Abstract
A 39-year-old man presented with angina and a high-sensitivity troponin-T of 61 ng/L. Initial workup revealed the presence of left ventricular hypertrophy, an anomalous left main coronary artery, and no coronary atherosclerosis. This case demonstrates how multimodality imaging was used to elucidate the primary cause of the patient’s angina.
Key Words: cardiac magnetic resonance, cardiomyopathy, computed tomography, coronary artery anomaly, echocardiography
Graphical Abstract
History of Presentation
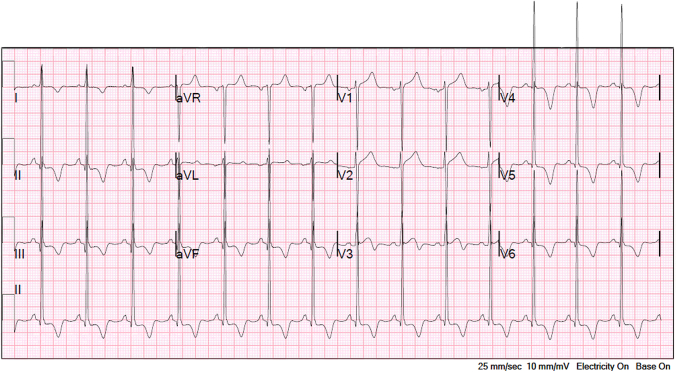
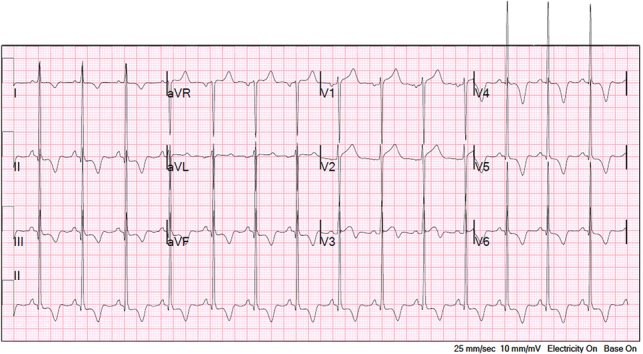
A 39-year-old man presented with symptoms of nonpleuritic, nonpositional, substernal chest pressure provoked by exertion and alleviated with rest. The patient denied a history of syncope or exertional syncope. Examination was notable for a crescendo-decrescendo murmur in the right upper sternal boarder that was accentuated with the Valsalva maneuver. Electrocardiogram was consistent with sinus rhythm, left ventricular hypertrophy, and secondary ST-T–wave changes from hypertrophy (Figure 1). High-sensitivity troponin-T was 57 to 61 ng/L with serial testing.
Take-Home Messages
-
•
Coronary computed tomography angiography can identify anatomic features of anomalous coronary arteries that are associated with increased risk of SCD. In patients without high-risk anatomic features, exercise stress echocardiography or nuclear myocardial perfusion imaging can be used to evaluate for ischemia resulting from the coronary anomaly, which in turn would help guide the need for surgical intervention.
-
•
Patients with symptomatic hypertrophic obstructive cardiomyopathy who have persistent angina despite the use of first-line agents titrated to the maximally tolerated dose should be referred to an HCM Center of Excellence for consideration of advanced therapies.
Figure 1.
Electrocardiogram
Past Medical History
The patient has no history of medical problems and no family history of cardiomyopathy or premature sudden cardiac death (SCD).
Differential Diagnosis
The differential diagnosis includes acute coronary syndrome, myocarditis, ischemia with nonobstructive coronary arteries, symptomatic severe valvular heart disease, hypertrophic cardiomyopathy (HCM), or infiltrative cardiomyopathy.
Investigations
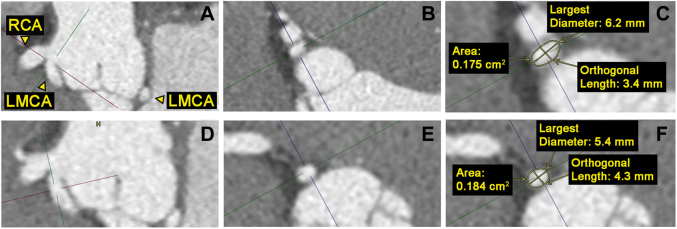
Coronary computed tomography angiography revealed an anomalous left main coronary artery (ALMCA) arising from the right coronary cusp. The ALMCA shares a common ostium with the right coronary artery and takes a retroaortic course to supply a left anterior descending artery and a small-caliber, nondominant circumflex artery. The ALMCA did not have a slit-like orifice,1 proximal narrowing, or elliptic vessel shape in the proximal segment2 (Figure 2). The takeoff angle was approximately 18.6°.3 There was no evidence of a myocardial bridge or coronary atherosclerosis (Video 1, Video 2, Video 3).
Figure 2.
Coronary Computed Tomography Angiography Multiplanar Reconstruction of the ALMCA
(A) Sagittal plane of the ostial orifice. (B) Axial plane of ostial orifice. (C) Measurements of the ALMCA ostial orifice. (D) Sagittal plane of the proximal segment. (E) Axial plane of the proximal segment. (F) Measurements of the ALMCA proximal segment. No evidence of proximal narrowing or a proximal segment elliptic vessel shape based on a height/width ratio of 1.25.2 ALMCA = anomalous left main coronary artery; LMCA = left main coronary artery; RCA = right coronary artery.
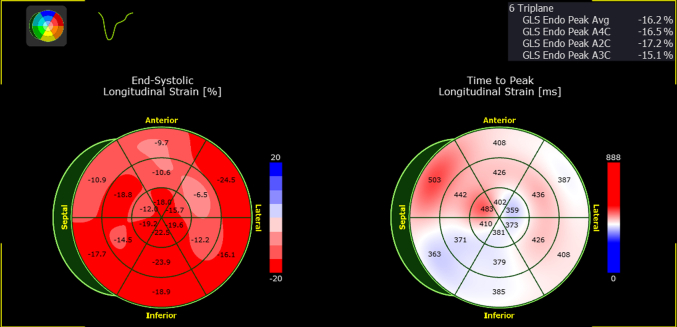
Transthoracic echocardiogram demonstrated increased septal wall thickness of 1.59 cm at end diastole, a left ventricular ejection fraction of 79% by the Simpson biplane method, and a resting left ventricular outflow tract peak gradient of 12 mm Hg. The global longitudinal peak strain was reduced and measured –16.2% (Figure 3). There was grade I diastolic dysfunction, normal right ventricular size and systolic function, and no significant valvular disease.
Figure 3.
Global Longitudinal Peak Strain Polar Map
There are regions of reduced strain associated with areas of increased hypertrophy and/or presence of late gadolinium enhancement on cardiac magnetic resonance.
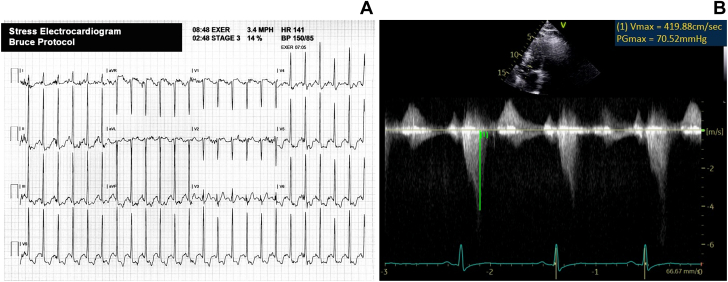
Exercise stress echocardiography demonstrated no regional wall motion abnormalities (Video 4, Video 5, Video 6, Video 7) and a left ventricular outflow tract obstruction with a peak gradient of 71 mm Hg (Figure 4). There was a 3-mm ST -segment depression associated with limiting substernal chest discomfort at a peak workload of 10.3 metabolic equivalents during exercise using the Bruce protocol.
Figure 4.
Exercise Stress Echocardiography Findings
(A) The 12-lead electrocardiogram obtained at peak exercise. (B) Continuous-wave Doppler through the left ventricular outflow tract, which demonstrates a peak gradient of 71 mm Hg obtained immediately following peak exercise.
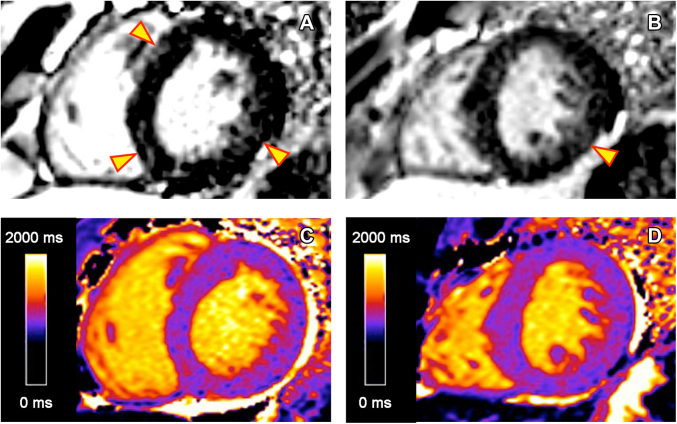
Cardiac magnetic resonance revealed a maximal wall thickness of 1.5 cm in the basal anteroseptal wall with a neutral septal morphology, left ventricular ejection fraction of 75%, no apical aneurysm, no regional wall motion abnormalities, and no systolic anterior motion of the anterior mitral leaflet (Video 8). There was patchy late gadolinium enhancement (LGE) in the midmyocardium with a burden of <15% (Figure 5). The global myocardial T1 time was normal (966-981 ms), but it was elevated in focal regions where LGE was present (1,176 ms in the basal inferoseptal wall).
Figure 5.
Cardiac Magnetic Resonance LGE and Native T1 Maps
Images demonstrate patchy LGE and focal increased native T1 signal in the midmyocardium corresponding to regions of hypertrophy in the basal anteroseptal and inferoseptal walls and the basal to midinferolateral walls, as noted by the arrows. (A) LGE in basal segments. (B) LGE in mid segments. (C) Native T1 map of the basal myocardium. (D) Native T1 map of the midmyocardium. LGE = late gadolinium enhancement.
Additional testing for evaluation of conditions known to cause cardiomyopathy were unremarkable, including C-reactive protein level of <0.3 mg/dL, eosinophil count of 0.04 to 0.25 × 103/μL, ferritin level of 49.6 ng/mL, kappa/lambda light chain ratio of 1.0, no monoclonal protein on serum immunofixation, and an endobronchial ultrasound with lymph node biopsy that demonstrated no granulomas. Genetic testing revealed no pathogenic variants associated with HCM. There were no episodes of ventricular tachycardia during ambulatory electrocardiogram monitoring.
Management
The patient’s angina was believed to be resulting from symptomatic hypertrophic obstructive cardiomyopathy. The patient was started on metoprolol succinate 50 mg once daily, and his dose was titrated to 200 mg once daily. The patient had no indications for placement of an implantable cardioverter-defibrillator for primary prevention of SCD.4 Finally, the patient has been advised to refrain from participating in moderate- to high-intensity activity.
Follow-Up
The patient’s symptoms have improved with the use of a β-blocker, but he continues to have Canadian Cardiovascular Society grade II angina despite use of the maximally tolerated dose. In light of this, the patient has been referred to a HCM Center of Excellence for consideration of a myosin inhibitor.
Discussion
The coexistence of hypertrophic obstructive cardiomyopathy and an anomalous coronary artery is a rare phenomenon, with only a few cases reported in the literature. Both findings are associated with increased risk of SCD. Patients with an ALMCA arising from the right sinus have a Class I indication for surgical intervention if they report ischemic symptoms or have ischemia during diagnostic testing.5 Despite the presence of significant ST-segment depression with exercise stress testing, the absence of anterior, anteroseptal, and anterolateral wall motion abnormalities on stress echocardiography suggest that the ALMCA is not responsible for the patient’s angina or ST-segment changes. We suspect that the patient’s ST-segment changes are resulting from multiple mechanisms, including subendocardial ischemia, coronary microvascular dysfunction, and/or repolarization abnormality, which are all known manifestations of HCM. We have not referred the patient for surgical repair of his ALMCA because the coronary anomaly lacks high-risk anatomic features (no interarterial/intramural course or slit-like orifice) associated with SCD,2 and there is an alternative etiology that explains the patient’s angina and abnormal ST-segment changes with exercise. The patient was referred to an HCM Center of Excellence for consideration of a myosin inhibitor for the management of persistent angina despite the use of metoprolol at the maximally tolerated dose.
Because the patient’s maximal wall thickness just met the threshold for the diagnosis of HCM, we felt that strain and cardiac magnetic resonance were helpful to confirm the etiology of this patient’s cardiomyopathy. Athlete’s heart does not explain this patient’s left ventricular hypertrophy given the presence of LGE, reduced global longitudinal peak strain, and diastolic dysfunction. Sarcoidosis was deemed to be less likely based on the absence of extracardiac sarcoidosis, regional wall motion abnormalities, arrhythmias, and a strain pattern not consistent with published findings in sarcoidosis.6
Conclusions
This case highlights how noninvasive multimodality cardiovascular imaging was used to evaluate a rare diagnostic dilemma, to stratify this patient’s risk for SCD, and to determine the likely etiology of this patient’s symptoms and cardiomyopathy.
Funding Support and Author Disclosures
The views expressed in this paper are those of the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency, the Department of Defense, nor any agencies under the U.S. Government. Publication fees were paid by the San Antonio Uniformed Services Health Education Consortium. Dr O’Gorman owns stock in Abbott Labs, Boston Scientific, Eli Lilly & Co, Bristol Myers & Squibb Co, United Healthcare, Thermo Fisher Scientific, and Intuitive Surgical INC. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Coronary Computed Tomography Angiogram Axial Images
Coronary Computed Tomography Angiogram Sagittal Images
Coronary Computed Tomography Angiogram 3-Dimensional Reconstruction
Exercise Stress Echocardiography Apical 4-Chamber View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Apical 2-Chamber View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Parasternal Long-Axis View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Parasternal Short-Axis View
(Left) Rest and (right) immediate poststress.
Cardiac Magnetic Resonance Cine of the Long Axis
References
- 1.Hirono K., Hata Y., Miyao N., et al. Anomalous origin of the right coronary artery evaluated with multidetector computed tomography and its clinical relevance. J Cardiol. 2016;68(3):196–201. doi: 10.1016/j.jjcc.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Gräni C., Buechel R., Kaufmann P., et al. Multimodality imaging in individuals with anomalous coronary arteries. JACC Cardiovasc Imaging. 2017;10(4):471–481. doi: 10.1016/j.jcmg.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Cheezum M.K., Ghoshhajra B., Bittencourt M.S., et al. Anomalous origin of the coronary artery arising from the opposite sinus: prevalence and outcomes in patients undergoing coronary CTA. Eur Heart J Cardiovasc Imaging. 2017;18:224–235. doi: 10.1093/ehjci/jev323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ommen S., Mital S., Burke M., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76(25):e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano C., Bruno G., Arciniegas Calle M.C., et al. Diagnostic and predictive value of speckle tracking echocardiography in cardiac sarcoidosis. BMC Cardiovasc Disord. 2020;20(1):21. doi: 10.1186/s12872-019-01323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary Computed Tomography Angiogram Axial Images
Coronary Computed Tomography Angiogram Sagittal Images
Coronary Computed Tomography Angiogram 3-Dimensional Reconstruction
Exercise Stress Echocardiography Apical 4-Chamber View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Apical 2-Chamber View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Parasternal Long-Axis View
(Left) Rest and (right) immediate poststress.
Exercise Stress Echocardiography Parasternal Short-Axis View
(Left) Rest and (right) immediate poststress.
Cardiac Magnetic Resonance Cine of the Long Axis