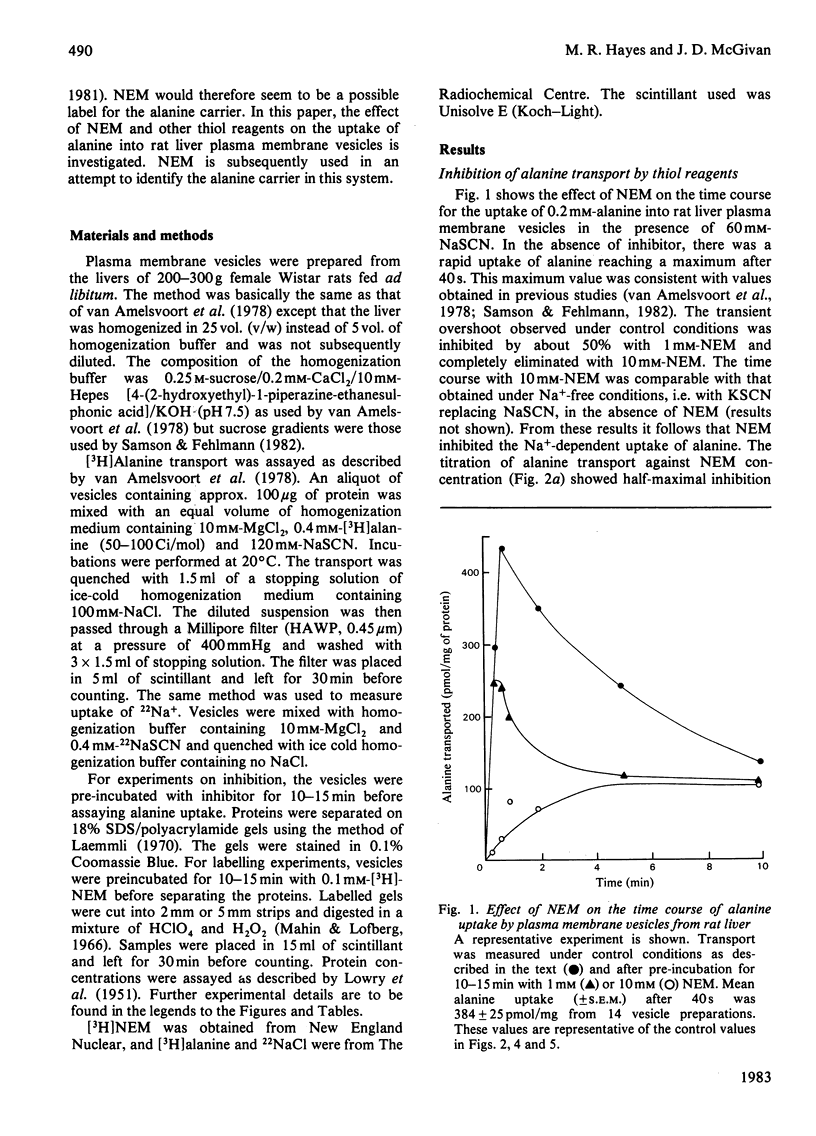
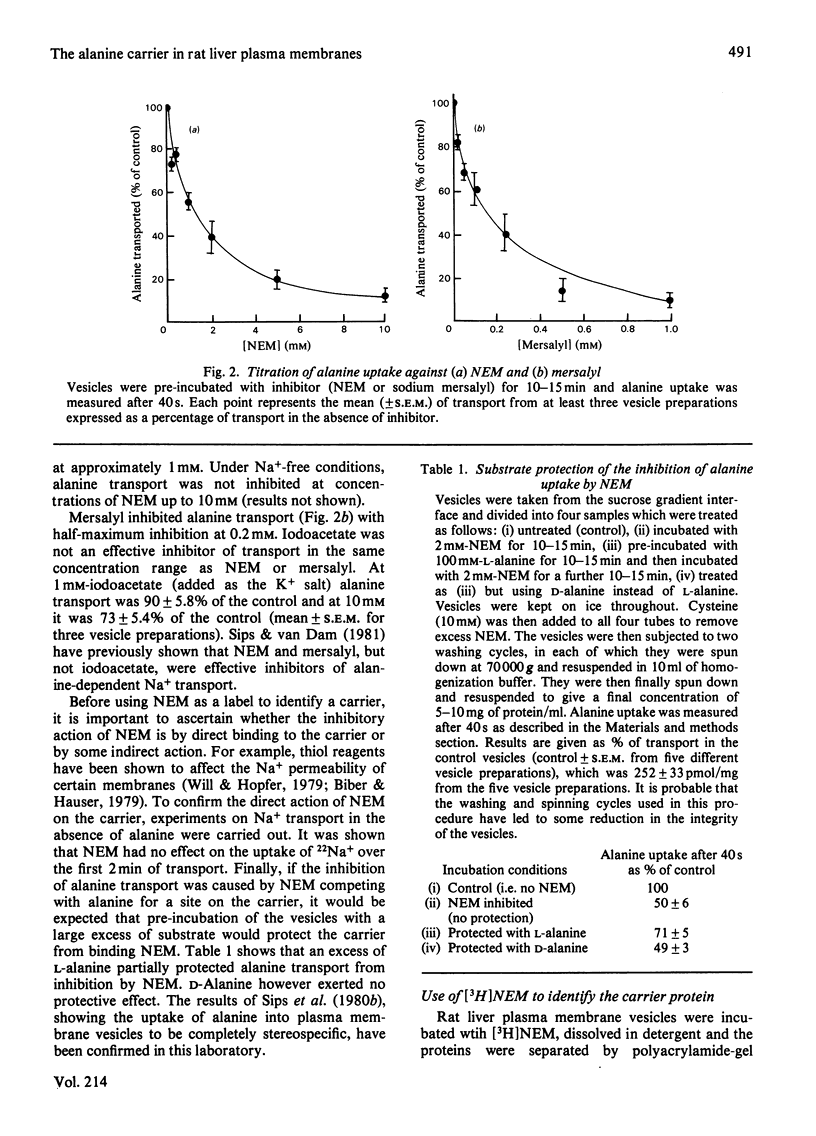
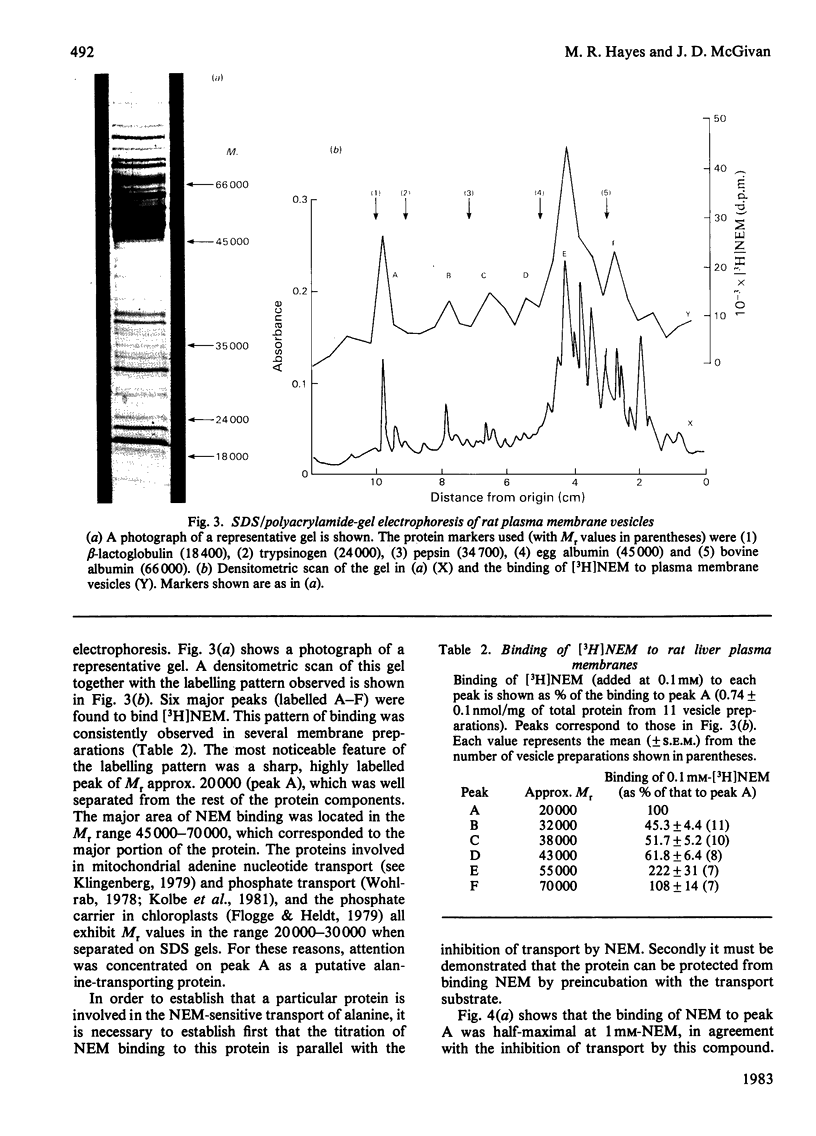
Abstract
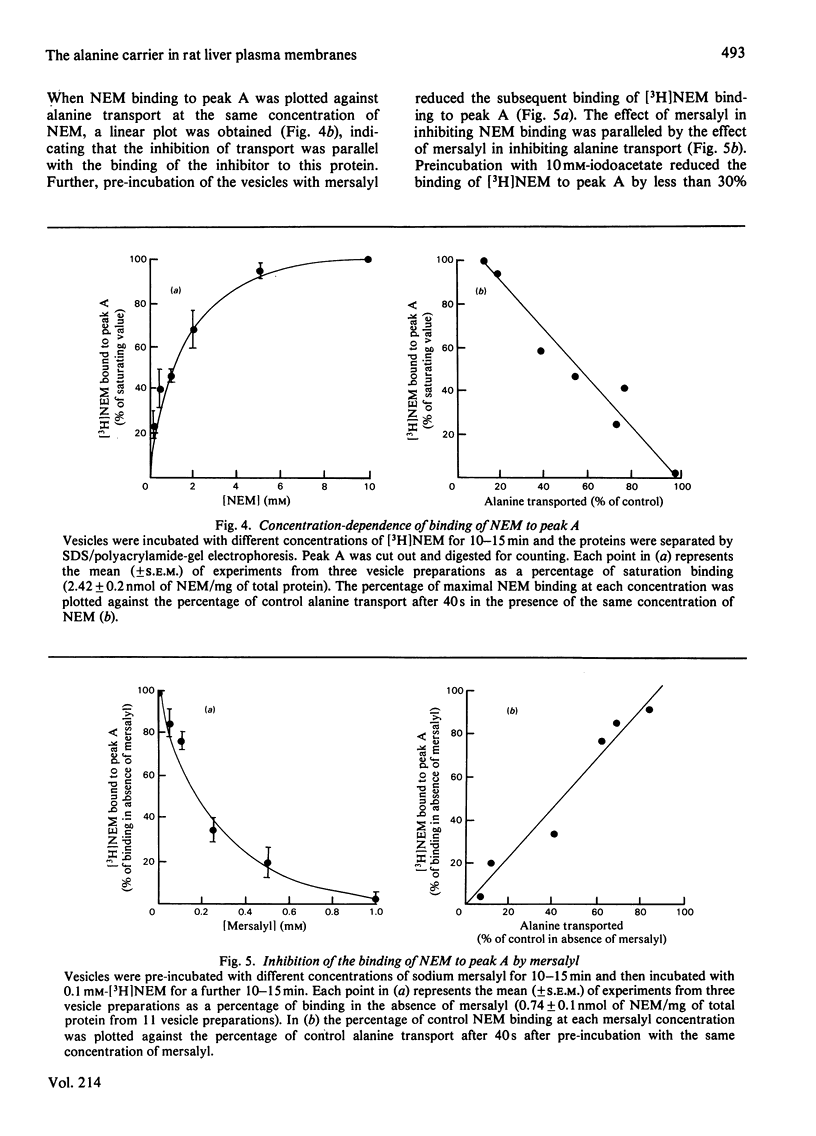
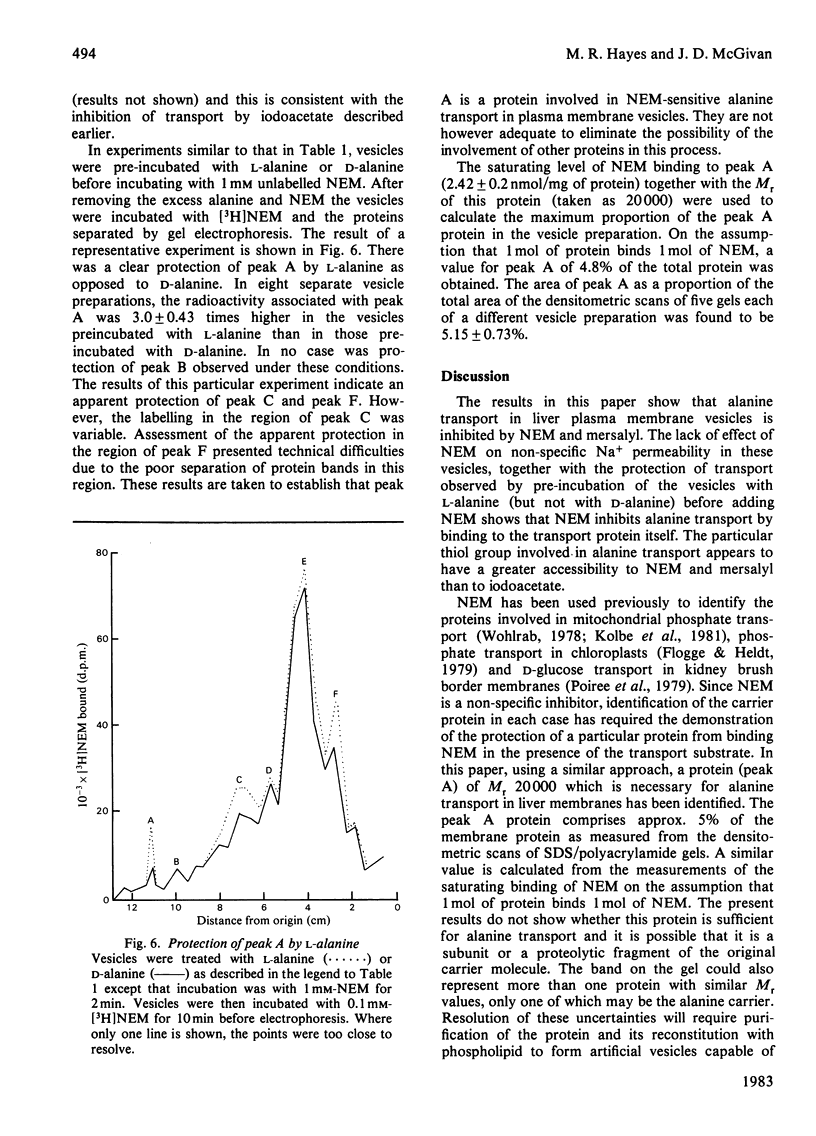
The Na+-dependent uptake of alanine into plasma membrane vesicles from rat liver was inhibited by N-ethylmaleimide (NEM) and by mersalyl. NEM did not inhibit alanine-independent Na+ uptake and the inhibition of alanine transport by NEM was protected by pre-incubation with an excess of substrate. It was therefore concluded that NEM acted by binding to the alanine carrier. A protein of Mr 20 000 was found to bind NEM with a concentration dependence parallel to the NEM inhibition of alanine transport. The inhibition of binding of [3H]NEM to this protein by mersalyl had a concentration dependence similar to that of the inhibition of transport by mersalyl. Preincubation with L-alanine, but not with D-alanine, led to protection of the Mr 20 000 protein from binding NEM. It is concluded that this protein is an essential component of the alanine transport system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber J., Hauser H. The role of SH-groups in the concentrative transport of D-glucose into brush border membrane vesicles. FEBS Lett. 1979 Dec 15;108(2):451–456. doi: 10.1016/0014-5793(79)80586-4. [DOI] [PubMed] [Google Scholar]
- Bradford N. M., McGivan J. D. The transport of alanine and glutamine into isolated rat intestinal epithelial cells. Biochim Biophys Acta. 1982 Jul 14;689(1):55–62. doi: 10.1016/0005-2736(82)90188-2. [DOI] [PubMed] [Google Scholar]
- Cecchini G., Payne G. S., Oxender D. L. Reconstitution of neutral amino acid transport systems from Ehrlich ascites tumor cells. Membr Biochem. 1978;1(3-4):269–278. doi: 10.3109/09687687809063851. [DOI] [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L. Biphasic stimulation of amino acid uptake by glucagon in hepatocytes. Biochem Biophys Res Commun. 1980 Sep 16;96(1):61–68. doi: 10.1016/0006-291x(80)91181-x. [DOI] [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L., Li T. K. Comparative studies of alanine and alpha-aminoisobutyric acid uptake by freshly isolated rat liver cells. J Biol Chem. 1979 Mar 10;254(5):1653–1658. [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L., Li T. K. Direct measurement of active transport systems for alanine in freshly isolated rat liver cells. Biochem Biophys Res Commun. 1977 Jun 6;76(3):751–757. doi: 10.1016/0006-291x(77)91564-9. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Le Cam A., Kitabgi P., Rey J. F., Freychet P. Regulation of amino acid transport in the liver. Emergence of a high affinity transport system in isolated hepatocytes from fasting rats. J Biol Chem. 1979 Jan 25;254(2):401–407. [PubMed] [Google Scholar]
- Hayes M. R., McGivan J. D. Differential effects of starvation on alanine and glutamine transport in isolated rat hepatocytes. Biochem J. 1982 Apr 15;204(1):365–368. doi: 10.1042/bj2040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Isolation of the alanine carrier from the membranes of a thermophilic bacterium and its reconstitution into vesicles capable of transport. J Supramol Struct. 1977;6(1):77–84. doi: 10.1002/jss.400060106. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Johnstone R. M. Partial purification of amino acid transport systems in Ehrlich ascites tumor cell plasma membranes. Membr Biochem. 1982;4(3):189–218. doi: 10.3109/09687688209065431. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N., Handlogten M. E. Cysteine as a system-specific substrate for transport system ASC in rat hepatocytes. Biochem Biophys Res Commun. 1979 May 28;88(2):744–751. doi: 10.1016/0006-291x(79)92110-7. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [PubMed] [Google Scholar]
- Kolbe H. V., Böttrich J., Genchi G., Palmieri F., Kadenbach B. Isolation and reconstitution of the phosphate-transport system from pig heart mitochondria. FEBS Lett. 1981 Feb 23;124(2):265–269. doi: 10.1016/0014-5793(81)80152-4. [DOI] [PubMed] [Google Scholar]
- Kusaka I., Kanai K. Purification and characterization of alanine carrier isolated from H-proteins of Bacillus subtilis. Eur J Biochem. 1978 Feb 1;83(1):307–311. doi: 10.1111/j.1432-1033.1978.tb12095.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Freychet P. Neutral amino acid transport. Characterization of the A and L systems in isolated rat hepatocytes. J Biol Chem. 1977 Jan 10;252(1):148–156. [PubMed] [Google Scholar]
- Lin C. S., Klingenberg M. Characteristics of the isolated purine nucleotide binding protein from brown fat mitochondria. Biochemistry. 1982 Jun 8;21(12):2950–2956. doi: 10.1021/bi00541a023. [DOI] [PubMed] [Google Scholar]
- Poiree J. C., Mengual R., Sudaka P. Identification of a protein component of horse kidney brush border D-glucose transport system. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1387–1392. doi: 10.1016/0006-291x(79)91189-6. [DOI] [PubMed] [Google Scholar]
- Samson M., Fehlmann M. Plasma membrane vesicles from isolated hepatocytes retain the increase of amino acid transport induced by dibutyryl cyclic AMP in intact cells. Biochim Biophys Acta. 1982 Apr 23;687(1):35–41. doi: 10.1016/0005-2736(82)90167-5. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Brown D., Oonk R., Orci L. Orientation of rat-liver plasma membrane vesicles. A biochemical and ultrastructural study. Biochim Biophys Acta. 1982 Nov 22;692(3):447–454. doi: 10.1016/0005-2736(82)90396-0. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Groen A. K., Tager J. M. Plasma-membrane transport of alanine is rate-limiting for its metabolism in rat-liver parenchymal cells. FEBS Lett. 1980 Oct 6;119(2):271–274. doi: 10.1016/0014-5793(80)80269-9. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Van Amelsvoort J. M., Van Dam K. Amino acid transport in plasma-membrane vesicles from rat liver. Characterization of L-alanine transport. Eur J Biochem. 1980 Apr;105(2):217–224. doi: 10.1111/j.1432-1033.1980.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Sips H. J., van Dam K. Amino acid-dependent sodium transport in plasma membrane vesicles from rat liver. J Membr Biol. 1981;62(3):231–237. doi: 10.1007/BF01998168. [DOI] [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]
- Wohlrab H. Mitochondrial phosphate transport: correlation between alkylation of membrane proteins with N-[3H-A1ethylmaleimide and inhibition of transport. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1430–1435. doi: 10.1016/0006-291x(78)91380-3. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort J. M., Sips H. J., van Dam K. Sodium-dependent alanine transport in plasma-membrane vesicles from rat liver. Biochem J. 1978 Sep 15;174(3):1083–1086. doi: 10.1042/bj1741083. [DOI] [PMC free article] [PubMed] [Google Scholar]