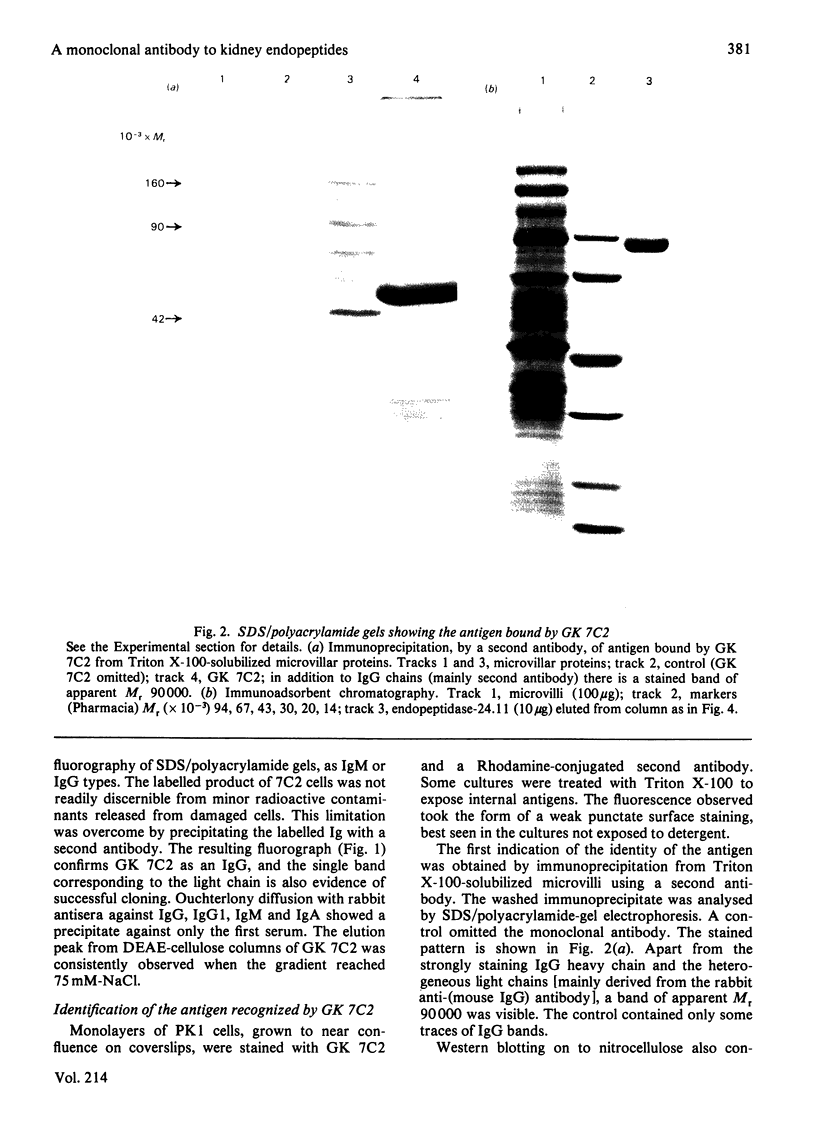
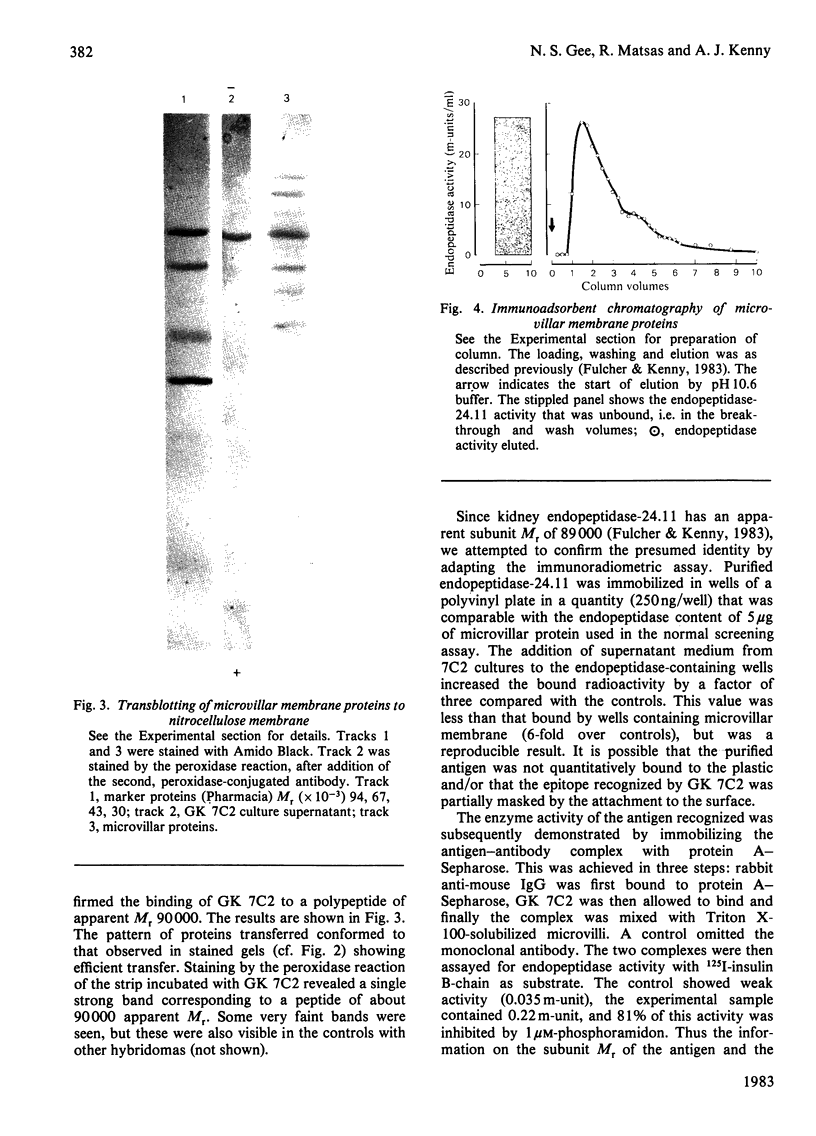
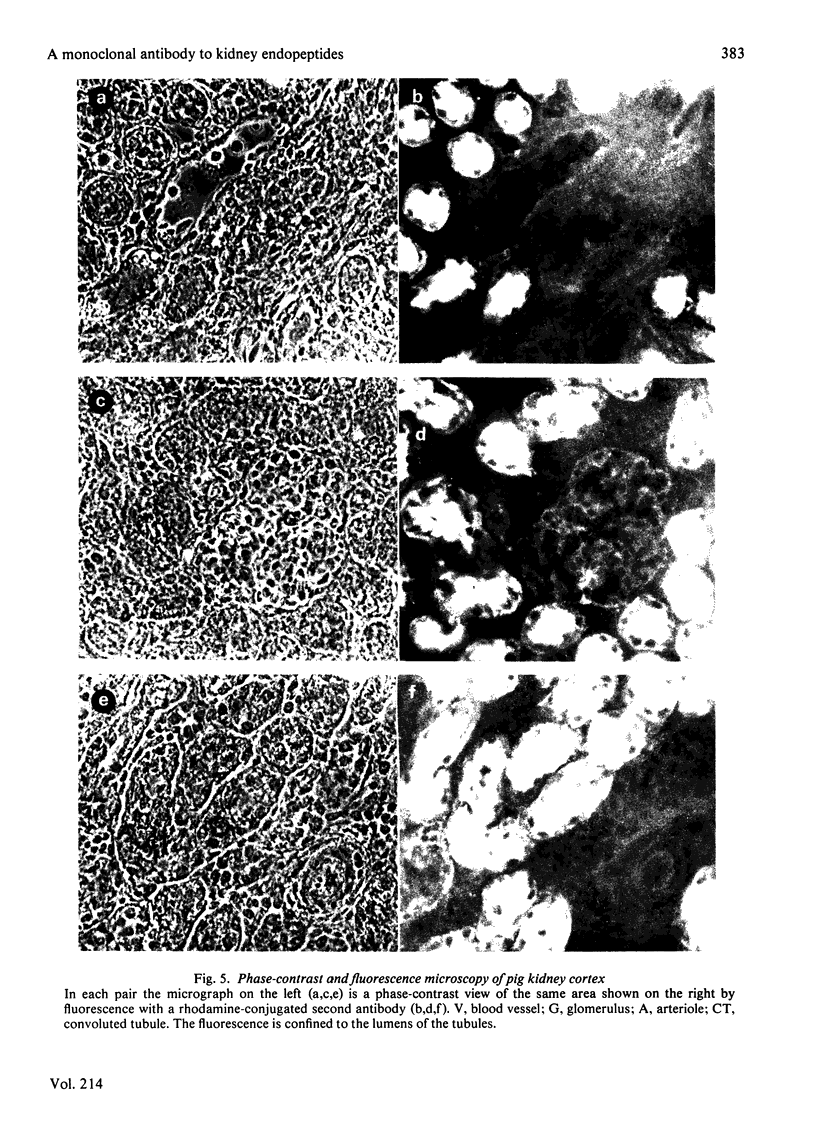
Abstract
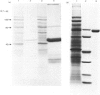
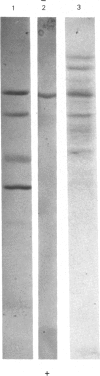
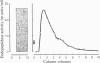
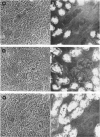
Hybridoma methodology has been used to produce a monoclonal antibody, GK 7C2, that binds specifically to microvillar endopeptidase-24.11 (EC 3.4.24.11). The antibody (an immunoglobulin G) was generated by fusion of mouse plasmacytoma cells with splenocytes from a Balb/c mouse immunized with pig kidney microvillar membranes. The identity of the antigen recognized by GK 7C2 was established by immuno-precipitation from detergent-solubilized pig kidney microvilli. The protein had an apparent Mr of 90 000 and contained endopeptidase activity sensitive to phosphoramidon. The identity was confirmed by immunoadsorbent purification of endopeptidase-24.11 by a column to which GK 7C2 had been attached. The endopeptidase, purified in a yield of 40%, was electrophoretically homogeneous and of specific activity comparable with that purified by other means. Fluorescence microscopy established that GK 7C2 bound specifically to the luminal membranes of kidney tubules and the intestinal mucosa. Thus endopeptidase-24.11 is located in the brush-border membranes of both cell types.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Wilk S., Orlowski M. Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):206–214. doi: 10.1016/0006-291x(81)91508-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Tamerius J. D., Hellström I. Indirect 125I-labeled protein A assay for monoclonal antibodies to cell surface antigens. J Immunol Methods. 1979;31(3-4):201–209. doi: 10.1016/0022-1759(79)90132-7. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Vyas J. P., Kenny A. J. A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J. 1980 Nov 1;191(2):645–648. doi: 10.1042/bj1910645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J. Endopeptidases in the brush border of the kidney proximal tubule. Ciba Found Symp. 1977;(50):209–215. doi: 10.1002/9780470720318.ch12. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S. Microvillar endopeptidase, an enzyme with special topological features and a wide distribution. Ciba Found Symp. 1983;95:12–33. doi: 10.1002/9780470720769.ch3. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schwartz J. C. Properties of "enkephalinase" from rat kidney: comparison of dipeptidyl-carboxypeptidase and endopeptidase activities. Biochem Biophys Res Commun. 1982 May 31;106(2):276–285. doi: 10.1016/0006-291x(82)91106-8. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Purification and specificity of a membrane-bound metalloendopeptidase from bovine pituitaries. Biochemistry. 1981 Aug 18;20(17):4942–4950. doi: 10.1021/bi00520a021. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Polyacrylamide-protein immunoadsorbents prepared with glutaraldehyde. FEBS Lett. 1972 Jun 1;23(1):24–28. doi: 10.1016/0014-5793(72)80274-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]