Abstract
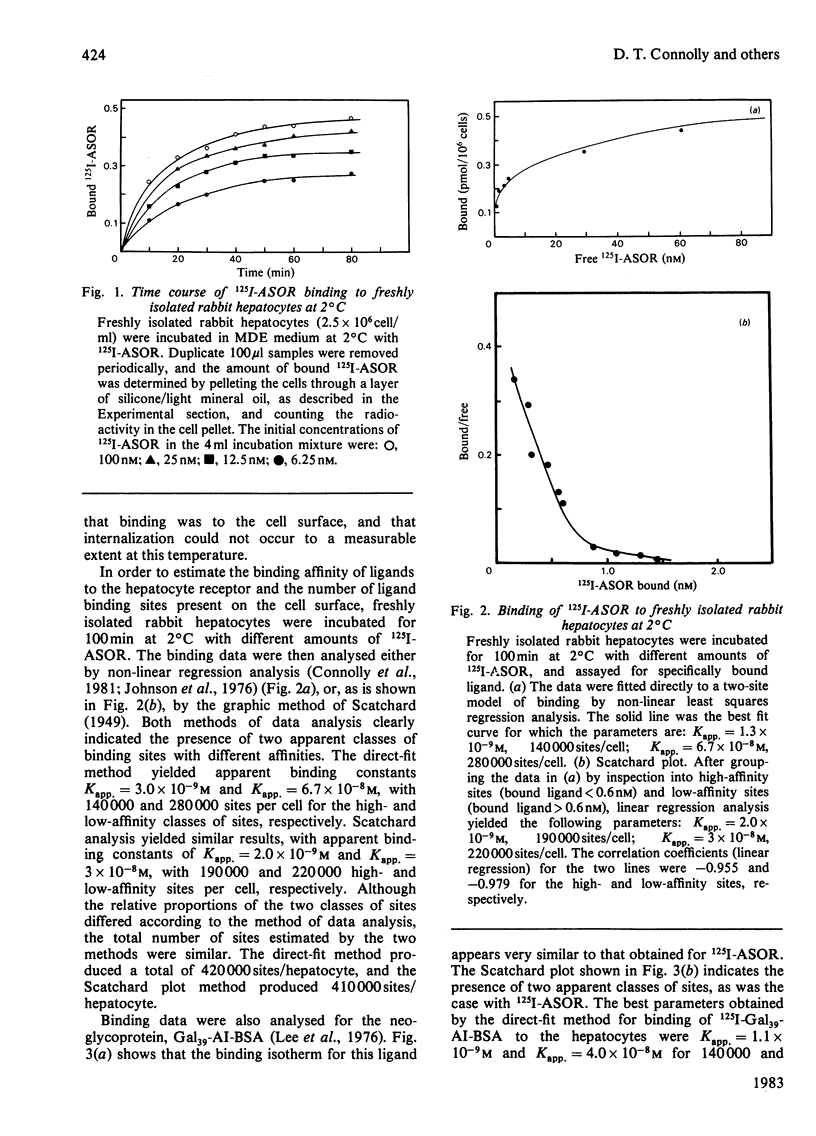
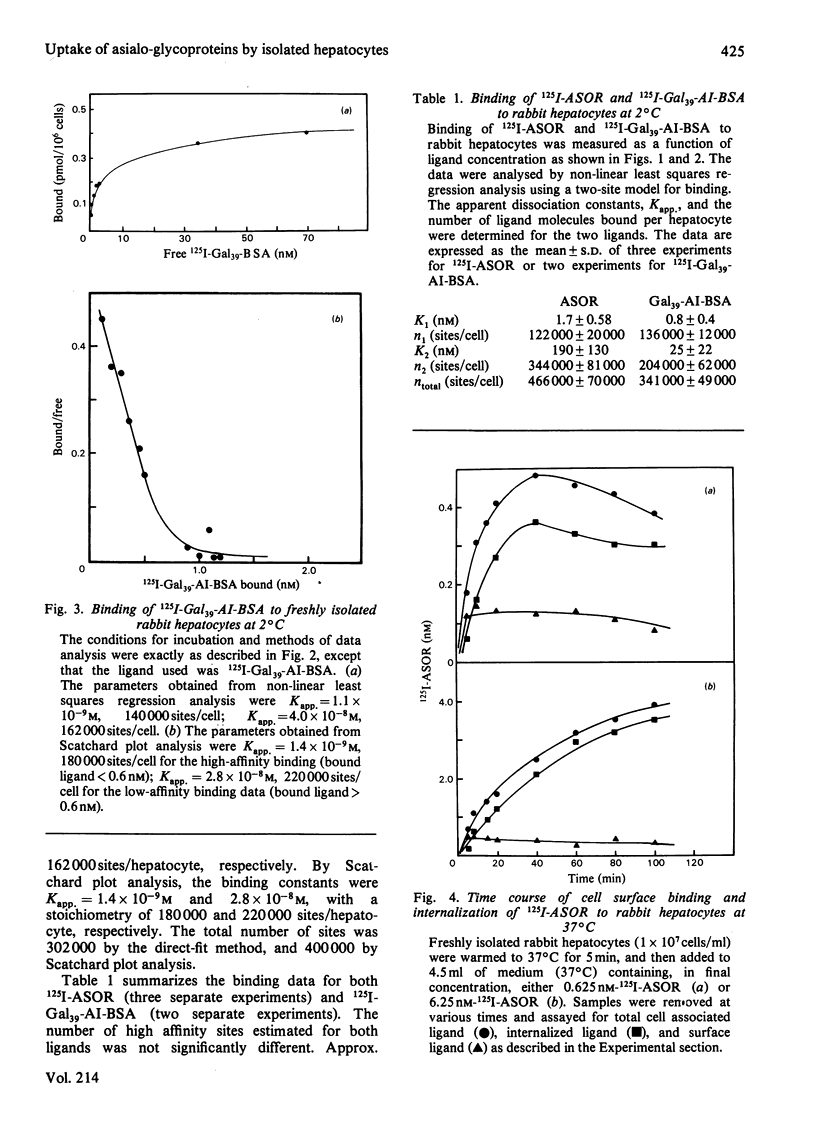
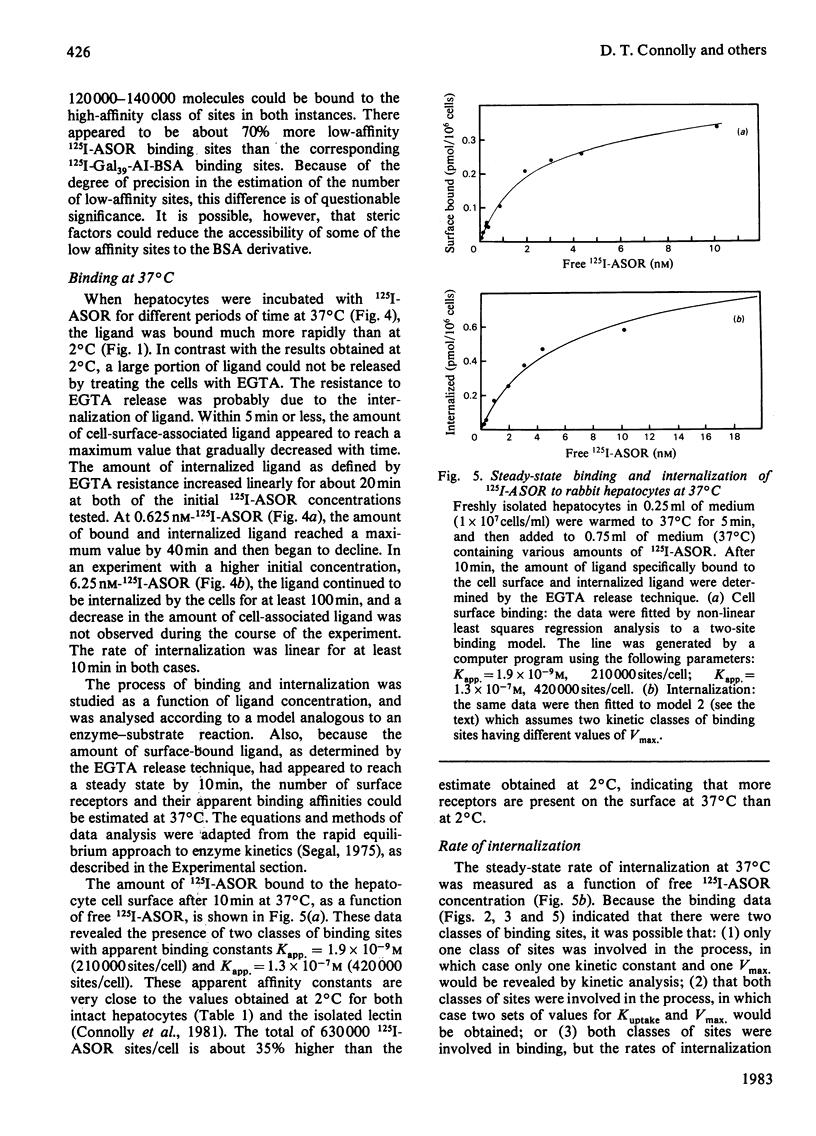
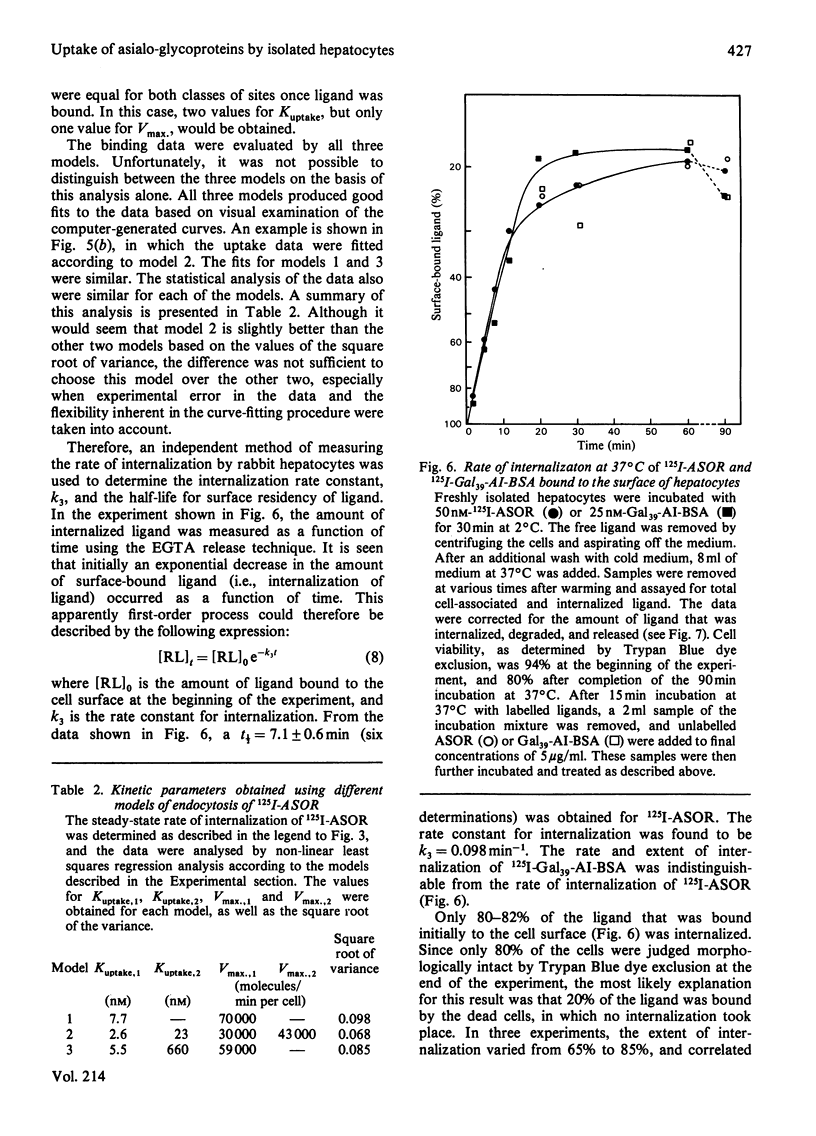
Rabbit hepatocytes were isolated by a collagenase perfusion technique, and used to study the binding and endocytosis of the glycoprotein, asialo-orosomucoid, and the neoglycoprotein, Gal39-bovine serum albumin. Both of these proteins contain exposed galactosyl residues, and were avidly bound by the lectin on the hepatic parenchymal cell surface. Steady state and kinetic experiments performed at 2 degrees C and at 37 degrees C revealed the presence of two apparent classes of binding sites totalling 4.7 X 10(5) sites/cell at 2 degrees C, and 6.3 X 10(5) sites/cell at 37 degrees C. At 37 degrees C, both classes of sites participated in internalization of bound ligand. The cells were capable of internalizing about 60 000 molecules/min per cell. The process appeared to be first-order, with a rate constant k = 0.098 min-1 and t1/2 = 7.1 +/- 0.6 min. Binding could be inhibited by galactose-containing compounds, EGTA, and by anti-(hepatic lectin) immunoglobulin G. The inhibition by antibody appeared to be reversible upon removal of antibody-containing medium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980 Nov;22(2 Pt 2):611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- Bridges K., Harford J., Ashwell G., Klausner R. D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. T., Townsend R. R., Kawaguchi K., Bell W. R., Lee Y. C. Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem. 1982 Jan 25;257(2):939–945. [PubMed] [Google Scholar]
- Dunn W. A., LaBadie J. H., Aronson N. N., Jr Inhibition of 125I-asialofetuin catabolism by leupeptin in the perfused rat liver and in vivo. J Biol Chem. 1979 May 25;254(10):4191–4196. [PubMed] [Google Scholar]
- Johnson M. L., Halvorson H. R., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: analysis of linkage functions for constituent energy terms. Biochemistry. 1976 Nov 30;15(24):5363–5371. doi: 10.1021/bi00669a024. [DOI] [PubMed] [Google Scholar]
- Lee R. T. Binding site of the rabbit liver lectin specific for galactose/N-acetylgalactosamine. Biochemistry. 1982 Mar 2;21(5):1045–1050. doi: 10.1021/bi00534a034. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Myers R. W., Lee Y. C. Further studies on the binding characteristics of rabbit liver galactose/N-acetylgalactosamine-specific lectin. Biochemistry. 1982 Nov 23;21(24):6292–6298. doi: 10.1021/bi00267a039. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Stowell C. P., Krantz M. J. 2-Imino-2-methoxyethyl 1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976 Sep 7;15(18):3956–3963. doi: 10.1021/bi00663a008. [DOI] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Chindemi P. A., Debanne M. T., Hatton M. W. Dual nature of the hepatic lectin pathway for human asialotransferrin type 3 in the rat. J Biol Chem. 1982 May 25;257(10):5431–5436. [PubMed] [Google Scholar]
- Schnaar R. L., Weigel P. H., Kuhlenschmidt M. S., Lee Y. C., Roseman S. Adhesion of chicken hepatocytes to polyacrylamide gels derivatized with N-acetylglucosamine. J Biol Chem. 1978 Nov 10;253(21):7940–7951. [PubMed] [Google Scholar]
- Schwartz A. L., Rup D., Lodish H. F. Difficulties in the quantification of asialoglycoprotein receptors on the rat hepatocyte. J Biol Chem. 1980 Oct 10;255(19):9033–9036. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Steer C. J., Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. J Biol Chem. 1980 Apr 10;255(7):3008–3013. [PubMed] [Google Scholar]
- Stockert R. J., Gärtner U., Morell A. G., Wolkoff A. W. Effects of receptor-specific antibody on the uptake of desialylated glycoproteins in the isolated perfused rat liver. J Biol Chem. 1980 May 10;255(9):3830–3831. [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Weigel P. H. Characterization of the asialoglycoprotein receptor on isolated rat hepatocytes. J Biol Chem. 1980 Jul 10;255(13):6111–6120. [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Endocytosis and degradation mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. J Biol Chem. 1982 Feb 10;257(3):1201–1207. [PubMed] [Google Scholar]


