers to submit using a specific form, with a dedicated team to work with manufacturers | Med Knowledge: standardized process with website instructions on how to submit via manufacturer Web Portal or email. Research Associate available for manufacturer support.Prizm: products listed on AccessGUDID are automatically included | Standardized process in which the manufacturer uses a submission form template. There are staff members who support a successful submission | Standardized process with website instructions on how to submit. A team of associates will directly support manufacturers. Information is evaluated for inclusion |
| Designation as a PDT, DTx, or digital health app | Prescription vs over-the-counter status is delineated | FDB Prizm and FDB MedKnowledge have fields to indicate prescription requirement status. MedKnowledge includes PDT | Brand/generic status is delineated in the database. Prescription status is determined by FDA labeling | Prescription vs over-the-counter status is delineated in the data table |
| Requirements or “qualifiers” used to list a product as a PDT | Identification number and price | MedKnowledge: FDA clearance or authorization, listed on AccessGUDID, prescription only, product information including instructions for use/directions, manufacturer name/contact info, pricing, and UDI | Device manual | Identifier, product information (including instructions for use/directions), price, and pricing effective date |
| Do you collect, evaluate, and cite evidence used to evaluate PDT? | No | No | No | No |
| Is the FDA status of a PDT product (FDA authorized, FDA cleared, or FDA approved) listed? | No | Yes | Unknown | No |
| If a PDT is approved as an adjunct to medication therapy, is it delineated within the compendia? | No | No | Unknown | Listed together if packaged together |
| If a PDT is authorized to help improve medication management or adherence, is that delineated? | No | No | Unknown | No |
| How is the duration or days supply delineated? | Not listed | Duration noted in the label name | Listed as “one” for each device | Not listed |
| What “dosage form” is used? | No dosage form; considered a “non-drug item” | Products are listed as miscellaneous | No dosage form; considered a “non-drug item” | Products are listed as miscellaneous |
| Can the product identifiers be classified by disease state, therapeutic class, type of digital product, or another differentiator? | Unknown | Products vary and classifications are determined at the time of evaluation for database inclusion | Unknown | No |
| Does your compendium provide UDI numbers for DTx products alongside other product identifiers? | No | Yes | Unknown | No |
| How quickly is it listed in your compendia and client data feeds? | Listed in compendia within a week or less | Varies depending on availability of product information, product complexity, and product launch/market date | Same day | Within a few days |
| Outreach to developers to support DTx listing | Yes, there is a team that will support the manufacturer | If information is lacking or absent, FDB will contact the labeler for information, such as pricing | Yes, there is a team that will support the manufacturer | Yes, there is a team that will support the manufacturer |
| How are updates and revised listings for PDT, which have frequent product revisions, such as software or clinical updates, handled? How do you distinguish what a substantive change is? How frequently does your company review new releases? | A substantive change would be a new or different product identification number | Labelers are required to review products listed on the FDB database every 6 months. Product information can be submitted to FDB via the manufacturer web portal at any time, but update frequency depends on the type of revision. With a substantive change, a new identifier is needed | Clinical and patient safety data, drug pricing, and images are updated daily. When a new drug launches or an important change is announced, clinicians can access all pertinent information | A substantive change would be a new or different product identification number |
DTx = digital therapeutics; FDA = US Food and Drug Administration; FDB = First Databank; PDT = prescription digital therapeutics; UDI = unique device identifier.
REQUIRED INFORMATION FOR PDT INCLUSION
Compendia companies differ on what information is required to have DTxs published in the compendia. To be listed in the compendia, both FDB and Gold Standard indicate that FDA product labeling is required. FDB further indicates that a PDT must be listed on AccessGUDID, the National Library of Medicine’s medical device database. When interviewed, at least 1 compendium mentioned that these additional elements were needed to list a PDT:
Identifier
Price
Pricing effective date
Package size
Product information
Instructions for use
Manufacturer name
Contact information
Components with unique device indication (UDI) (if available)
Days supply/duration of therapy
Version number
Gold Standard requires a device manual for a product to be included, and FDB added that manufacturers should provide information about the FDA approval/FDA clearance pathway for the product, such as 510(k), premarket approval, or de novo classification.
EVALUATION OF CLINICAL EVIDENCE
None of the compendia collect or evaluate clinical evidence, such as the quantity, rigor, and quality of evidence or risk of bias of the clinical evidence related to using DTxs or PDTs. Compendia do not publish information about whether a PDT is intended to help improve medication management or adherence, nor will a specific PDT be linked to adjunct medication therapies within most compendia.
One unique characteristic of DTxs and PDTs are frequent software updates or revisions. For drug products, although changes in the route of administration or dosage form are considered substantive and are captured, a change in where the product is manufactured would not be captured by the drug compendia. As noted by the compendia, many of these DTx changes are not considered substantive and are not captured in the compendia. Medi-Span and Cerner Multum both provided examples of substantive changes that would require updates, expressing that a new or different product identifier would be a reason for a compendia update. Compendia should not be viewed as an avenue to access specific details (eg, version or web vs app platforms) for DTx or PDT products.
PRESCRIPTION VS OVER-THE-COUNTER STATUS
Each compendium has a field to differentiate prescription vs over-the-counter status in its database, which can differentiate PDT and DTx products. However, a further distinction between DTxs and digital health apps is not currently possible. FDB noted that they list the FDA 510(k) status for PDTs. Medi-Span stated that the FDA status of a DTx or PDT is not listed.
Originally designed to capture drug information, some compendia fields, such as dosing and duration, are not readily adaptable to DTxs. For example, FDB and Medi-Span responded that DTxs are assigned a “miscellaneous” dosage form for DTxs. Gold Standard and Cerner Multum refer to each DTx as a “non-drug item.” Finally, FDB noted that the duration of use may be included within the “Label Name” field.
PRODUCT CLASSIFICATION AND UNIQUE DEVICE IDENTIFIERS
Each compendium publishes a proprietary field to classify and group “like” products, such as the generic product identifier in Medi-Span, generic code number in FDB, specific product ID in Gold Standard, or Main Multum Drug Code in Cerner Multum. Each identifier differs and may vary in length (from 5 to 14 digits). Therefore, the structure for categorizing DTxs does not yet appear well-defined in any compendium or consistent in format or length across compendia.
For drug products, drug databases use the National Drug Code (NDC) number as the unique identifier. DTx products do not use NDC numbers. Some DTx products use a UDI number; however, compendia products do not consistently provide the UDI numbers for DTx products alongside other product identifiers. Today, compendia convert UDIs to 11-digit identifiers to publish for reimbursement purposes. In the future, compendia may publish UDIs, which should be aligned across all drug compendia to eliminate opportunities for confusion or discrepancy.
PROCESS FOR PDT LISTING IN DRUG COMPENDIA DATABASES
Despite the limitations discussed, listing PDT and DTx products in the drug compendia databases is an important process for manufacturers and care delivery. Compendia provide the data that feed EHRs, pharmacy dispensing software systems, and payer databases. For some compendia, like Gold Standard, new products may be listed on the same day as the manufacturer submission. Medi-Span notes that products are listed within a few days, whereas Cerner states it may take up to a week. FDB states that time will vary based on the availability of product information and its completeness. Each compendium has a proprietary form that can be used for submission, with each compendium noting that associates are available to support manufacturers who may be unfamiliar with compendia processes.
MEDICAL VS PHARMACY BENEFIT
DTx and PDTs may be covered under the medical or pharmacy benefit. As Medi-Span shared, many PDT products are covered under the medical benefit. Although listing in the pricing compendia is critical for pharmacy benefit coverage, products covered under a medical benefit may also benefit from being listed in pricing compendia. For example, payers may leverage compendia information when designing generic product identifier/generic code number-NDC-HCPCS crosswalks (a coding translation that maps similar or equivalent codes between 2 different code sets) or to update fee schedules. Although listing in compendia is particularly important for products covered under the pharmacy benefit, listing may not be as beneficial for products covered under the medical benefit because those products are reimbursed based on HCPCS codes that occur outside of the pharmacy adjudication process.12,15 This insight is applicable to the other compendia as well.
STRENGTHS AND LIMITATIONS
There are several potential limitations to this review. Information collected relies on self-reporting by compendia organizations. One company provided written responses, whereas 3 agreed to a structured interview, allowing the opportunity for more detailed follow-up questions and the ability to clarify responses. Although both the compendia organizations and the AMCP DTxAG members reviewed the information to ensure that it reflected their expertise, additional verification steps were not applied. This rapidly evolving marketplace may uncover additional criteria over time and lead to more standardized processes. In addition, as DTxs and PDTs have evolved, so has the compendia listing for these products. For example, FDB noted recent changes to include PDTs in FDB’s MedKnowledge drug database. Previously, compendia customers subscribing only to FDB’s MedKnowledge drug database would not have access to DTx listings published in FDB Prizm. In addition, other compendia organizations noted changes or that certain product characteristics had not reached critical mass. Therefore, this landscape may be updated as organizations make additional changes.
This is an important step forward in bridging a critical gap in how DTx and the PDTs can be assessed and standardized by payers. This work could benefit not only drug compendia and payers but also manufacturers who are interested to get into the prescription space but may not be so familiar with how this step in the process works.
Recommendations for Compendia Companies to Support Managed Care
In the current health care ecosystem, compendia listings are critical for access to therapies and processing pharmacy claims. Listing services are primarily designed to include pharmaceuticals. As the DTx sector grows and consumer demand rises, compendia listing services will need to evolve to accommodate these new therapies and treatment modalities and facilitate patient access and efficient claims processing.
Compendia companies should reduce barriers to patient access and enable the successful adjudication of claims by
Ensuring consistent inclusion of UDIs across the various compendia
Including FDA approval pathway status for listed DTx products
Providing clear, uniform descriptions or categories for PDT and DTx products
Incorporating product categories (eg, disease state, therapeutic class, or other differentiation) to more effectively allow comparisons of treatment alternatives
Evaluating clinical evidence associated with PDTs and consider listing these products within their clinical compendia databases
Providing clear and consistent PDT dosing information and dispensing units
Conclusions
This landscape assessment shows that the interviewed compendia companies have improved the incorporation of these therapies into their databases although listings remain variable, with potentially critical adjudication information missing, such as FDA market authorization information or UDIs. For the health care system to reap the tremendous benefit that DTxs and PDTs have to offer patients and payers in terms of cost savings, information must be in an accessible format recognized by clinical and operational processes within the health care delivery system.
Managed care pharmacy looks to compendia companies to support the implementation of existing interventions. More consistent and complete information on DTx and PDT products is needed to help with patient access to these products and support existing processes for product reimbursement.
ACKNOWLEDGMENTS
Engagement in and sponsorship of this Advisory Group should not imply endorsement by the participants’ respective organizations. We would specifically like to acknowledge these members of the AMCP DTxAG, the IT and Database Subgroup: Mark Atalla, PharmD, MBA (Founder, Purposed, Inc.); Chris Christensen (DTx Market Access Lead, Otsuka); Mark Hopman, RPh, MBA (Vice President, Market Access, Pear Therapeutics); Jim Kenney, RPh, MBA (President, JTKENNEY, LLC); Helen Kim, PharmD, BCGP (Clinical Pharmacist, Optum Specialty Pharmacy); Abigail Lore, MPA (Director, Director of Policy and Research, PhRMA); Gregory Lyles (Vice President, Market Access, Akili Interactive Labs, Inc); Michael Pace, MBA (CEO & Founder, PalmHealthCo); Tim Regan, BSPharm (Vice President, Xcenda); Tim Rudolphi, MBA (CEO, metaMe Health); Laura Topor, BA (Senior Manager, Cognizant); Andrew Wall, MS (Founder/Chief Innovation Officer, Healthmap Solutions); and Mitzi Wasik, PharmD, MBA (Vice President, Product and Platform Strategy, SS&C Health).
Funding Statement
The AMCP DTxAG, the IT and Database Subgroup, and the development of the Viewpoint were supported by Akili Interactive, Mahana Therapeutics, metaMe Health, Otsuka, Pear Therapeutics, Pfizer Inc, PhRMA, and Xcenda.
REFERENCES
- 1.Makin S. The emerging world of digital therapeutics. Nature. 2019;573(7775):S106-9. doi:10.1038/d41586-019-02873-1 [DOI] [PubMed] [Google Scholar]
- 2.Digital Therapeutics Alliance. DTA’s adoption & interpretation of ISO’s DTx definition. Published June 2023. Accessed May 28, 2024. https://dtxalliance.org/wp-content/uploads/2023/06/DTA_FS_New-DTx-Definition.pdf
- 3.Academy of Managed Care Pharmacy. Prescription digital therapeutics. Accessed May 28, 2024. https://www.amcp.org/policy-advocacy/legislative-regulatory-issues/prescription-digital-therapeutics
- 4.Digital Therapeutics Alliance. Fact Sheet: DTx product categories. Published January 2021. Accessed May 28, 2024. https://dtxalliance.org/wp-content/uploads/2021/01/DTA_FS_DTx-Product-Categories_010521.pdf
- 5.Academy of Managed Care Pharmacy. AMCP Partnership Forum: The evolving role of digital therapeutics. J Manag Care Spec Pharm. 2022;28(7):804-10. doi:10.18553/jmcp.2022.22093 [DOI] [PubMed] [Google Scholar]
- 6.Academy of Managed Care Pharmacy. AMCP Partnership Forum: Digital therapeutics—what are they and where do they fit in pharmacy and medical benefits? J Manag Care Spec Pharm. 2020;26(5):674-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digital Therapeutics Alliance. Digital therapeutics definitions and core principles. Published November 2019. Accessed February 16, 2023. https://dtxalliance.org/wp-content/uploads/2021/01/DTA_DTx-Definition-and-Core-Principles.pdf
- 8.Digital Therapeutics Alliance. Setting the stage for a fit-for-purpose DTx evidentiary standard. Published December 2022. Accessed January 10, 2024. https://dtxalliance.org/wp-content/uploads/2022/12/DTA-Clinical-Evidence-Paper_12.22.pdf
- 9.Fierce Pharma. Advancing access to digital therapeutics. Published December 19, 2022. Accessed January 10, 2024. https://www.fiercepharma.com/sponsored/advancing-access-digital-therapeutics
- 10.IQVIA. Digital health trends 2021. Published July 22, 2021. Accessed January 10, 2024. https://www.iqvia.com/insights/the-iqvia-institute/reports/digital-health-trends-2021
- 11.Goth G. PBM ‘digital formularies’ promote healthier habits with vetted apps. Society for Human Resource Management (SHRM). Published October 12, 2020. Accessed January 10, 2024. https://www.shrm.org/resourcesandtools/hr-topics/benefits/pages/pbm-digital-formularies-promote-healthier-habits-with-vetted-apps.aspx [Google Scholar]
- 12.Pena G. More Blues agree to pay for prescription digital therapeutics. Modern Healthcare. Published November 14, 2022. Accessed January 10, 2024. https://www.modernhealthcare.com/insurance/blue-cross-plans-expand-access-prescription-digital-health-tools [Google Scholar]
- 13.Larson C. Massachusetts’ Medicaid to cover certain prescription digital therapeutics offered by Pear Therapeutics Inc. Published October 24, 2021. Accessed January 10, 2024. https://bhbusiness.com/2021/10/24/massachusetts-medicaid-to-cover-certain-prescription-digital-therapeutics-offered-by-pear-therapeutics-inc/ [Google Scholar]
- 14.Cantrell SA. Centers for Medicare & Medicaid Services’ first biannual 2022 Healthcare Common Procedure Coding system public meeting. Academy of Managed Care Pharmacy. Published June 9, 2022. Accessed January 10, 2024. https://www.amcp.org/policy-advocacy/letters-statements-analysis/amcp-cms-recommendations-prescription-dtx [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. Medicare Program; public meeting for new revisions to the Healthcare Common Procedure Coding System (HCPCS) coding-June 7-10, 2022. Federal Register. Published May 6, 2022. Accessed January 10, 2024. https://www.federalregister.gov/documents/2022/05/06/2022-09780/medicare-program-public-meeting-for-new-revisions-to-the-healthcare-common-procedure-coding-system


