Abstract
We discuss a case of a 60-year-old man with hypertrophic cardiomyopathy treated with the novel cardiac myosin inhibitor, mavacamten. Dynamic electrocardiogram patterns of left ventricular hypertrophy and left ventricular strain coincided with the patient starting mavacamten, discontinuing the drug, and then restarting mavacamten, highlighting electrocardiograms as accessible and inexpensive potential tools to monitor drug efficacy. This case also shows the ability of myosin inhibition to positively alter the adverse electrical changes associated with hypertrophic cardiomyopathy.
Key Words: chronic heart failure, electrocardiogram, hypertrophic cardiomyopathy
Graphical Abstract
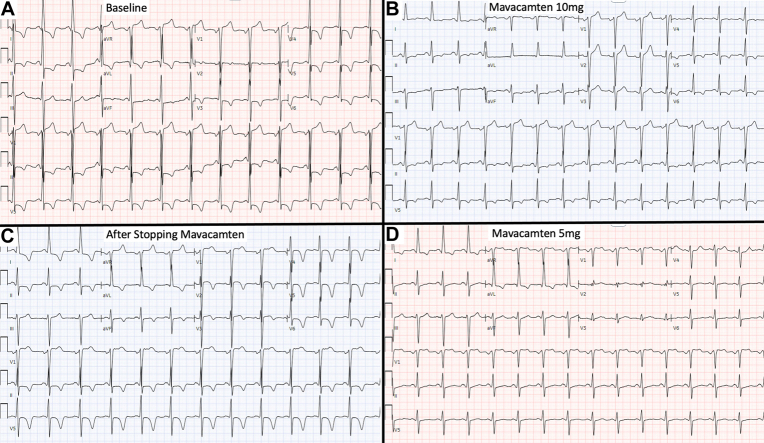
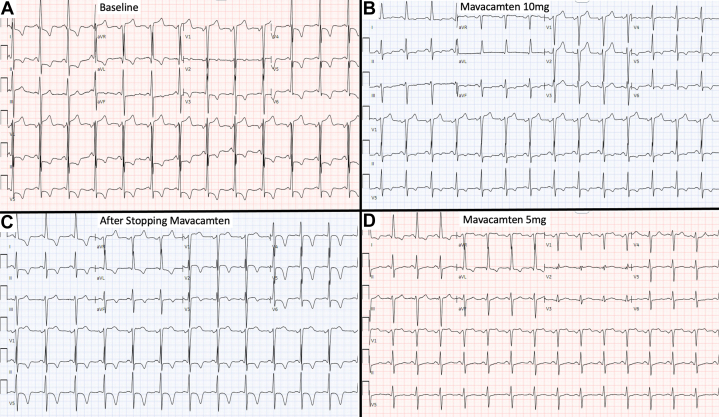
A 60-year-old man with obstructive hypertrophic cardiomyopathy (HCM) showed dynamic electrocardiogram (ECG) changes after starting, discontinuing, and restarting the novel cardiac myosin inhibitor mavacamten. ECG abnormalities commonly observed in HCM include patterns of left ventricular hypertrophy (LVH) with left ventricular (LV) strain. These findings are present in the patient’s baseline ECG (Figure 1A), which met voltage criteria for LVH per Cornell, Sokolow-Lyon, and Peguero-Lo Presti criteria and showed repolarization abnormalities signifying LV strain. ECG approximately 2 years following initiation of mavacamten (Figure 1B) showed improvement in LVH and LV strain patterns. The drug was temporarily discontinued due to an asymptomatic drop in LV ejection fraction. ECG 2 months later (Figure 1C) noted recurrence of LV strain patterns and again met LVH voltage criteria. Transthoracic echocardiography at that time (Video 1) showed asymmetric septal hypertrophy consistent with HCM. The patient ultimately restarted mavacamten after normalization of LV systolic function. ECG 3 months later (Figure 1D) again showed marked improvement in the LVH and LV strain patterns. Transthoracic echocardiography at that time (Video 2) showed persistent asymmetric septal hypertrophy despite improvement in electrical changes on ECG.
Figure 1.
Dynamic ECG Changes Associated With Mavacamten
(A) Baseline electrocardiogram (ECG) shows deep S-waves in V1-V2 and large R waves in V4-V6, and aVL meeting voltage criteria for left ventricular hypertrophy (LVH) per Cornell, Sokolow-Lyon, and Peguero-Lo Presti criteria. Repolarization abnormalities signifying left ventricular (LV) strain were also present, including ST-segment depressions and T-wave inversion in I, aVL, and V3-V6. (B) ECG after initiation of mavacamten and uptitration to 10 mg daily shows improvement in LVH (only meets Peguero-Lo Presti criteria) and LV strain patterns. (C) ECG after discontinuation of mavacamten noted recurrence of LV strain pattern (marked T-wave inversions in I, aVL, and V2-V6), and deep S-waves in V1-V3 with large R waves in V5-V6 and aVL, once again meeting LVH voltage criteria per Cornell, Sokolow-Lyon, and Peguero-Lo Presti criteria. (D) ECG after restarting mavacamten again shows marked improvement in the LV strain pattern (resolution of T-wave inversions in precordial leads) and decreased S- and R-wave amplitudes (LVH only per Peguero-Lo Presti criteria).
Mavacamten selectively targets the myosin motor of the sarcomere and promotes a super relaxed state of myosin. This leads to reduced adenosine triphosphatase consumption and myocardial contractility, which are the primary derangements in HCM. Moreover, animal studies have shown that mavacamten partially normalizes the Ca2+ sensitivity of thin filaments and improves myocardial relaxation.1 Imaging subanalyses of the pivotal mavacamten clinical trials have shown reductions in LVH and plasma biomarkers of myocardial stress and injury along with improvements in diastolic function.2 However, the ECG correlates of these structural changes have not been well described.
This case highlights several learning points. First, it emphasizes some of the typical ECG findings associated with HCM, including LVH with LV strain patterns. It also highlights that the ECG can be considered a biomarker of treatment with the novel cardiac myosin inhibitor, mavacamten. As ECG is accessible, inexpensive, and noninvasive, it may emerge as a convenient patient-level tool for monitoring response to cardiac myosin inhibitor therapy. Although a few limited case series have shown normalization of LVH and LV strain pattern in a subset of patients after starting mavacamten,3 no data currently exist describing the dynamic changes of ECG patterns in patients who stop and restart this medication. This case shows the ability of myosin inhibition to positively alter the adverse electrical changes associated with LVH in the setting of HCM.
Funding Support and Author Disclosures
Dr Reza has received consulting/speaking honoraria from Roche Diagnostics, Zoll, American Regent, and Bristol Myers Squibb; has received research support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL166961 (the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health) and Bristol Myers Squibb (grant to the institution). Dr Owens has received consultant/advisor fees and consulting/research support from Cytokinetics, MyoKardia/Bristol Myers Squibb, Pfizer, Lexicon Pharmaceuticals, Tenaya Therapeutics, Stealth BioTherapeutics, Renovacor, Edgewise Therapeutics, and BioMarin Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Drs Reza and Owens contributed equally to this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
References
- 1.Nag S., Gollapudi S.K., Del Rio C.L., Spudich J.A., McDowell R. Mavacamten, a precision medicine for hypertrophic cardiomyopathy: from a motor protein to patients. Sci Adv. 2023;9(30) doi: 10.1126/sciadv.abo7622. [DOI] [PubMed] [Google Scholar]
- 2.Saberi S., Cardim N., Yamani M., et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. 2021;143(6):606–608. doi: 10.1161/CIRCULATIONAHA.120.052359. [DOI] [PubMed] [Google Scholar]
- 3.Abdelfattah O.M., Lander B., Demarco K., Richards K., Dubose D., Martinez M.W. Mavacamten short-term hemodynamic, functional, and electrocardiographic outcomes: initial real-world post-trial experience. JACC Adv. 2023;2(10) doi: 10.1016/j.jacadv.2023.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.