Abstract
Background:
Despite the majority of studies have identified smoking as a risk factor for coronary artery calcification (CAC), some studies have not identified this relationship. Differences on results reached by studies on the association of alcohol consumption with CAC exist. Moreover, studies have almost exclusively investigated the association between smoking and alcohol consumption independently. Whether an interaction effect of alcohol on the association of smoking and CAC exists has hardly been investigated.
Methods:
The data of 2431 adult patients who visited Fuwai Hospital, Chinese Academy of Medical Sciences from September, 2001 to December, 2023 and had Agaston coronary artery calcification score (CACS) reported were utilized. Patients who (1) underwent percutaneous coronary intervention, coronary bypass graft and heart transplantation, or (2) were complicated by acute medical conditions, chronic kidney disease or malignant neoplasms were excluded. Data from 1528 patients were eventually analyzed. Logistic regression was employed to investigate the association of smoking and alcohol consumption with presence of CAC and severe CAC. Interaction effects of alcohol consumption history on the association of current smoking and both presence of and severe CAC were examined.
Results:
Smoking history was significantly associated with presence of CAC and severe CAC. Current alcohol consumption was also significantly associated with presence of CAC and severe CAC. After adjusting for confounders, alcohol consumption history demonstrated an interaction effect on the association of current smoking with both presence of and severe CAC. Using non-alcohol consumers not smoking at the time of the study as reference, current smokers with an alcohol consumption history suffered from an increased risk of presence of CAC and severe CAC.
Conclusions:
Both smoking history and current alcohol consumption were associated with presence of and severe CAC. Alcohol consumption history demonstrated an interaction effect on the association of current smoking with both presence of and severe CAC.
Keywords: smoking, alcohol consumption, coronary artery calcification, coronary artery calcification score, interaction
1. Introduction
Coronary artery calcification (CAC) has been proven to be associated with increased cardiovascular risk. Current studies have demonstrated that the coronary artery calcification score (CACS) plays an important role in the diagnosis of early, subclinical coronary heart disease [1] as well as risk stratification of diabetic [2], hypertensive [3], elderly [4] individuals and smokers [5]. CAC is associated with stent under-expansion, which in turn is associated with in-stent restenosis and thrombosis [6]. Moreover, CAC is associated with the prognosis of certain non-cardiovascular diseases (e.g., malignant neoplasms, hip fracture and chronic obstructive pulmonary diseases) [7].
Significant heterogeneities exist between individuals without CAC (defined as CACS = 0) and those with CAC (defined as CACS 0). In the Rotterdam Elderly Study [8], every group of patients with CACS 0 was found to be associated with a significant increased risk of adverse cardiovascular events as compared with those with CACS = 0. Statins were found to be associated with a reduction of risk for major adverse cardiovascular events in patients with CACS 0 whereas such an association was not observed in those with CACS = 0 [9]. More importantly, McClelland et al. [10] found that at a given time point, after adjusting for confounders, liquor consumption was significantly associated with the severity of CAC in populations with CACS 0; yet no association was found between liquor consumption and CACS 0 in the entire population that encompassed both individuals with (defined as CACS 0) and without (defined as CACS = 0) CAC. This indicated that at a given time point, risk factors associated with the presence of CAC in the entire population and those associated with the severity of CAC in the population with CAC may not necessarily be the same, providing theoretical plausibility of investigating the risk factors for the presence of and severe CAC separately.
Studies to date have generally demonstrated the presence of an association between smoking and CAC, despite the absence of evidence of such associations in certain populations. van der Toorn et al. [11] found that smoking was associated with CAC volume increment in female populations while such an association was not found in male populations. Capisizu et al. [12] found that smoking was significantly associated with severe CAC. Bagyura et al. [13] found that smoking was significantly associated with CACS 100 after adjusting for other risk factors. Kiss et al. [14] found that smoking was an independent risk factor of CACS 300. Molina et al. [15] found that current or former smokers were associated with the presence of CAC as well as higher CACS. Min et al. [16] found smoking to be a risk factor in the transformation into CACS 0 at follow-up in individuals with baseline CACS = 0.
Yet, there are also studies that failed to find an association between smoking and CAC in certain respects. The aforementioned study by Kiss et al. [14] also found no association between smoking and CACS 0 after adjusting for confounders. A multi-center study conducted by Zhang et al. [17] found that smoking history (i.e., former or current smoking) exhibited no significant association in patients receiving hemodialysis or peritoneal dialysis. The aforementioned study by Min et al. [16] also found no association between smoking and CAC progression in individuals with baseline CACS 0. Yang et al. [18] also found no association between smoking and conversion from CACS = 0 to CACS 0 at follow-up.
Despite the presence of studies investigating the association between alcohol consumption and CAC, their results have been inconsistent, with some studies revealing the association between alcohol consumption and CAC in some respects (e.g., the severity of CAC in CACS 0). McClelland et al. [10] found that liquor consumption amount correlated significantly with CACS 0 after adjusting for other risk factors; consumption of two drinks of liquor per day was significantly associated with the severity of CAC in individuals with CACS 0 as well as rapid change (30 units per year) in CAC during follow-up among individuals with baseline CACS 0; total daily alcohol consumption volume correlated positively with annual change in CACS. The Coronary Artery Risk Development in Young Adults (CARDIA) study revealed that liquor consumption was significantly associated with presence of CAC; heavy drinking (14 drinks per week) was an independent risk factor of presence of CAC; the statistically significant increasing trend of likelihood of the presence of CAC as the amount of alcohol consumption increased [19]. Serum calciprotein particle maturation time (T50) is negatively associated with calcification propensity. Eelderink et al. [20] found that serum T50 was negatively associated with alcohol consumption, suggesting the positive association between the latter and calcification propensity.
There are also studies that failed to find an association between alcohol consumption and the presence of CAC. The aforementioned study by McClelland et al. [10] also found no association between alcohol consumption and baseline presence of CAC. This phenomenon was independent of the type of alcohol. In addition, no J-shaped association was observed between alcohol consumption and baseline presence of CAC. Another study found no association between CAC amount and alcohol consumption in asymptomatic individuals at high risk of coronary heart disease [21]. Alcohol consumption as well as the type and amount of alcohol consumed were all found to be uncorrelated with the presence of CAC in a cohort of healthy American military personnels [22]. A U-shaped association between alcohol consumption amount and risk of severe CAC has also been found [23].
As has been mentioned above, different types of drinks containing alcohol demonstrated different associations with CAC. What complicates the association of alcohol consumption and CAC was that substances coexistent in certain drinks containing alcohol may also exert influence upon the calcification process. An animal study conducted by Liou et al. [24] found that xanthohumol, a chemical found in hops, could alleviate vascular calcification mediated by vitamin D and nicotine. Hops, on the other hand, is an indispensable ingredient in producing beer. Therefore, beer consumption could influence the association between smoking and vascular calcification. In a nutshell, the association between alcohol consumption and vascular calcification is complex and may influence the association of other risk factors and vascular calcification. It is therefore necessary to examine the association of current smoking with the presence and severity of CAC separately in patients with and without alcohol consumption history. If there is a difference in risk of an event (e.g., presence of CAC) associated with current smoking between those with and those without alcohol consumption history, then alcohol consumption history can be said to have an interaction effect on the association of current smoking and that event.
In summary, the association between smoking and alcohol consumption with both the presence and severity of CAC has not been fully elucidated. Further investigation into the association of smoking and alcohol consumption and both the presence as well as the severity of CAC is warranted. In particular, examination into the interaction effect of alcohol consumption on the association of current smoking and presence of as well as the severe CAC is warranted.
2. Materials and Methods
2.1 Study Population
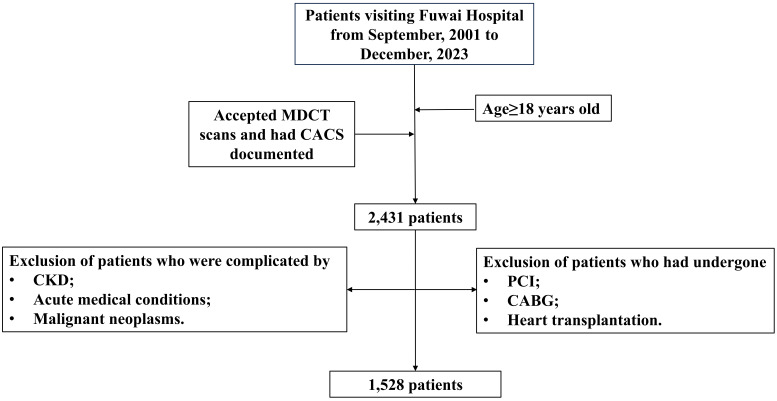
This study screened 2431 patients that visited Fuwai Hospital, Chinese Academy of Medical Sciences from September, 2001 to December, 2023. Inclusion criteria of this study were as follows: (1) Age 18 years old; (2) underwent multidetector row helical computed tomography (MDCT) and had Agaston CACS reported. Exclusion criteria were having undergone percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or heart transplantation, complicated by acute medical conditions or chronic kidney disease (CKD) or malignant neoplasms. 1528 patients were eventually selected in this study. The selection process is diagrammatically outlined in Fig. 1.
Fig. 1.
Schematic representation of the selection of study population. Abbreviations: CABG, coronary artery bypass graft; CACS, coronary artery calcification score; CKD, chronic kidney disease; MDCT, multidetector row helical computed tomography; PCI, percutaneous coronary intervention.
2.2 Data Collection
This study collected patients’ data from the electronic medical record system. Demographic data, history of comorbidities, alcohol consumption and smoking history, medication history, laboratory test results, total Agaston CACS calculated from MDCT were collected. Of note, smoking history was defined as ever (former or current) smoking, current smoking was defined as smoking in the month before the study. Likewise, alcohol consumption history was defined as ever (former or current) alcohol drinking while current alcohol consumption was defined as alcohol drinking in the month before the study. Presence of CAC was defined as total CACS 0 while severe CAC was originally defined as total CACS 1000 and then adjusted to be defined as total CACS 1100.
2.3 Statistical Analyses
Patients were divided into two groups based on the presence of CAC–i.e., patients with CACS = 0 and CACS 0. For continuous variables, normalities were assessed comprehensively by test results from the Shapiro-Wilk test or Kolmogorov-Smirnov test (chosen as appropriate), histogram, probability-probability (P-P) plots and quantile-quantile (Q-Q) plots. Continuous variables that followed normal distributions were reported as mean standard deviation and were tested for inter-group difference via t tests whereas those that did not follow normal distributions were reported as median (1st quartile, 3rd quartile) and were tested for inter-group difference via Wilcoxon sum-of-rank test. For categorical variables, the current study used frequency (proportion) to report their distributions in the two CAC groups while Chi-square tests were employed to test the significances of inter-group differences. Binary logistic regression was employed to analyze the unadjusted association of smoking history, current smoking, alcohol consumption history and current alcohol consumption with the presence and severe CAC as well as the associations of aforementioned smoking and alcohol consumption-related variables with CAC adjusting for confounders. Interaction term between alcohol consumption history and current smoking status was added to analyze the interaction effect of alcohol consumption on the association of smoking and CAC.
This study partitioned patients into three groups according to their CACS (CACS = 0, 0 CACS 1000, CACS 1000). Cumulative odds logistic regression was used for analyzing the association of the association of smoking-related histories like smoking history and current smoking with severe CAC as well as the association of alcohol consumption-related histories like alcohol consumption history and current alcohol consumption. Interaction terms of alcohol consumption history and current smoking status were added in the model to analyze the interaction effect of alcohol consumption. Based upon the results above, patients were rearranged into three groups (CACS = 0, 0 CACS 1100, CACS 1100). Cumulative odds logistic regression was employed to analyze the unadjusted association of smoking history, current smoking status, alcohol consumption history and current alcohol consumption with the presence of and severe CAC as well as the associations of the aforementioned smoking and alcohol consumption-related variables with CAC adjusting for confounders. Interaction terms between alcohol consumption history and current smoking status were again used to analyze the interaction effect of alcohol consumption.
Multiple imputation was employed to tackle missing data. In the imputation stage, fully conditional specification (FCS) logistic regression was used for imputation of discrete variables while FCS predictive mean matching (PMM) was used for imputation of continuous ones. Following statistical analyses on each of the imputed datasets separately, the results generated were pooled via Rubin’s rule if the variable to be pooled followed a t distribution or was demonstrated to have asymptotic normality. If the variable to be pooled followed a Chi-square distribution, the D2 method [25] was employed to pool the results.
Statistical Analysis System (SAS) version 9.4 TS1M5 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. p values 0.05 are considered statistically significant.
3. Results
3.1 Characteristics of the Studied Population
Data of 1528 patients were eventually used for analyses. Table 1 summarizes clinical characteristics of the two CAC groups as well as the statistical test results of inter-group differences. Results indicate that compared to those without CAC, patients with CAC had older age, higher levels of free fatty acids, fasting glucose and glycohemoglobin, declined renal function (measured as lower estimated glomerular filtration rate) and lower apoA1 levels. In addition, the proportion of male, hypertensive, diabetic, former or current smokers, former or current alcohol consumers, aspirin and statin users were higher. No significant differences were found in terms of the triglyceride level, height and weight of the two groups. A decreasing trend was observed in low-density lipoprotein cholesterol (LDL-C) level of CAC patients as compared with those without CAC (p = 0.0572). This phenomenon might be associated with the higher proportion of statin users in patients with CAC.
Table 1.
Clinical characteristics of studied population.
| Clinical characteristics | CACS = 0 (n = 681) | CACS 0 (n = 847) | p value |
| Age (Years) | 51.02 10.06 | 59.67 11.25 | 0.001 |
| Male gender (%) | 455 (66.81%) | 654 (77.21%) | 0.001 |
| Hypertension (%) | 411 (60.37%) | 622 (73.38%) | 0.001 |
| Diabetes (%) | 127 (18.66%) | 307 (36.25%) | 0.001 |
| Smoking history (%) | 273 (40.05%) | 424 (50.11%) | 0.001 |
| Current smoking (%) | 183 (26.85%) | 263 (31.01%) | 0.125 |
| Alcohol consumption history (%) | 195 (28.67%) | 332 (39.21%) | 0.001 |
| Current alcohol consumption (%) | 159 (23.33%) | 246 (29.03%) | 0.026 |
| Aspirin (%) | 117 (17.20%) | 263 (31.03%) | 0.001 |
| Statins (%) | 129 (19.01%) | 325 (38.32%) | 0.001 |
| eGFR (mL/(min × 1.73 m2)) | 95.31 15.95 | 89.51 16.66 | 0.001 |
| apoA1 (g/L) | 1.43 0.29 | 1.39 0.30 | 0.011 |
| Free fatty acid (mmol/L) | 0.48 0.22 | 0.52 0.23 | 0.001 |
| TC (mmol/L) | 4.72 1.09 | 4.53 1.20 | 0.001 |
| LDL-C (mmol/L) | 2.79 0.84 | 2.70 1.00 | 0.057 |
| HDL-C (mmol/L) | 1.26 0.37 | 1.22 0.34 | 0.062 |
| Height (cm) | 169.58 8.70 | 170.03 7.76 | 0.298 |
| Weight (kg) | 75.62 14.33 | 75.64 12.92 | 0.971 |
| Glycohemoglobin (%) | 5.66 (5.40, 6.00) | 5.90 (5.60, 6.60) | 0.001 |
| TG (mmol/L) | 1.48 (1.04, 2.23) | 1.44 (1.03, 2.18) | 0.327 |
| Blood glucose (mmol/L) | 5.28 (4.85, 5.90) | 5.63 (5.08, 6.81) | 0.001 |
Continuous variables that followed normal distributions were reported as mean standard deviation and were tested for inter-group differences via t tests whereas those that did not follow normal distributions were reported as median (1st quartile, 3rd quartile) and were tested for inter-group differences via Wilcoxon sum-of-rank test. For categorical variables, frequency (proportion) were used for reporting while Chi-square tests were employed to test the significances of inter-group differences. To tackle missing data, the results generated on each imputed dataset were pooled via Rubin’s rule if the variable to be pooled followed a t distribution or was demonstrated to have asymptotic normality. If the variable to be pooled followed a Chi-square distribution, the D2 method was employed to pool the results.
Abbreviations: apoA1, apolipoprotein A1; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; CACS, coronary artery calcification score. “Aspirin” and “Statins” refer to usage of the medications.
3.2 Univariable Logistic Regression Analyses of Smoking-Related Histories and Presence of CAC
Table 2 summarizes the unadjusted associations of smoking-related histories and presence of CAC. Results in Table 2 are consistent with those in Table 1, indicating that smoking history was a significant risk factor of presence of CAC, yet the association between current smoking and presence of CAC was insignificant.
Table 2.
Results of univariable logistic regression analyses of smoking and presence of CAC.
| Variable | CACS = 0 (n = 681) | CACS 0 (n = 847) | Regression coefficient | OR (95% CI) | p value |
| Smoking history | 273 (40.05%) | 424 (50.11%) | 0.41 | 1.50 (1.22, 1.85) | 0.001 |
| Current smoking | 183 (26.85%) | 263 (31.01%) | 0.20 | 1.23 (0.97, 1.55) | 0.086 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score.
3.3 Univariable Logistic Regression Analyses of Smoking-Related Histories and Severe CAC
Table 3 summarizes unadjusted associations of smoking-related histories and severe CAC given the definition of severe CAC as CACS 1000. Results indicate that smoking history was significantly associated with severe CAC whereas current smoking was insignificantly associated with severe CAC.
Table 3.
Results of univariable logistic regression analyses of smoking and severe CAC*.
| Variable | CACS 1000 (n = 1459) | CACS 1000 (n = 69) | Regression coefficient | OR (95% CI) | p value |
| Smoking history | 663 (45.47%) | 34 (48.80%) | 0.38 | 1.46 (1.20, 1.79) | 0.000 |
| Current smoking | 429 (29.41%) | 17 (23.93%) | 0.17 | 1.17 (0.94, 1.46) | 0.165 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score.
*Defined as CACS 1000.
3.4 Univariable Logistic Regression Analyses of Alcohol Consumption-Related Histories and Presence of CAC
Table 4 summarizes unadjusted associations of alcohol consumption-related histories and presence of CAC. Results identified both alcohol consumption history and current alcohol consumption as significant risk factors for the presence of CAC.
Table 4.
Results of univariable logistic regression analyses of alcohol consumption and presence of CAC.
| Variable | CACS = 0 (n = 681) | CACS 0 (n = 847) | Regression coefficient | OR (95% CI) | p value |
| Alcohol consumption history | 195 (28.67%) | 332 (39.21%) | 0.47 | 1.60 (1.27, 2.02) | 0.001 |
| Current alcohol consumption | 159 (23.33%) | 246 (29.03%) | 0.30 | 1.34 (1.06, 1.71) | 0.015 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score.
3.5 Univariable Logistic Regression Analyses of Alcohol-Related Histories and Severe CAC
Detailed in Table 5, results of this section revealed that both alcohol consumption history and current alcohol consumption were significantly associated with severe CAC.
Table 5.
Results of univariable logistic regression analyses of alcohol consumption and severe CAC*.
| Variable | CACS 1000 (n = 1459) | CACS 1000 (n = 69) | Regression coefficient | OR (95% CI) | p value |
| Alcohol consumption history | 500 (34.24%) | 28 (40.42%) | 0.45 | 1.57 (1.25, 1.96) | 0.0001 |
| Current alcohol consumption | 387 (26.53%) | 18 (25.67%) | 0.26 | 1.30 (1.03, 1.64) | 0.027 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score. *Defined as CACS 1000.
3.6 Interaction Effect of Alcohol Consumption History on the Association of Current Smoking and Presence of CAC
Table 6 summarizes the association of alcohol consumption history as well as current smoking with the presence of CAC when factoring in the interaction of alcohol consumption history and current smoking. Results indicated that after adjusting for confounders, both alcohol consumption history and current smoking were associated with the presence of CAC. Moreover, the association of current smoking and presence of CAC was influenced by alcohol consumption history. To further facilitate the elaboration of the interaction effect, patients with no alcohol consumption history who were not currently smoking were used as reference while current smokers were grouped into two subpopulations: those with and those without alcohol consumption history. Relevant results are summarized in Table 7. Results indicated that while current smokers with or without alcohol consumption history were both exposed to an increased risk of CAC, current smokers with concurrent alcohol consumption history were associated with an increased risk of CAC.
Table 6.
Multivariable logistic regression analyses of alcohol consumption history and current smoking with presence of CAC.
| Variable | Regression coefficient | p value |
| Alcohol consumption history* | 0.78 | 0.001 |
| Current smoking** | 0.50 | 0.016 |
| Alcohol consumption history×Current smoking*** | –0.63 | 0.032 |
| Male gender | 0.85 | 0.001 |
| Diabetes | 0.57 | 0.001 |
| Age | 0.09 | 0.001 |
| LDL-C | 0.29 | 0.001 |
| Statins | 0.84 | 0.001 |
Abbreviations: CAC, coronary artery calcification; LDL-C, low-density lipoprotein cholesterol. “Statins” refer to usage of the medication.
*Adjusting for current smoking and its interaction with alcohol consumption history, gender, age, diabetes, LDL-C and use of statins.
**Adjusting for alcohol consumption history and its interaction with current smoking, gender, age, diabetes, LDL-C and use of statins.
***Refers to the interaction term of alcohol consumption history and current smoking.
Table 7.
Risk of presence of CAC for different subpopulations (non-smokers with no alcohol consumption history as reference).
| Subpopulation | OR (95% CI) | p value* |
| Smoking (-), Alcohol (-) | Reference | - |
| Smoking (+), Alcohol (-) | 1.66 (1.10, 2.49) | 0.016 |
| Smoking (+), Alcohol (+) | 1.92 (1.32, 2.81) | 0.001 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio. Smoking (-), Alcohol (-): Non-alcohol-consumers who currently do not smoke. Smoking (+), Alcohol (-): Current smokers with no alcohol consumption history. Smoking (+), Alcohol (+): Alcohol consumers who currently smoke.
*Adjusting for age, gender, diabetes, low-density lipoprotein cholesterol and use of statins.
3.7 Interaction Effect of Alcohol Consumption History on the Association of Current Smoking and Severe CAC
Association of alcohol consumption history and current smoking with severe CAC when interaction was factored in are summarized in Table 8. Results indicated that both alcohol consumption history and current smoking were risk factors for severe CAC. In addition, p value of the interaction term was slightly larger than 0.05, suggesting the probable presence of an interaction effect of alcohol consumption history on the association of current smoking with severe CAC when severe CAC was defined as CACS 1000. In other words, similar to the case in the presence of CAC, association of current smoking with severe CAC might also be influenced by alcohol consumption history. For clarity, patients who did not smoke currently and had no alcohol consumption history were used as reference and those either with or without alcohol consumption history were grouped into the two foregoing subpopulations. Odds ratios of the subpopulations for severe CAC are detailed in Table 9. Similar to the situation in the presence of CAC, results of this section indicated while current smokers with or without alcohol consumption history were both exposed to an increased risk of severe CAC, current smokers with concurrent alcohol consumption history were associated with an increased risk of severe CAC.
Table 8.
Multivariable logistic regression analyses of alcohol consumption history and current smoking with severe CAC*.
| Variable | Regression coefficient | p value |
| Alcohol consumption history** | 0.70 | 0.001 |
| Current smoking*** | 0.39 | 0.046 |
| Alcohol consumption history×Current smoking**** | –0.51 | 0.059 |
| Male gender | 0.93 | 0.001 |
| Diabetes | 0.62 | 0.001 |
| Age | 0.09 | 0.001 |
| LDL-C | 0.31 | 0.001 |
| Statins | 0.77 | 0.001 |
Abbreviations: CAC, coronary artery calcification; LDL-C, low-density lipoprotein cholesterol. “Statins” refer to usage of the medication.
*Defined as CACS 1000.
**Adjusting for current smoking and its interaction with alcohol consumption history, gender, age, diabetes, LDL-C and use of statins.
***Adjusting for alcohol consumption history and its interaction with current smoking, gender, age, diabetes, LDL-C and use of statins.
****Refers to the interaction term of alcohol consumption history and current smoking.
Table 9.
Risk of severe CAC* for different subpopulations (non-smokers with no alcohol consumption history as reference).
| Subpopulation | OR (95% CI) | p value** |
| Smoking (-), Alcohol (-) | Reference | - |
| Smoking (+), Alcohol (-) | 1.47 (1.01, 2.15) | 0.046 |
| Smoking (+), Alcohol (+) | 1.78 (1.25, 2.53) | 0.001 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio. Smoking (-), Alcohol (-): Non-alcohol-consumers who currently do not smoke. Smoking (+), Alcohol (-): Current smokers with no alcohol consumption history. Smoking (+), Alcohol (+): Alcohol consumers who currently smoke.
*Defined as CACS 1000.
**Adjusting for age, gender, diabetes, low-density lipoprotein cholesterol and use of statins.
3.8 Univariable Logistic Regression Analyses of Smoking-Related Histories and Severe CAC after Regrouping
In order to further analyze the association of smoking, alcohol consumption and severe CAC while investigating the interaction effect of alcohol consumption history, a regrouping of patients was conducted, i.e., grouping the patients into three groups according to CACS (CACS = 0, 0 CACS 1100, CACS 1100), which served as the basis of the following analyses. Table 10 summarizes results of univariable analyses of associations of smoking-related histories and severe CAC. Results again revealed that smoking history was a risk factor of severe CAC whereas current smoking was not significantly associated with severe CAC.
Table 10.
Results of univariable logistic regression analyses of smoking-related histories and severe CAC*.
| Variable | CACS 1100 (n = 1471) | CACS 1100 (n = 57) | Regression coefficient | OR (95% CI) | p value |
| Smoking history | 668 (45.44%) | 29 (50.30%) | 0.39 | 1.48 (1.21, 1.81) | 0.001 |
| Current smoking | 433 (29.41%) | 13 (22.68%) | 0.16 | 1.17 (0.94, 1.47) | 0.162 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score.
*Defined as CACS 1100.
3.9 Univariable Logistic Regression Analyses of Alcohol Consumption-Related Histories and Severe CAC after Regrouping
Results of univariable analyses of associations of alcohol consumption-related histories and severe CAC are summarized in Table 11. They also revealed that both alcohol consumption history and current alcohol consumption were significantly associated with severe CAC.
Table 11.
Results of univariable logistic regression analyses of alcohol consumption-related histories and severe CAC*.
| Variable | CACS 1100 (n = 1471) | CACS 1100 (n = 57) | Regression coefficient | OR (95% CI) | p value |
| Alcohol consumption history | 505 (34.34%) | 22 (39.00%) | 0.44 | 1.56 (1.25, 2.00) | 0.001 |
| Current alcohol consumption | 391 (26.55%) | 14 (25.02%) | 0.26 | 1.30 (1.03, 1.64) | 0.026 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio; CACS, coronary artery calcification score. *Defined as CACS 1100.
3.10 Interaction Effect of Alcohol Consumption History on the Association of Current Smoking and Severe CAC after Regrouping
Table 12 summarizes the results of the adjusted association of alcohol consumption history and current smoking with severe CAC as well as the interaction effect after regrouping. Results indicate that both alcohol consumption history and current smoking status were significantly associated with severe CAC after adjusting for confounders. A significant interaction effect of alcohol consumption history was observed after regrouping, confirming its presence. Similar to the analyses performed above, this study also chose current non-smokers free of alcohol consumption history as reference and calculated the risks of severe CAC for current smokers with alcohol consumption history and current smokers without alcohol consumption history, which are summarized in Table 13. Results indicate that after adjusting for confounders, current smoking was a significant risk factor in both alcohol consumers and non-alcohol consumers.
Table 12.
Multivariable logistic regression analyses of alcohol consumption history and current smoking with severe CAC* after regrouping.
| Variable | Regression coefficient | p value |
| Alcohol consumption history** | 0.72 | 0.001 |
| Current smoking*** | 0.42 | 0.031 |
| Alcohol consumption history×Current smoking**** | –0.55 | 0.046 |
| Male gender | 0.89 | 0.001 |
| Diabetes | 0.59 | 0.001 |
| Age | 0.09 | 0.001 |
| LDL-C | 0.30 | 0.001 |
| Statins | 0.78 | 0.001 |
Abbreviations: CAC, coronary artery calcification; LDL-C, low-density lipoprotein cholesterol. “Statins” refer to usage of the medication.
*Defined as CACS 1100.
**Adjusting for current smoking and its interaction with alcohol consumption history, gender, age, diabetes, LDL-C and use of statins.
***Adjusting for alcohol consumption history and its interaction with current smoking, gender, age, diabetes, LDL-C and use of statins.
****Refers to the interaction term of alcohol consumption history and current smoking.
Table 13.
Risk of severe CAC* for different subpopulations after regrouping (non-smokers with no alcohol consumption history as reference).
| Subpopulation | OR (95% CI) | p value** |
| Smoking (-), Alcohol (-) | Reference | - |
| Smoking (+), Alcohol (-) | 1.52 (1.04, 2.23) | 0.031 |
| Smoking (+), Alcohol (+) | 1.81 (1.27, 2.58) | 0.001 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; OR, odds ratio. Smoking (-), Alcohol (-): Non-alcohol-consumers who currently do not smoke. Smoking (+), Alcohol (-): Current smokers with no alcohol consumption history. Smoking (+), Alcohol (+): Alcohol consumers who currently smoke.
*Defined as CACS 1100.
**Adjusting for age, gender, diabetes, low-density lipoprotein cholesterol and use of statins.
4. Discussion
CAC refers to calcium deposition on the coronary arterial wall and is an important type of vascular calcification. Vascular calcification can be classified into (atherosclerotic) intimal calcification, medial calcification and genetic calcification [26]. Being a measure quantifying the severity of CAC, CACS can also be used for measuring coronary atherosclerotic burden [27] and overall atherosclerotic burden [28].
Generally, studies to date investigating the association of smoking and CAC have confirmed their associations in multiple respects, despite the attenuation or even lack of presence of such associations in certain populations. Molina et al. [15] conducted a retrospective study on 1162 South Asian individuals in North America. Results pointed out that smoking history (current or former smoking) was a risk factor for CAC. Wetscherek et al. [29] also found smoking history to be a risk factor of CAC in univariate analysis. Moreover, Zhang et al. [30] concluded that smoking history was a risk factor of CAC after adjusting for confounders in a cohort of 4989 asymptomatic individuals who participated in screening for lung cancer at a Chinese cancer hospital. This association was also presented in other cohorts. Prior to Zhang et al. [30], Trab et al. [31] analyzed data of 163 schizophrenics in Denmark and also found smoking history (defined as current or former smoking) a risk factor of presence of CAC after adjusting for confounders. However, Kiss et al. [14] analyzed data of 511 participants of Budakalász Health Survey, a cardiovascular screening program in a central Hungarian town and found that smoking was not significantly associated with CAC after the adjustment of confounders. Yet the concept of “smoking” was not clearly defined in the study by Kiss et al. [14]. In all, it can be concluded from prior studies that smoking history is associated with CAC. Our results are consistent with them.
The association of current smoking status and CAC has also been examined. The Jackson Heart Study conducted by Oshunbade et al. [32] enrolled 4432 black adults free of coronary heart disease and recorded their baseline smoking status as well as their CACS at follow-up. Results indicated that compared with non-smokers, former smokers or current smokers at baseline were exposed to a significantly increased risk of CAC at follow-up. However, the association of current smoking and presence of CAC at the same time point was not investigated in the Jackson Heart Study. Lee et al. [33] found on a cohort of 1914 Korean patients with CKD that compared with never smokers, current smokers were associated with a significantly increased risk of CAC after adjusting for confounders. Multivariable analysis results from this study are different from those of the aforementioned studies, especially that of Lee et al. [33] This is exemplified in several aspects. (1) Study population of our study differs from the study by Lee et al. [33]. While both the current study and the study by Lee et al. [33] are conducted on eastern Asian populations, this study is conducted on patients visiting a hospital primarily for cardiovascular diseases while the population studied by Lee et al. [33] was confined to patients with CKD. (2) Comparisons of the two studies were different. This study compared the risk of presence of CAC of patients who smoked currently against those who did not smoke currently while the comparison conducted by Lee et al. [33] was current smokers vs. never smokers. (3) Last but not least, the confounders which were adjusted for were different. While Lee et al. [33] did not adjust their results for any variable related to alcohol consumption, alcohol consumption history was adjusted in this study.
Researchers have also conducted investigations of the association between smoking and severe CAC. Capisizu et al. [12] conducted a single-center study on 222 individuals. Results indicated smoking history was significantly associated with severe CAC (defined as CACS 400). The aforementioned study conducted by Kiss et al. [14] on 511 Budakalász Health Survey participants also found smoking significantly associated with CACS 300 after adjusting for confounders. But, as has already been pointed out in this article, the concept of “smoking” was not clearly defined [14]. In all, with a much larger sample size than previously conducted studies, this study also found that smoking history was significantly associated with severe CAC, despite different definitions of the disease.
Research on current smoking status and severe CAC is also present. Similar to Kiss et al. [14], Bagyura et al. [13] also investigated data of Budakalász Health Survey and eventually enrolled 280 participants with age 35 years old (for males) or 40 years old (for females) and neither history of cardiovascular events or diseases (including chronic heart failure, angina pectoris, myocardial infarction, PCI, CABG, cardiomyopathy, peripheral vascular diseases and arrythmias) nor inflammatory disorders (defined as use of glucocorticoids and other immunomodulators as well as C-reactive protein 30 mg/L). Univariable analyses revealed that current smoking (defined as at least smoking one cigarette per day at the time of the study) was not associated with severe CAC (defined as CACS 100). However, results of multivariable analyses found that despite this insignificance lingered after adjusting for age, gender, interaction of neutrophil/lymphocyte ratio and tertile of visceral adiposity index as well as body mass index, further adjustment of either cardiometabolic diseases (hyperlipidemia, hypertension and diabetes) or cardiometabolic diseases + glycohemoglobin + C-reactive protein attained results indicating that current smoking was significantly associated with severe CAC, with OR stood at 3.20 and 3.97 respectively [13]. Differences exist between results of the study by Bagyura et al. [13] and the current study, which may be associated with the following factors. (1) Sample size of this study outnumbers that of by Bagyura et al. [13]. (2) Studied population of this study was different from that by Bagyura et al. [13] in the following aspects. (A) While this study was conducted on Chinese individuals, Bagyura et al. [13] analyzed data from an eastern European population. (B) Studied participants of Bagyura et al. [13] came from a geographically delimited region (a town) while participants of our study came from all over China. (C) Bagyura et al. [13] studied populations from a health survey while this study was based on patients visiting a hospital with special expertise on Cardiology. (3) Exclusion criteria of the study by Bagyura et al. [13] confined it to populations free of cardiovascular diseases or events while this study only excluded patients with prior PCI, CABG because of the inability to calculate CACS of PCI recipients or the altered coronary flow that may exert influence on CAC in patients with CABG history. (4) Differences in the definition of severe CAC existed between the two studies. (5) Alcohol consumption history was not adjusted for with respect to the result obtained by Bagyura et al. [13] whereas our study adjusted the results for it. Therefore, these comparisons indicate that race and comorbidities may influence the association of current smoking and severe CAC, a condition with possibly different definitions among studies. The types of confounders adjusted for also has an impact on the results.
The association of alcohol consumption and CAC is more obscure than that of smoking and CAC. Studies investigating the former have generally revealed the presence of association. McClelland et al. [10] investigated 6814 American individuals aged between 45 and 84 years old and were free from clinically apparent cardiovascular diseases, including White, African American, Hispanics and Chinese American. Results indicated that after adjusting for confounders, amount of liquor consumption correlated significantly with CAC. However, for usual alcohol consumption, the amount of alcohol consumed and former alcohol consumption were both insignificantly associated with CAC after adjustment of confounders. When it comes to the specific type of alcohol consumed, different degrees of amount of wine, beer, liquor consumed as well as total amount of alcohol consumed were not significantly associated (including associated in a J-shaped manner) with CAC after adjusting for confounders. Compared with those that never consumed liquor, populations that consumed liquor in the month prior to the study took place suffered from a significant increase of risk of the presence of CAC. Moreover, the association of liquor consumption and presence of CAC was generally found to be present among Chinese individuals [10]. The CARDIA study enrolled healthy black and white individuals aged between 33 and 45 years old [19]. Results indicated that heavy alcohol drinking (14 drinks per week) was independently associated with CAC. A statistically significant trend regarding the amount of alcohol consumed and likelihood of CAC in the entire population was observed. This trend was also statistically significant in both black male and black female subpopulations while the significance was absent in white male and white females. Liquor consumption also significantly correlated with the presence of CAC [19]. However, in a cohort of 731 healthy American military personnels that aged between 39 to 45 years old and were almost exclusively white (72%), researchers found none of several consumption-related variables (alcohol consumption, type or alcohol consumed and amount of alcohol consumed) were significantly associated with the presence of CAC [22]. The population studied by McClelland et al. [10] shared a fraction of similarity with the current study in that Chinese Americans in the study by McClelland et al. [10] accounted for 11.81% of the entire study population while the current study was solely based on Chinese individuals. This can explain the difference obtained between the study by McClelland et al. [10] and this study. This study is a retrospective study that utilized data from medical records where type of alcohol consumed was not recorded. However, the study found alcohol consumption history significantly associated with presence of CAC after adjusting for confounders. The discrepancy between results obtained by this study and the study by McClelland et al. [10] may be associated with racial difference between the study populations. More specifically, McClelland et al. [10] found liquor consumption to be associated with CAC in the Chinese population. This might have contributed to the significance found in this study.
The current study also investigated the association of alcohol consumption and the severity of CAC, which was also studied by prior studies. For instance, McClelland et al. [10] found consumption of more than two drinks of liquor per day was associated with the severity of CAC in individuals with CACS 0; consumption of more than two drinks of liquor per day was also associated with the drastic change in CAC during follow-up (30 units per year) in individuals with baseline CACS 0 [10]. However, there are also studies that failed to find associations between alcohol consumption and severe CAC. A study has found a lack of association between alcohol consumption and amount of CAC in a cohort of asymptomatic individuals at high risk of coronary heart disease [21]. Vliegenthart et al. [23] found that the amount of alcohol consumed to be U-shaped correlated with severe CAC, despite the statistical insignificance of the correlation. They analyzed data of 1795 participants from the Rotterdam Coronary Calcification Study and found that compared with non-drinkers, participants who drank one drink per day had lower odds of being afflicted by severe CAC (defined as CACS 400) (OR = 0.60), while odds ratios for those who drank 1–2 drinks per day and 2 drinks per day stood at 0.51 and 0.90 respectively. However, none of the trio were statistically significant. While both the current study and the study by Vliegenthart et al. [23] defined severe CAC as a situation where CACS exceeded a certain threshold, differences in the threshold existed. This, along with the difference in study population (i.e., the one by Vliegenthart et al. [23] was residents in Rotterdam, The Netherlands while the one of this study was Chinese), may have contributed to the differences in the current study and the one by Vliegenthart et al. [23].
Until now, the majority of studies investigating the association of smoking and alcohol consumption with CAC analyzed them independently. However, evidence from basic biological studies have casted light on the potential dependence of the association of smoking and vascular calcification on drinks containing alcohol. The aforementioned animal study by Liou et al. [24] found that xanthohumol, a chemical found in hops, an indispensable ingredient in producing beer, could alleviate vascular calcification mediated by vitamin D and nicotine. Therefore, beer consumption could influence the association between smoking and vascular calcification. It is therefore worthwhile to investigate in human subjects if alcohol consumption could influence the association between smoking and CAC. In other words, clinical evidence regarding the potential interaction effect of alcohol consumption on the association of smoking and CAC deserves to be gathered, which was part of the current study. Lee et al. [33] found that compared with never smokers, current smokers were exposed to a significant increase in risk of CAC while the risk increment of former smokers was insignificant. This study therefore analyzed if the interaction effect of alcohol consumption history existed with respect to the association of current smoking and CAC. While reaffirming that both current smoking and alcohol consumption history were risk factors of the presence of and severe CAC, results of this study found current smokers with concomitant alcohol consumption history were afflicted by an increase in risk of both presence of and severe CAC. These results also suggest that detailed biological mechanisms of the effect of alcohol (including ethanol and other chemicals that coexist) and smoking on vascular calcification should be further investigated. From a public health point of view, while significant weights have been laid upon tobacco control when it comes to the prevention of CAC and coronary atherosclerosis, results of this study have suggested a non-negligible weight of alcohol consumption in the formulation of policies tackling atherosclerotic diseases. When it comes to the prevention of CAC, emphasis should be laid upon current smokers who simultaneously had alcohol consumption history. From a clinical point of view, results of this study call on clinicians to be aware of the increased risk of CAC associated with both smoking and alcohol consumption, especially among those who simultaneously have a history of alcohol consumption and currently smoke.
Limitations of this study include: (1) This study is a single-center study based on patients that visited a hospital with expertise on Cardiology. Whether the results generalize to other populations remains to be investigated. (2) This study is a retrospective study based on medical records with the possibility of recall bias. In addition, the amount of information available has provided room for unmeasured confounders. (3) As a measure of CAC, CACS itself is subject to limitations. For instance, patients having undergone PCI (especially coronary stent implantation) are ineligible for calculating CACS.
Our study has provided clinical evidence of the interaction effect of alcohol consumption on the association between current smoking and CAC. Future research could be oriented in the following directions: (1) Further explore the underlying biological mechanism of the interaction effect. (2) Further examine the association of smoking and alcohol consumption with the onset and exacerbation in cohorts who have their CAC repeatedly gauged. (3) Examine the necessity to provide targeted care by setting more stringent goals in controlling atherosclerosis for current smokers with alcohol consumption history.
5. Conclusions
Both smoking history and alcohol consumption history correlated positively with the presence of and severe CAC. Alcohol consumption history has been shown to alter the association of current smoking and both the presence of and severe CAC. Alcohol consumption history was associated with a more pronounced risk for the presence of and severe CAC in current smokers.
Availability of Data and Materials
Data of the current study could be made available in response to reasonable request proposed to the corresponding author.
Acknowledgment
Not applicable.
Funding Statement
This study was funded by National Key Research and Development Program of China (2022YFC3602400, 2022YFC3602405); Key Project of the National Health Commission of The People's Republic of China (2020-ZD13). Funders of the study had no role in study design, data collection, data analysis, data interpretation, or drafting of the manuscript.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aimin Dang, Email: amdangfw@163.com.
Naqiang Lv, Email: lvnaqiang@gmail.com.
Author Contributions
YZJ: Conceptualization, Methodology (original design of study), Data curation and Investigation (data acquisition and data cleaning), Formal analysis (data analyses and mathematical modelling via SAS), Software (compiling SAS codes), Visualization, Writing (drafting of original manuscript and edited manuscript submitted for publication). XRH: Data acquisition, Writing - review & editing. YZG: Data acquisition, Writing (editing manuscript). JXL: Data acquisition, Writing (editing manuscript). YFL: Data acquisition, Writing (editing manuscript). WZ: Data acquisition, Writing (editing manuscript). AMD: Conceptualization, Methodology (original design of study), Supervision, Project administration, Funding acquisition, Writing-review & editing. NQL: Funding acquisition, Project administration, Conceptualization, Methodology (original design of study), Writing (editing manuscript). All authors have full and direct access to and verified the underlying data in this study, and were responsible for the decision to submit the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
This study was approved by Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval No.: 2021-1461). Informed consent was waived since data from the electronic database of medical records do not involve any personally identifiable information.
Funding
This study was funded by National Key Research and Development Program of China (2022YFC3602400, 2022YFC3602405); Key Project of the National Health Commission of The People’s Republic of China (2020-ZD13). Funders of the study had no role in study design, data collection, data analysis, data interpretation, or drafting of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Faggiano P, Dasseni N, Gaibazzi N, Rossi A, Henein M, Pressman G. Cardiac calcification as a marker of subclinical atherosclerosis and predictor of cardiovascular events: A review of the evidence. European Journal of Preventive Cardiology . 2019;26:1191–1204. doi: 10.1177/2047487319830485. [DOI] [PubMed] [Google Scholar]
- [2].Agarwal S, Cox AJ, Herrington DM, Jorgensen NW, Xu J, Freedman BI, et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care . 2013;36:972–977. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shemesh J, Motro M, Morag-Koren N, Konen E, Grossman E. Relation of coronary artery calcium to cardiovascular risk in patients with combined diabetes mellitus and systemic hypertension. The American Journal of Cardiology . 2012;109:844–850. doi: 10.1016/j.amjcard.2011.10.047. [DOI] [PubMed] [Google Scholar]
- [4].Elias-Smale SE, Proença RV, Koller MT, Kavousi M, van Rooij FJA, Hunink MG, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. Journal of the American College of Cardiology . 2010;56:1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- [5].Leigh A, McEvoy JW, Garg P, Carr JJ, Sandfort V, Oelsner EC, et al. Coronary Artery Calcium Scores and Atherosclerotic Cardiovascular Disease Risk Stratification in Smokers. JACC. Cardiovascular Imaging . 2019;12:852–861. doi: 10.1016/j.jcmg.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang BC, Wang C, Li WH, Li DY. Clinical outcome of drug-eluting versus bare-metal stents in patients with calcified coronary lesions: a meta-analysis. Internal Medicine Journal . 2015;45:203–211. doi: 10.1111/imj.12622. [DOI] [PubMed] [Google Scholar]
- [7].Handy CE, Desai CS, Dardari ZA, Al-Mallah MH, Miedema MD, Ouyang P, et al. The Association of Coronary Artery Calcium With Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC. Cardiovascular Imaging . 2016;9:568–576. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vliegenthart R, Oudkerk M, Hofman A, Oei HHS, van Dijck W, van Rooij FJA, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation . 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- [9].Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, et al. Impact of Statins on Cardiovascular Outcomes Following Coronary Artery Calcium Scoring. Journal of the American College of Cardiology . 2018;72:3233–3242. doi: 10.1016/j.jacc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McClelland RL, Bild DE, Burke GL, Mukamal KJ, Lima JA, Kronmal RA, et al. Alcohol and coronary artery calcium prevalence, incidence, and progression: results from the Multi-Ethnic Study of Atherosclerosis (MESA) The American Journal of Clinical Nutrition . 2008;88:1593–1601. doi: 10.3945/ajcn.2008.26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Toorn JE, Vernooij MW, Ikram MA, Kavousi M, Bos D. Progression of arterial calcifications: what, where, and in whom? European Radiology . 2024 doi: 10.1007/s00330-023-10566-7. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Capisizu AS, Stanciu SM, Cuzino D. A Pilot Study on the Association between Cardiovascular Risk Factors and Coronary Artery Calcification in a Group of Patients Investigated via Cardiac Computed Tomography in a European Country with High Cardiovascular Risk. Biomedicines . 2023;11:2926. doi: 10.3390/biomedicines11112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bagyura Z, Kiss L, Lux Á, Csobay-Novák C, Jermendy ÁL, Polgár L, et al. Neutrophil-to-Lymphocyte Ratio Is an Independent Risk Factor for Coronary Artery Disease in Central Obesity. International Journal of Molecular Sciences . 2023;24:7397. doi: 10.3390/ijms24087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kiss LZ, Bagyura Z, Csobay-Novák C, Lux Á, Polgár L, Jermendy Á, et al. Serum Uric Acid Is Independently Associated with Coronary Calcification in an Asymptomatic Population. Journal of Cardiovascular Translational Research . 2019;12:204–210. doi: 10.1007/s12265-018-9843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Molina CR, Mathur A, Soykan C, Sathe A, Kunhiraman L. Risk Factor Interactions, Non-High-Density Lipoprotein Cholesterol to Apolipoprotein B Ratio, and Severity of Coronary Arteriosclerosis in South Asian Individuals: An Observational Cohort Study. Journal of the American Heart Association . 2023;12:e027697. doi: 10.1161/JAHA.122.027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Min JK, Lin FY, Gidseg DS, Weinsaft JW, Berman DS, Shaw LJ, et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? Journal of the American College of Cardiology . 2010;55:1110–1117. doi: 10.1016/j.jacc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- [17].Zhang H, Li G, Yu X, Yang J, Jiang A, Cheng H, et al. Progression of Vascular Calcification and Clinical Outcomes in Patients Receiving Maintenance Dialysis. JAMA Network Open . 2023;6:e2310909. doi: 10.1001/jamanetworkopen.2023.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang SC, Wu YJ, Wang WH, Wu FZ. Gender Differences in Subclinical Coronary Atherosclerosis in the Asian Population With a Coronary Artery Calcium Score of Zero. The American Journal of Cardiology . 2023;203:29–36. doi: 10.1016/j.amjcard.2023.06.074. [DOI] [PubMed] [Google Scholar]
- [19].Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. American Journal of Epidemiology . 2005;161:423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- [20].Eelderink C, Te Velde-Keyzer CA, Frenay ARS, Vermeulen EA, Bachtler M, Aghagolzadeh P, et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population: The PREVEND Study. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40:1942–1951. doi: 10.1161/ATVBAHA.120.314187. [DOI] [PubMed] [Google Scholar]
- [21].Yang T, Doherty TM, Wong ND, Detrano RC. Alcohol consumption, coronary calcium, and coronary heart disease events. The American Journal of Cardiology . 1999;84:802–806. doi: 10.1016/s0002-9149(99)00440-3. [DOI] [PubMed] [Google Scholar]
- [22].Tofferi JK, Taylor AJ, Feuerstein IM, O’Malley PG. Alcohol intake is not associated with subclinical coronary atherosclerosis. American Heart Journal . 2004;148:803–809. doi: 10.1016/j.ahj.2004.05.023. [DOI] [PubMed] [Google Scholar]
- [23].Vliegenthart R, Oei HHS, van den Elzen APM, van Rooij FJA, Hofman A, Oudkerk M, et al. Alcohol consumption and coronary calcification in a general population. Archives of Internal Medicine . 2004;164:2355–2360. doi: 10.1001/archinte.164.21.2355. [DOI] [PubMed] [Google Scholar]
- [24].Liou SF, Nguyen TTN, Hsu JH, Sulistyowati E, Huang SE, Wu BN, et al. The Preventive Effects of Xanthohumol on Vascular Calcification Induced by Vitamin D_3 Plus Nicotine. Antioxidants . 2020;9:956. doi: 10.3390/antiox9100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Buuren S. Flexible Imputation of Missing Data. 2nd edn . Chapman and Hall/CRC; New York: 2018. [Google Scholar]
- [26].Finn AV. Coronary Calcium: A Comprehensive Understanding of Its Biology, Use in Screening, and Interventional Management. 1st edn. Elsevier Science & Technology; Amsterdam, Netherlands: 2019. [Google Scholar]
- [27].Obisesan OH, Osei AD, Uddin SMI, Dzaye O, Blaha MJ. An Update on Coronary Artery Calcium Interpretation at Chest and Cardiac CT. Radiology. Cardiothoracic Imaging . 2021;3:e200484. doi: 10.1148/ryct.2021200484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malguria N, Zimmerman S, Fishman EK. Coronary Artery Calcium Scoring: Current Status and Review of Literature. Journal of Computer Assisted Tomography . 2018;42:887–897. doi: 10.1097/RCT.0000000000000825. [DOI] [PubMed] [Google Scholar]
- [29].Wetscherek MTA, McNaughton E, Majcher V, Wetscherek A, Sadler TJ, Alsinbili A, et al. Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death. European Radiology . 2023;33:4723–4733. doi: 10.1007/s00330-023-09428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang ZW, Jin YJ, Zhao SJ, Zhou LN, Huang Y, Wang JW, et al. Prevalence and risk factors of coronary artery calcification on lung cancer screening with low-dose CT. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] . 2022;44:1112–1118. doi: 10.3760/cma.j.cn112152-20201114-00986. [DOI] [PubMed] [Google Scholar]
- [31].Trab T, Attar R, Jensen SE, Grøntved S, Frøkjær JB, Polcwiartek C, et al. Coronary artery calcium in patients with schizophrenia. BMC Psychiatry . 2021;21:422. doi: 10.1186/s12888-021-03412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oshunbade AA, Kassahun-Yimer W, Valle KA, Hamid A, Kipchumba RK, Kamimura D, et al. Cigarette Smoking, Incident Coronary Heart Disease, and Coronary Artery Calcification in Black Adults: The Jackson Heart Study. Journal of the American Heart Association . 2021;10:e017320. doi: 10.1161/JAHA.120.017320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee MJ, Park JT, Chang TI, Joo YS, Yoo TH, Park SK, et al. Smoking Cessation and Coronary Artery Calcification in CKD. Clinical Journal of the American Society of Nephrology . 2021;16:870–879. doi: 10.2215/CJN.15751020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of the current study could be made available in response to reasonable request proposed to the corresponding author.