Abstract
Brainstem cerebral cavernous malformations (CCM) are clinically more aggressive compared to superficial CCMs. Due to their location, resection can be challenging, making stereotactic radiosurgery (SRS) an attractive alternative for symptomatic patient. Brainstem CCM patients (n = 170) were treated with Gamma Knife SRS at 11 radiosurgical centers. Hemorrhagic risk reduction, risk factors of post-SRS hemorrhage, and clinical outcomes were retrospectively analyzed. Most patients had a single (165/170 patients) brainstem CCMs treated; the majority of CCMs (165/181) presented with bleeding. Single-session SRS decreased the risk of repeat hemorrhage in patients with hemorrhagic brainstem CCM (HR: 0.17, p < 0.001) using recurrent multivariate analysis. The annual hemorrhage rate decreased from 14.8 per 100 CCM-years before SRS to 2.3 after treatment. Using univariate Cox-analysis, the probability of a new hemorrhages after SRS was reduced for patient older than 35 years (HR = 0.21, p = 0.002) and increased with a margin dose > 13 Gy (HR = 2.57, p = 0.044). Adverse radiation effect (ARE) occurred in 9 patients (5.3%) and was symptomatic in four (2.4%). At a median follow-up of 3.4 years (Inter-quartile range: 5.4), 13 patients (8.0%) had a worsened clinical status, with the treated CCM being the cause in 5.6% (10) of the patients. Single-session SRS decreased the risk of repeat hemorrhage in patients with hemorrhagic brainstem CCM and conveyed this benefit with a low risk of advrse radiation effects (ARE) and worsening clinical status.
Keywords: Cerebral cavernous malformation, Hemorrhagic, Stereotactic radiosurgery, brainstem
Subject terms: Diseases, Neurology, Physics
Introduction
Brainstem cerebral cavernous malformations (CCMs) represent 18% of CCM’s1. Unlike superficial lesion, brainstem CCMs are more prone to hemorrhage and are thought to be more aggressive in their natural history course2. The risk of repeat hemorrhage is estimated to be as high as 30.8% at 5 years for patients with brainstem CCMs presenting with intracerebral hemorrhage or focal neurological deficit compared to 18.4% in other location3. Microsurgical resection is the preferred option to treat symptomatic or hemorrhagic CCM. However, the risk of surgery in this location is high with a mortality rate of 1.5% and a long-term morbidity of 16%,1 while in other cases some lesions are not amenable to surgery4.
Stereotactic radiosurgery (SRS) can be an alternative treatment option for brainstem CCM patients5–7. However, SRS’s role for such patients remains inadequately defined due to the lack of radiological endpoints and uncertainty about the extent to which CCM hemorrhage is reduced. Our intention with this retrospective, multicentric study is to better define the benefits and risks of treating brainstem CCM patients using Gamma Knife radiosurgery, and better define the role of SRS in the management of brainstem CCM patients8.
Methods
Study population and participant inclusion/exclusion criteria
This study follows the guidelines set forth by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)9. All methods were carried out in accordance with relevant guidelines, regulations and experimental protocols were approved university of Virginia licensing committee. Also, when necessary, the informed consent was obtained from all subjects and/or their legal guardian(s).
Eleven hospital from 9 countries participated through the International Radiosurgery Research Foundation (IRRF). Each center obtained individual IRB approval for sharing de-identified data. Internal consistency from each cohort was checked and any missing data or discrepancies were resolved by request to the centers. The centers contributed data on patients treated with SRS between 1995 and 2021. This is a focused analysis of patients with brainstem CCMs treated with single-session SRS. Patients lacking follow-up after SRS were excluded. Patients with prior resection had residual cavernous malformations that were treated with SRS.
SRS technique
The Leksell Gamma-Knife (Elekta AB) available at each participating with the Leksell G head frame (Elekta AB) center were used to deliver the prescribed radiation dose in a single fraction. Stereotactic, high-resolution brain magnetic resonance imaging (MRI) and/or computed tomography (CT) scanning were used for planning.
Study endpoints and follow-up
The difference between symptomatic intracerebral hemorrhage (ICH) rates prior to and after SRS was the primary endpoint; the occurrence and evolution of neurological deficit, and the development of adverse radiation effects (ARE) were secondary outcomes. Hemorrhage was defined by new/worsening neurological symptoms and evidence of new bleeding on MRI or CT8. Nonhemorrhagic focal neurological deficit (FND) was defined as a new or worsening focal neurological deficit attributable to the anatomic location of the lesion in the absence of radiological hemorrhage8,10. Neurological symptoms at last follow-up were classified as improved, stable, or worsened. A worsening clinical condition was defined as the occurrence of new, permanent symptoms and/or worsening of at least one neurological symptom. Neither epilepsy nor headache were taken into account to define neurological status. ARE were defined as new or worsening perilesional T2 hyperintensity post-SRS or cyst development.
Follow-up was performed according to local protocols, usually every 6 months after SRS for 2 years and annually thereafter. The Zabramski stage was defined per CCM before SRS and at last imaging follow-up11. In the case of neurological deterioration, the patients underwent radiographic studies at the time of the diagnosis of new neurological symptoms to evaluate for associated imaging changes. Volumetric changes were categorized based on pre-specified cutoff points: increase with enlargement by more than 20%, decrease with volumetric reduction exceeding 20% of baseline volume, and stable otherwise12.
Statistical analysis
The statistical analysis was performed using R language in R Studio13,14. No data were imputed. A p-value < 0.05 was considered statistically significant. The analyses were performed per patient or per cavernoma depending on the parameter.
Continuous variables were descriptively summarized using medians with interquartile ranges (IQR), and categorical factors were reported using percentages. Normality of continuous variables was assessed graphically and with the use of the Shapiro-Wilk test.
The reasoning and methodology behind the calculation of annual hemorrhage rates (AHR) and the statistical models chosen for the identification of risk factors for outcome measures has been previously described12. In brief, three pre-SRS AHR were calculated (from birth to SRS with all hemorrhages, from diagnosis to SRS with all hemorrhages, from diagnosis to SRS with first hemorrhage excluded if it led to diagnosis).The post-SRS AHR was calculated by dividing the cumulative number of hemorrhages by the cumulative number of contributed years of follow-up by each lesion. Each lesion contributed risk time from the date of SRS to the date of last follow-up, death or new procedure for CCM. To assess the treatment effect, we compared the pre-SRS AHR with the overall and first 2-year post-SRS AHR using the methodology described by Sahai et al.15.
Univariate and multivariate recurrent event analysis for overall hemorrhage was performed with the Prentice, William and Peterson Gap-Time model (PWP-GT), using the third method for AHR calculation6. Statistically significant factors and clinically relevant ones with a p-value less than 0.20 were included in the multivariate analysis. A Cox regression model was performed to identify risk factors associated with post-SRS hemorrhage16, and Kaplan-Meier curves for first hemorrhage after SRS were plotted. Logistic regression was performed to evaluate risk factors of ARE. Optimal cut-off points for continuous variable were calculated with the Youden index.
Results
Demographics
The study included 170 patients [female: 100 (58.8%)] with a median age of 37.3 (IQR: 27.5) years at diagnosis. One patient (0.6%) was diagnosed with a CCM2 mutation. The initial presentation was hemorrhage in 132 patients (77.6%), FND in 28 (16.5%), and seizure in 3 (1.8%). Seven patients (4.1%) were asymptomatic at the time of diagnosis.
Prior to SRS, 85 (50.0%) patients had a cranial nerve deficit, 50 (29.4%) a motor deficit, and 49 (28.8%) a sensory deficit. Table 1.
Table 1.
Demographic characteristics of the 170 included patients.
| Demographics | Number (%) |
|---|---|
| Age at diagnosis (years), median (IQR) | 37.3 (27.5) |
| Age at SRS (years), median (IQR) | 40.8 (28.2) |
| Sex | |
| Male | 70 (41.2%) |
| Female | 100 (58.8%) |
| Genetic mutation identified | 1 (0.6%) |
| Initial presentation | |
| Incidental* | 7 (4.1%) |
| Seizure* | 3 (1.8%) |
| Hemorrhage | 132 (77.6%) |
| Focal Neurological deficit* | 28 (16.5%) |
| Clinical symptoms pre-SRS | |
| None | 11 (6.5%) |
| Motor deficit | 50 (29.4%) |
| Sensory deficit | 49 (28.8%) |
| Cerebellar symptom | 15 (8.8%) |
| Cranial nerve deficit | 85 (50.0%) |
| Seizure | 6 (3.5%) |
| Headaches | 16 (9.4%) |
| Others † | 21 (12.4%) |
IQR: Interquartile range, SRS: Stereotactic radiosurgery.
*Not associated with acute or subacute hemorrhage; the patients had a hemorrhage later after diagnosis. †speech disorder, memory loss, unspecified gait trouble, unspecified vertigo.
Among the 170 patients, three were treated for two brainstem CCM, one patient for three CCM, and one patient for 5 CCM. Overall, 181 CCM were treated by SRS. The median time from diagnosis to SRS was 0.9 years (IQR: 4.3). Prior surgical resection occurred in 13 (7.2%) of CCM with a median delay from surgical resection to SRS of 3.2 years (IQR:5.6). A DVA was associated in 26 (14.4%) of the CCM and the CCM was adjacent to an ependymal surface in 42 (23.2%) cases. Table 2.
Table 2.
Clinical, radiological and treatment characteristics of 181 CCM CCM: cerebral cavernous malformation, IQR: interquartile range, SRS: stereotactic radiosurgery.
| Clinical and radiological data per CCM | Number (%) |
|---|---|
| Previous surgery | 13 (7.2%) |
| Location adjacent to ependymal plane | 42 (23.2%) |
| Associated developmental venous anomaly | 26 (14.4%) |
| Number of pre-SRS hemorrhages | |
| 0 | 26 (14.4%) |
| 1 | 103 (56.9%) |
| 2 | 37 (20.4%) |
| 3 | 12 (6.6%) |
| 4 | 1 (0.6%) |
| 5 | 2 (1.1%) |
| Median (IQR) time from diagnosis to SRS, years | 0.9 (4.3) |
| Zabramski Classification | |
| 1 | 49 (27.5%) |
| 2 | 108 (60.7%) |
| 3 | 18 (10.1%) |
| 4 | 3 (1.7%) |
| Unknown | 3 |
| Dosimetric parameter per CCM, median (IQR) | |
| Target volume cm3, median (IQR) | 0.4 (0.9) |
| Margin dose Gy, median (IQR) | 12.0 (2.0) |
| Isocenter, median (IQR) | 4.0 (5.0) |
| Isodose line %, median (IQR) | 50.0 (10.0) |
The CCM were treated with a median margin dose of 12.0 Gy (IQR:2.0) at a 50% (IQR:10) isodose line for a median volume of 0.4 cm3 (IQR:0.9). Table 2.
Prior to SRS, 26 (14.4%) CCM never experiences a defined acute and/or subacute hemorrhage, 103 (56.9%) CCM bled one time, 37 (20.4%) CCM bled two times, 12 (6.6%) CCM bled three times, 1 (0.6%) four times, and 2 (1.1%) five times.
Hemorrhage analysis
Among CCMs with hemorrhage prior to SRS (n = 155), the pre-SRS AHR varied based on the methodology used; 3.6 per 100 CCM-years (follow-up from birth: 6309.9 years; 227 hemorrhages), 42.0 per 100 CCM-years (follow-up from diagnosis: 540.5 years; 227 hemorrhages), and 14.8 per 100 CCM-years (follow-up from diagnosis: 540.5 years; 80 hemorrhages after the exclusion of hemorrhages leading to CCM diagnosis).
The overall post-SRS AHR was 2.3 per 100 CCM-years (follow-up post SRS: 736.8 years; 17 hemorrhages) and specificallyin the first 2 years 2.8 per 100 CCM-years (follow-up post SRS: 288.3years; 9 hemorrhages) and after the two first years 1.8 per 100 CCM-years (follow-up post SRS: 449.1 years; 8 hemorrhages). There was a statistically significant reduction in hemorrhage rate post-SRS (-6.4 per 100 CCM-years, 95% CI:3.87–11.13, p < 0.0001). Using recurrent analysis, SRS was associated with a reduction of hemorrhage risk (HR = 0.17, 95% CI = 0.09–0.34, p < 0.001) in multivariate analysis. Table 3.
Table 3.
Annual hemorrhage rate per 100 CCM-years for patient with hemorrhagic CCM prior to SRS.
| Annual hemorrhage rate per 100 CCM-years | ||||
|---|---|---|---|---|
| Pre-SRS | Post-SRS | |||
| < 2y post-SRS |
≥ 2y post-SRS |
Overall | ||
| Overall cohort (n = 155) | ||||
| From birth | 3.4 | 2.8 | 1.8 | 2.3 |
| From diagnosis with 1st hemorrhage included | 42.0 | |||
| From diagnosis with 1st hemorrhage excluded* | 14.8 | |||
| Single hemorrhage before SRS (n = 103) | ||||
| From birth | 2.53 | 1.6 | 1.4 | 1.5 |
| From diagnosis with 1st hemorrhage included | 46.8 | |||
| From diagnosis with 1st hemorrhage excluded* | 3.6 | |||
| ultiple hemorrhages before SRS (n = 52) | ||||
| From birth | 5.6 | 6.1 | 2.5 | 3.8 |
| From diagnosis with 1st hemorrhage included | 38.7 | |||
| From diagnosis with 1st hemorrhage excluded* | 22.5 | |||
*except when the diagnosis was before the first hemorrhage (n = 21 cases for overall series and single bleed patients).
CCM: Cerebral cavernous malformations, SRS: Stereotactic radiosurgery.
After SRS, among all the CCM (n = 181), new hemorrhages occurred in 19 CCM (10.5%) with 18 (9.9%) CCMs having bled one time and one (0.6%) CCM twice. Three CCM (3/26, 11.5%) without hemorrhage prior SRS, bled after SRS. The median delay from SRS to the first bleeding post-SRS was 3.2 (IQR:6.1) years. In one case, the DVA associated with the CCM was occluded and was considered the reason for the bleeding.
Using univariate Cox-analysis, the probability of a new hemorrhages after SRS was reduced for patient older than 35 years old (HR = 0.21, 95% CI = 0.08–0.56, p = 0.002) and increased with margin doses > 13 Gy (HR = 2.57, 95% CI = 1.03–6.45, p = 0.044. The number of hemorrhages prior to SRS was not associated with a risk modification of new bleeding after SRS. Table 4.
Table 4.
Risk factors associated to hemorrhage.
| Factor | Univariate analysis | Multivariate anlysis | ||
|---|---|---|---|---|
| p | HR (95%CI) | p | HR (95%CI) | |
| Recurrent event analysis using the Prentice Williams and Peterson-Gap time model for risk factors associated with hemorrhage in 155 brainstem CCM | ||||
| Gender female | 0.18 | 1.42 (0.86–2.35) | 0.38 | 1.25 (0.76–2.05) |
| Developemental venous anomaly | 0.35 | 1.32 (0.74–2.35) | ||
| Prior surgery | 0.22 | 1.43 (0.81–2.52) | ||
| SRS | < 0.001 | 0.16 (0.08–0.32) | < 0.001 | 0.17 (0.09–0.34) |
| Cox analysis for factor associated with new hemorrhage after SRS in 180 CCM* | ||||
| Gender female | 0.34 | 1.64 (0.59–4.59) | ||
| Developemental venous anomaly | 0.43 | 1.56 (0.51–4.72) | ||
| Age at SRS > 35 | 0.002 | 0.21 (0.08–0.56) | ||
| Volume > 0.3 cc | 0.36 | 1.61 (0.58–4.50) | ||
| Margin > 13 Gy | 0.044 | 2.57 (1.03–6.45) | ||
| Hemorrhage before SRS | ||||
| 0 | ||||
| 1 hemorrhages before SRS | 0.74 | 0.79 (0.20–3.11) | ||
| ≥ 2 hemorrhages before SRS | 0.35 | 1.87 (0.50–6.98) | ||
CI: Confidence interval, HR: Hazard ratio, Sterotactic radiosurgery.
*No multivariate analysis was performed due to the low number of events.
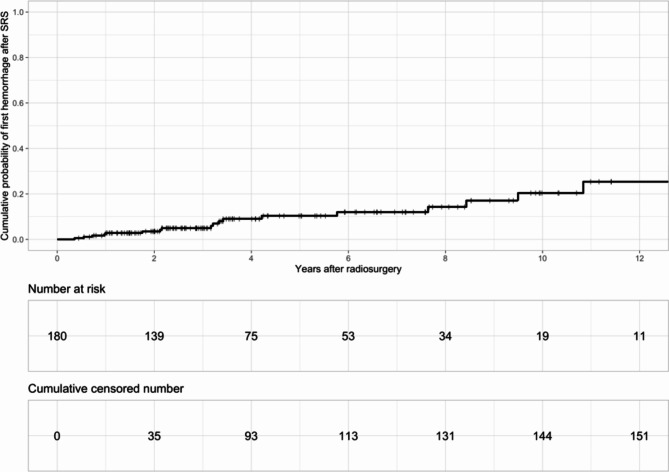
The probability of new hemorrhage after SRS was 4% (95% CI = 0.8–7.2), 11.2% (95% CI = 4.6–17.3) and 20.8% (95% CI = 7.7–32.0) at 2, 5, and 10 years, respectively. Figure 1.
Fig. 1.
Probability of new hemorrhage after SRS.
Imaging outcomes
The median follow-up imaging was 3.4 years (IQR = 5.1) after SRS. At last follow-up, the CCM volume was decreased in 77/179 (43.0%), stable in 99/179 (55.3%) and increased in 3/179 (1.7%).
The pre-SRS Zabramski score was 1 in 49/178 (27.5%), 2 in 108/178 (60.7%), 3 in 18/178 (10.1%) and 4 in 3/ 178 (1.7%). After SRS, the Zabramski score was unchanged in 100/146 (68.5%) CCM. Table 5.
Table 5.
Outcomes after SRS per patient and CCM.
| Outcomes per 181 CCM | |
|---|---|
| Adverse radiation effect | 9 (5.0%) |
| New hemorrhage after SRS | |
| 1 | 18 (9.9%) |
| 2 | 1 (0.6%) |
| CCM volume at last FU | |
| Decreased | 77 (43.0%) |
| Stable | 99 (55.3%) |
| Increased | 3 (1.7%) |
| Unknown | 2 |
| Zabramski post SRS | |
| 1 | 15 (10.2%) |
| 2 | 87 (59.2%) |
| 3 | 34 (23.1%) |
| 4 | 11 (7.5%) |
| Unknown | 34 |
| Zabramski evolution before and after SRS | |
| Unchanged | 100 (68.5%) |
| PreSRS = 1 to post SRS 2 | 12 (8.2%) |
| PreSRS = 1 to post SRS 4 | 5 (3.4%) |
| PreSRS = 1 to post SRS 1 | 5 (3.4%) |
| PreSRS = 2 to post SRS 1 | 18 (12.3%) |
| PreSRS = 2 to post SRS 4 | 4 (2.7%) |
| PreSRS = 3 to post SRS 2 | 1 (0.6%) |
| PreSRS = 4 to post SRS 1 | 1 (0.6%) |
| Unknown | 35 |
| Outcomes for 170 patients | |
| Symptom evolution among patient | |
| Neurologic improvement | 69 (42.3%) |
| Neurologic stability | 81 (49.7%) |
| Neurological worsening | 13 (8.0%) |
| Missing data | 7 |
| New neurological deficit after SRS* | 27 (15.9%) |
| Transient | 15 (8.8%) |
| Permanent | 12 (7.1%) |
| Deficit due to ARE | 2 (2.1%) |
| Deficit due to a new hemorrhage of treated CCM | 11 (6.5%) |
| Deficit due to hemorrhage and ARE | 2 (2.1%) |
| New neurological deficit from other reason | 12 |
| Death | |
*Headaches and seizures were not taken into account for this classification.
ARE: Adverse radiation effect, CCM: cerebral cavernous malformations, FU: Follow-up, SRS: Stereotactic radiosurgery.
Adverse Radiation effects
AREs occurred in 9 CCM and 9 patients (5.3%). ARE was symptomatic in four cases (2.2%). One (0.6%) patient developed a cyst that required stereotactic aspiration and thalamotomy for the associated tremor. T2 hyperintensities occurred in 8 (4.7%) patients. They were managed conservatively in 6 and with a transient course of corticosteroids in two. No factors were associated with the occurrence of ARE using logistic analysis. Table 6.
Table 6.
Logistic regression for the risk factor associated with an adverse radiation effect occurrence in 180CCM.
| Factor | Univariate analysis | |
|---|---|---|
| p | OR (95%CI) | |
| Gender female | 0.36 | 1.89 (0.52–8.84) |
| DVA | 0.22 | 2.38 (0.49–8.93) |
| Age > 35 | 0.98 | 1.01 (0.29-4.00) |
| Volume > 0.3 cc | 0.059 | 7.42 (1.38–138) |
| Margin dose > 13 Gy | 0.078 | 3.02 (0.87–10.9) |
| Hemorrhage pre-SRS | 0.61 | 1.74 (0.31–32.6) |
CI: Confidence Interval, DVA: Developmental venous anomaly, OR: Odd-ratio, SRS: Stereotactic radiosurgery.
Clinical outcomes per patient
New neurological deficits occurred in 27 (15.9%) patients, with 12 (7.1%) being permanent. The cause was new post-SRS hemorrhage in 11 (6.5%) (8 (4.4%) transient deficit and 3 (1.7%) permanent deficit), ARE in 2 (1.2%) (one (0.6%) transient and one (0.6%) permanent deficit). Two (2.1%) patients (with transient deficit) presented with ARE and a new hemorrhage. In three (1.8%) patients (with permanent deficit), the symptoms were linked to the CCM despite the lack of evidence of hemorrhage or ARE on imaging. Four (2.4%) patients developed new neurologic symptom unrelated to the CCM (one (0.6%) permanent and three (1.8%) transient); two (2.1%) patients (with permanent deficit) had hemorrhage from other CCMs. In three (1.8%) patients (with 2 (1.2%) permanent deficit and one (0.6%) transient), the reason for the new deficit is unknown; one (0.6%) patient had a worsening of his clinical presentation (dysesthesia and hypoesthesia in one hand) without clear radiologic explanation.
Overall, with all events considered, 69 patients (42.3%) demonstrated improvement, 81 (49.7%) exhibited stability, and 13 (8.0%) had a worsening of their clinical neurological status at a median follow-up of 3.4 years (IQR: 5.4). Among the 13 patient with worsening of their clinical neurological status, 10 (5.6%) were potentially associated with the treated CCM (new hemorrhage, ARE, unknown). The data of previous symptom evolution were missing for 7 patients. No patient died during the follow-up period.
Additional treatment
Two patients had repeat SRS, and three had an open neurosurgical procedure with a median delay of 7.5 years (IQR = 4.8) from initial SRS.
Discussion
One-hundred and seventy patients with 181 CCM were included in this study. The majority had experienced at least one hemorrhagic event (85.6% of the CCM) prior to treatment. Patients with brainstem CCMS are more likely to present with hemorrhage relative to superficial lesions, as observed in previous report2. There was a statistically significant reduction in AHR following SRS (-6.4 per 100 CCM-years, 95% CI = 3.87–11.13, p < 0.0001) and risk of bleeding (HR: 0.17 (95% CI = 0.09–0.34), p < 0.001) when using recurrent event analysis.
In univariate Cox analysis, patients treated with a margin dose > 13 Gy were more likely to bleed after SRS (HR: 2.57 (95% CI= (1.03–6.45), p = 0.044), a recent observation. This result replicated one previous study which included part of this population with hemorrhagic CCMs irrespective of their location12. Gamma radiation has been shown to promote angiogenesis through multiple pathways via the upregulation of angiogenic factors, including the vascular endothelial growth factor (VEGF), hypoxia inducible factor 1 alpha (HIF-1α), and basic fibroblast growth factor (bFGF)17–19. High radiation doses have been shown to induce overexpression of VEGF, leading to neovascularization and subsequent bleeding17. The risk factor of new hemorrhages after SRS was reduced for patient older than 35 years old (HR: 0.21, p = 0.014). The impact of age on hemorrhage risk is controversial; In a recent meta-analysis, age was not significantly associated with hemorrhage risk20. These differences could be linked to the differences in the population included in each study. Increasing age is also associated with less VEGF expression21. These observations and the role of VEGF need further investigation.
ARE occurred in 5.3% (9) of patients, with only 2.2% of them having associated symptoms. At last follow-up, 10 (5.6%) patients had worse clinical status due to the CCM- or treatment-related complications. No sub-classification of the CCM inside the brainstem was performed. This would be of interest to better define outcome in function of location. In an earlier study, SRS was responsible for a high rate of neurological and ARE complication22. Improvement in technique, such as lowering the prescription dose, avoiding targeting the hemosiderin rim and using high-resolution MRI, might be responsible for the lower complication rate describe in our study and recent reports23–25.
Brainstem CCMs patients should be managed with either active surveillance, SRS or resective surgery. Based on the existing literature, all 3 modalities of treatment are reasonable and should be considered for patients. Active surveillance, although seemingly harmless, carries a high risk of hemorrhage; recent evidence shows a 23% risk of hemorrhage in the natural course of brainstem CCMs26. Microsurgical resection, even in experienced hands, carries moderate to severe risk of periprocedural morbidity and mortality, along with a probability of incomplete resection27,28. The current guidelines recommended SRS after a second hemorrhage, in case of surgically inaccessible lesion29. As repeat hemorrhage is associated with higher risk of neurological deficit compared to a single event, SRS could potentially be offered after a first hemorrhage for inaccessible lesion30. Further works to define which patients have higher risk of rebleeding could help to improve outcomes in this pathology31.
Limitations
Even though the multi-centric design can partially mitigate the effect of individual center biases, its retrospective nature makes it subject to selection bias and institutional treatment practices. Patients had their images with various MRI sequences and/or CT scans over a long time. This could have introduced bias in the evaluation of hemorrhage and ARE. Due to the natural history of CCM in regards with hemorrhage, this study may not be adequately powered to provide long-term patient outcomes. However, our scrutiny would have mitigated its effect. The low number of patients limited the analysis of risk factors associated with new hemorrhage after SRS or ARE. Treatments occurred over a large period of time, differences in treatment planning (MRI or CT based, MRI sequences used, SRS technology, exclusion of hemosiderin rim) could have occurred and cannot be taken into account in this study.
Conclusion
Single-session SRS appreciably decreases the risk of repeat hemorrhage in patients with hemorrhagic brainstem CCM. Prescription doses ≤ 13 Gy could reduce SRS-related complications and the risk of repeat hemorrhage. The rate of permanent complication linked to the treated CCM is 5.6% after SRS.
Acknowledgements
Dr Dumot gratefully acknowledges receipt of a grant for mobility from the “Hospices civils de Lyon”, France, from the “Institut Servier”, France, from the “Societe française of Neurochirurgie (SFNC)”, France, from the “Fondation Planiol”, France and from the “Phillip foundation”.
Author contributions
S.D., G.M., J.S. (1,2 and last) planned the study, analyzed the data and wrote the manuscriptAll other authors collected data helped to prepare the manuscript.
Data availability
Data available on requestContact corresponding author: Sam Dayawansa at “samwansa20@gmail.com”.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
First co-author.
References
- 1.Gross, B. A., Batjer, H. H., Awad, I. A., Bendok, B. R. & Du, R. Brainstem cavernous malformations: 1390 surgical cases from the literature. World Neurosurg.80(1–2), 89–93. 10.1016/j.wneu.2012.04.002 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Porter, R. W. et al. Cavernous malformations of the brainstem: Experience with 100 patients. J. Neurosurg.90(1), 50–58. 10.3171/jns.1999.90.1.0050 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Horne, M. A. et al. Clinical course of untreated cerebral cavernous malformations: A meta-analysis of individual patient data. Lancet Neurol.Bold">15(2), 166–173. 10.1016/S1474-4422(15)00303-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia R.M., Ivan M.E., & Lawton, M.T. Brainstem cavernous malformations: Surgical results in 104 patients and a proposed grading system to predict neurological outcomes. Neurosurgery. 76(3), 265–277. 10.1227/NEU.0000000000000602 (2015). discussion 277–278. [DOI] [PubMed]
- 5.Fritschi, J. A., Reulen, H. J., Spetzler, R. F. & Zabramski, J. M. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir. (Wien). 130(1–4), 35–46. 10.1007/BF01405501 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Pandey, P., Westbroek, E., Gooderham, P. & Steinberg, G. Cavernous malformation of Brainstem, Thalamus, and basal ganglia: A series of 176 patients. NEUROSURGERY. 72(4), 573–588. 10.1227/NEU.0b013e318283c9c2 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka, D., Lunsford, L. D., Coffey, R. J., Bissonette, D. J. & Flickinger, J. C. Stereotactic radiosurgery of angiographically occult vascular malformations: indications and preliminary experience. Neurosurgery. 27(6), 892–900. 10.1097/00006123-199012000-00006 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Akers, A., Al-Shahi Salman, R. & Awad, A. Synopsis of guidelines for the Clinical Management of Cerebral cavernous malformations: Consensus recommendations based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery. 80(5), 665. 10.1093/neuros/nyx091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm, E. et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol.61(4), 344–349. 10.1016/j.jclinepi.2007.11.008 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Al-Shahi Salman, R., Berg, M. J., Morrison, L., Awad, I. A. & Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Sci. Advisory Board. Stroke. 39(12), 3222–3230. 10.1161/STROKEAHA.108.515544 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Zabramski, J. M. et al. The natural history of familial cavernous malformations: Results of an ongoing study. J. Neurosurg.80(3), 422–432. 10.3171/jns.1994.80.3.0422 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Dumot, C. et al. Stereotactic radiosurgery for haemorrhagic cerebral cavernous malformation: A multi-institutional, retrospective study. Stroke Vasc Neurol. Published online. doi: (2023). 10.1136/svn-2023-002380 [DOI] [PMC free article] [PubMed]
- 13.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL (2020). https://www.R-project.org/
- 14.RStudio, T. & RStudio RStudio: Integrated Development Environment for R. PBC, Boston, MA URL http://www.rstudio.com/. (2020).
- 15.Sahai, H. & Khurshid, A. Statistics in Epidemiology: Methods, Techniques, and Applications (CRC, 1996).
- 16.Amorim, L. D. A. F. & Cai, J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int. J. Epidemiol.44(1), 324–333. 10.1093/ije/dyu222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell.5(5), 429–441. 10.1016/s1535-6108(04)00115-1 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Park, C. M. et al. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.66(17), 8511–8519. 10.1158/0008-5472.CAN-05-4340 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Hlatky, L., Tsionou, C., Hahnfeldt, P. & Coleman, C. N. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res.54(23), 6083–6086 (1994). [PubMed] [Google Scholar]
- 20.Gross, B. A. & Du, R. Hemorrhage from cerebral cavernous malformations: a systematic pooled analysis. J. Neurosurg.126(4), 1079–1087. 10.3171/2016.3.JNS152419 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 373(6554), eabc8479. 10.1126/science.abc8479 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Pollock, B. E. et al. Stereotactic radiosurgery for cavernous malformations. J. Neurosurg.93(6), 987–991. 10.3171/jns.2000.93.6.0987 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Lunsford, L. D., Niranjan, A., Kano, H., Monaco Iii, E. A. & Flickinger, J. C. Leksell Stereotactic Radiosurgery for cavernous malformations. Prog Neurol. Surg.34, 260–266. 10.1159/000493072 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Nagy, G. et al. Contemporary radiosurgery of cerebral cavernous malformations: Part 1. Treatment outcome for critically located hemorrhagic lesions. J. Neurosurg.1306(6), 1817–1825. 10.3171/2017.5.JNS17776 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Karaaslan, B. et al. Stereotactic radiosurgery for cerebral cavernous malformation: Comparison of hemorrhage rates before and after stereotactic radiosurgery. J. Neurosurg.136(3), 655–661. 10.3171/2021.2.JNS21138 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Xu, X. Y. et al. Nomogram for predicting an individual prospective hemorrhage risk in untreated brainstem cavernous malformations. J. Neurosurg.138(4), 910–921. 10.3171/2022.8.JNS221228 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Hori, T. et al. Long-term outcomes after surgery for brainstem cavernous malformations: Analysis of 46 consecutive cases. J. Neurosurg.138(4), 900–909. 10.3171/2022.7.JNS22314 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Walcott, B. P., Choudhri, O. & Lawton, M. T. Brainstem cavernous malformations: Natural history versus surgical management. J. Clin. Neurosci.32, 164–165. 10.1016/j.jocn.2016.03.021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niranjan, A. & Lunsford, L. D. Stereotactic radiosurgery guidelines for the management of patients with intracranial cavernous malformations. Prog Neurol. Surg.27, 166–175. 10.1159/000341792 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Li, D. et al. Neurological outcomes of untreated brainstem cavernous malformations in a prospective observational cohort and literature review. Stroke Vasc Neurol.6(4), 501–510. 10.1136/svn-2020-000608 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, D. et al. Natural history of brainstem cavernous malformations: Prospective hemorrhage rate and adverse factors in a consecutive prospective cohort. J. Neurosurg.134(3), 917–928. 10.3171/2019.12.JNS192856 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on requestContact corresponding author: Sam Dayawansa at “samwansa20@gmail.com”.