Abstract
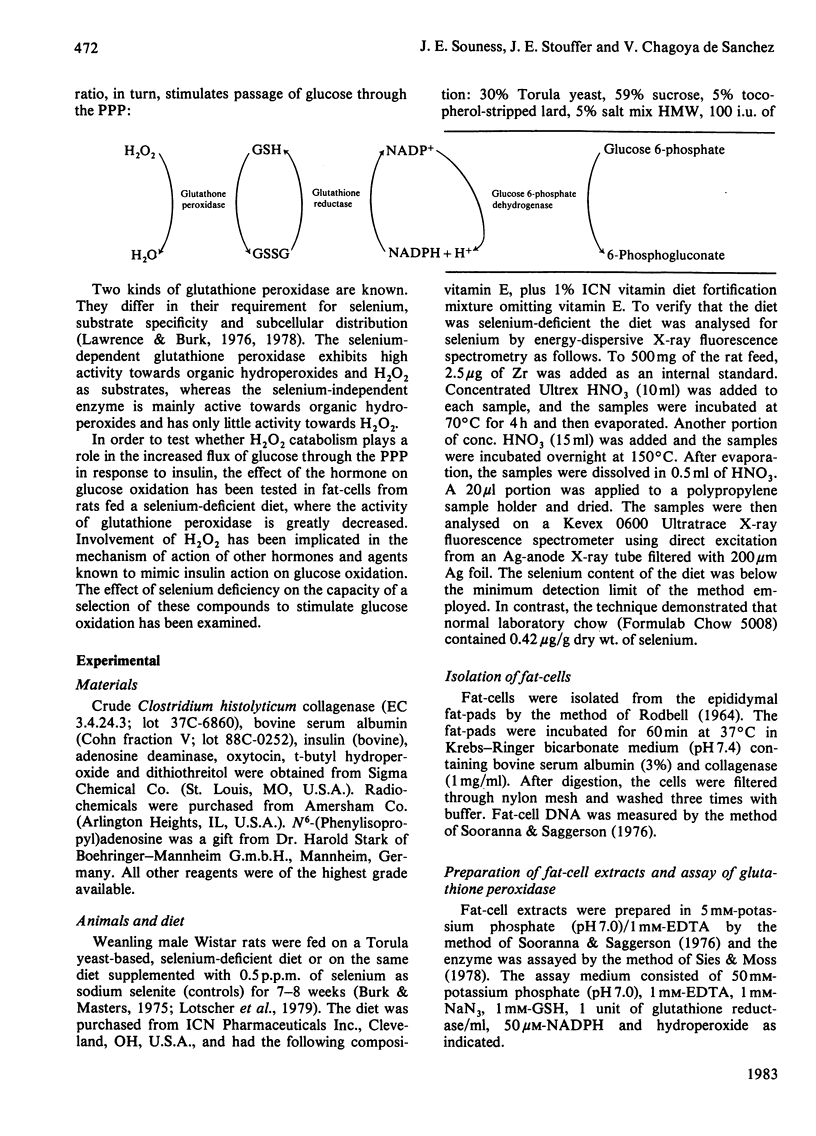
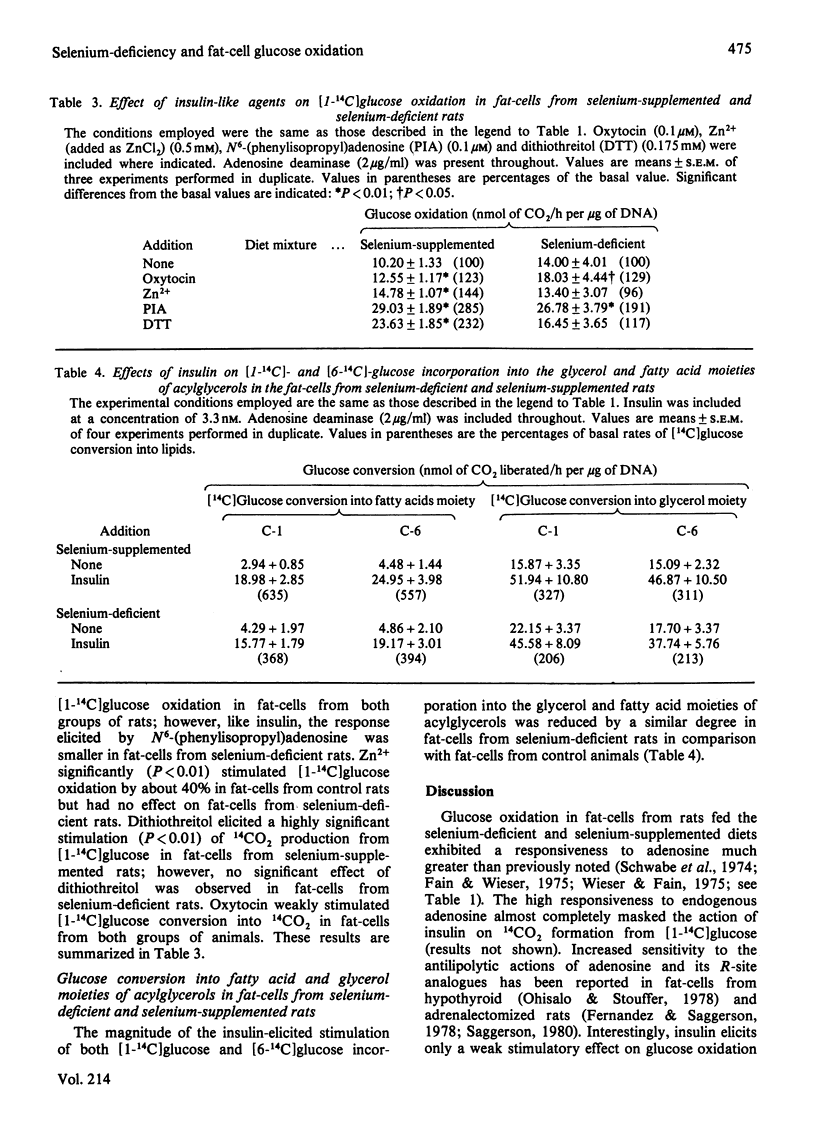
When rats are fed a selenium-deficient diet, the glutathione peroxidase activity of epididymal fat-cells decreases to 5-9% of that of control rats fed the same diet supplemented with 0.5 p.p.m. of selenium as sodium selenite. [1-14C]Glucose oxidation in fat-cells from rats fed a selenium-deficient diet is unresponsive to the action of t-butyl hydroperoxide, which stimulates 14CO2 formation from [1-14C]glucose 4-fold in control rats. Insulin enhances [1-14C]glucose oxidation and incorporation into lipids in fat-cells from both groups of rats; however, the response elicited is reduced in fat-cells prepared from selenium-deficient animals. The 'C-1/C-6 ratio' (ratio of glucose C-1 to glucose C-6 oxidized) is enhanced by insulin to a similar degree in fat-cells from both groups of animals. The stimulatory action of Zn2+ and dithiothreitol on [1-14C]glucose oxidation observed in fat-cells from selenium-supplemented rats is greatly reduced in fat-cells from selenium-deficient rats. [1-14C]Glucose oxidation in fat-cells from both groups of animals is highly sensitive to the stimulatory action of adenosine. It is concluded that the enhanced formation and glutathione-linked destruction of H2O2 plays, at the most, only a minor role in the stimulation of the flux of glucose through the pentose phosphate pathway elicited by insulin, although elimination of glutathione peroxidase activity may influence the action of insulin on glucose oxidation. Production and subsequent destruction of H2O2 may play an important role in the stimulatory action of Zn2+ and dithiothreitol on fat-cell [1-14C]glucose oxidation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burk R. F., Masters B. S. Some effects of selenium deficiency on the hepatic microsomal cytochrome P-450 system in the rat. Arch Biochem Biophys. 1975 Sep;170(1):124–131. doi: 10.1016/0003-9861(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Insulin action and the regulation of hexose transport. Diabetes. 1980 May;29(5):399–409. doi: 10.2337/diab.29.5.399. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Malbon C. C., Kerman K., Gitomer W., Pilch P. F. Effect of thyroid status on insulin action in rat adipocytes and skeletal muscle. J Clin Invest. 1980 Sep;66(3):574–582. doi: 10.1172/JCI109889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobretsov G. E., Borschevskaya T. A., Petrov V. A., Vladimirov Y. A. The increase of phospholipid bilayer rigidity after lipid peroxidation. FEBS Lett. 1977 Dec 1;84(1):125–128. doi: 10.1016/0014-5793(77)81071-5. [DOI] [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- Fernandez B. M., Saggerson E. D. Alterations in response of rat white adipocytes to insulin, noradrenaline, corticotropin and glucagon after adrenalectomy. Correction of these changes by adenosine deaminase. Biochem J. 1978 Jul 15;174(1):111–118. doi: 10.1042/bj1740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried S. K., Lavau M., Pi-Sunyer F. X. Role of fatty acid synthesis in the control of insulin-stimulated glucose utilization by rat adipocytes. J Lipid Res. 1981 Jul;22(5):753–762. [PubMed] [Google Scholar]
- Goko H., Takashima S., Kawamuro A., Matsuoka A. Insulin-like effects of dithiothreitol on isolated rat adipocytes. Biochem J. 1981 Nov 15;200(2):425–428. doi: 10.1042/bj2000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Rivera M., Brand K. Interrelationship and control of glucose metabolism and lipogenesis in isolated fat-cells. Effect of the amount of glucose uptake on the rates of the pentose phosphate cycle and of fatty acid synthesis. Biochem J. 1972 Aug;128(5):1089–1096. doi: 10.1042/bj1281089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978 Feb;108(2):211–215. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. M., Contoreggi C. S. The mechanism of the insulin-like effects of ionic zinc. J Biol Chem. 1982 Apr 25;257(8):4362–4368. [PubMed] [Google Scholar]
- May J. M. The role of glutathione in rat adipocyte pentose phosphate cycle activity. Arch Biochem Biophys. 1981 Mar;207(1):117–127. doi: 10.1016/0003-9861(81)90016-3. [DOI] [PubMed] [Google Scholar]
- May J. M., de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979 Apr 10;254(7):2214–2220. [PubMed] [Google Scholar]
- McDonald J. M., Bruns D. E., Jarett L. The ability of insulin to alter the stable calcium pools of isolated adipocyte subcellular fractions. Biochem Biophys Res Commun. 1976 Jul 12;71(1):114–121. doi: 10.1016/0006-291x(76)90256-4. [DOI] [PubMed] [Google Scholar]
- Muchmore D. B., Little S. A., de Haën C. A dual mechanism of action of ocytocin in rat epididymal fat cells. J Biol Chem. 1981 Jan 10;256(1):365–372. [PubMed] [Google Scholar]
- Muchmore D. B., Little S. A., de Haën C. Counterregulatory control of intracellular hydrogen peroxide production by insulin and lipolytic hormones in isolated rat epididymal fat cells: a role of free fatty acids. Biochemistry. 1982 Aug 3;21(16):3886–3892. doi: 10.1021/bi00259a025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Lane R. H., Lynn W. S. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27(22):2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Lynn W. S. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone's effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977 Nov;184(1):69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Richardson D. K., Czech M. P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am J Physiol. 1978 Feb;234(2):E182–E189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Increased antilipolytic effect of the adenosine 'R-site' agonist N6-(phenylisopropyl)adenosine in adipocytes from adrenalectomized rats. FEBS Lett. 1980 Jun 16;115(1):127–128. doi: 10.1016/0014-5793(80)80741-1. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Schönhöfer P. S., Ebert R. Facilitation by adenosine of the action of insulin on the accumulation of adenosine 3':5'-monophosphate, lipolysis, and glucose oxidation in isolated fat cells. Eur J Biochem. 1974 Aug 1;46(3):537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Moss K. M. A role of mitochondrial glutathione peroxidase in modulating mitochondrial oxidations in liver. Eur J Biochem. 1978 Mar 15;84(2):377–383. doi: 10.1111/j.1432-1033.1978.tb12178.x. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Interactions of insulin and adrenaline with glycerol phosphate acylation processes in fat-cells from rat. FEBS Lett. 1976 Apr 15;64(1):36–39. doi: 10.1016/0014-5793(76)80242-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Beutler E. Useful agents for the study of glutathione metabolism in erythroyctes. Organic hydroperoxides. Biochem J. 1974 May;139(2):289–295. doi: 10.1042/bj1390289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser P. B., Fain J. N. Insulin, prostaglandin E1, PHENYLISOPROPYLADENOSINE AND NICOTINIC ACID AS REGULATORS OF FAT CELL METABOLISM. Endocrinology. 1975 May;96(5):1221–1225. doi: 10.1210/endo-96-5-1221. [DOI] [PubMed] [Google Scholar]



