Abstract
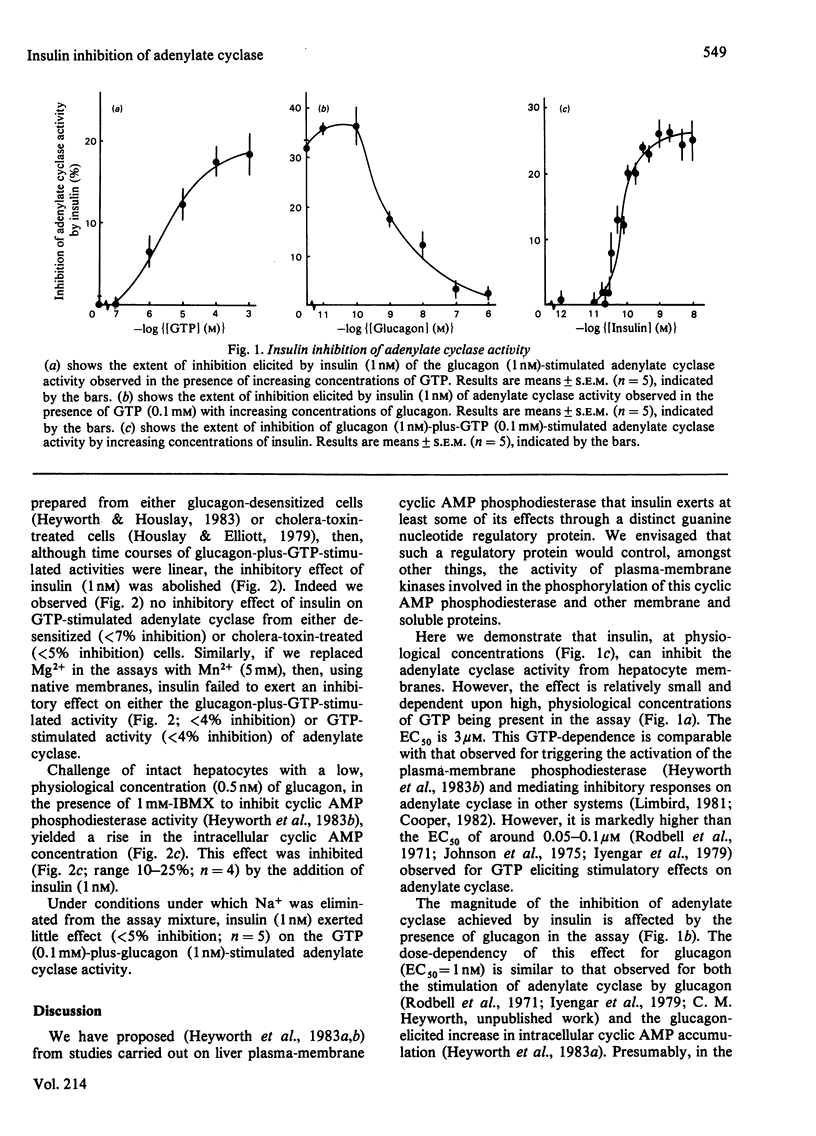
Insulin failed to exert an effect on the basal and glucagon- and guanosine 5'-[beta, gamma-imido]-triphosphate-stimulated adenylate cyclase activities of hepatocyte membranes. In the presence of high GTP (0.1 mM) concentrations, however, insulin was shown to inhibit adenylate cyclase activity. This effect was dose-dependent, exhibiting an EC50 (median effective concentration) of 3 microM for GTP. Elevated glucagon concentrations blocked the inhibitory effect of insulin in a dose-dependent fashion, with an EC50 of 1 nM. The insulin inhibition was dose-dependent (EC50 = 90 pM). The inhibitory effects of insulin were abolished using membranes from either glucagon-desensitized hepatocytes or cholera-toxin-treated hepatocytes. If either Mn2+ replaced Mg2+ in adenylate cyclase assays or Na+ was removed from the assay mixtures then insulin failed to exert any inhibitory effect. It is suggested that insulin exerts its action on adenylate cyclase through an inhibitory guanine nucleotide protein. This is integrated with the proposal [Heyworth, Rawal & Houslay (1983) FEBS Lett. 154, 87-91; Heyworth, Wallace & Houslay (1983) Biochem. J. in the press] that insulin mediates a variety of cellular effects through a specific guanine nucleotide regulatory protein and associated protein kinase(s).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmore P. F., Assimacopoulos-Jeannet F., Chan T. M., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Insulin inhibition of alpha-adrenergic and glucagon actions in normal and calcium-depleted hepatocytes. J Biol Chem. 1979 Apr 25;254(8):2828–2834. [PubMed] [Google Scholar]
- Braun S., Arad H., Levitzki A. The interaction of Mn2+ with turkey erythrocyte adenylate cyclase. Biochim Biophys Acta. 1982 Jul 12;705(1):55–62. doi: 10.1016/0167-4838(82)90335-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M. Bimodal regulation of adenylate cyclase. FEBS Lett. 1982 Feb 22;138(2):157–163. doi: 10.1016/0014-5793(82)80431-6. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Jagus R., Somers R. L., Rodbell M. Cholera toxin modifies diverse GTP-modulated regulatory proteins. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1179–1185. doi: 10.1016/0006-291x(81)91572-2. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J. L., Van Obberghen E., Freychet P., Thamm P., Saunders D., Brandenburg D., Orci L. Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5921–5925. doi: 10.1073/pnas.79.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Renner R. Insulin action on the adenyl cyclase system: Antagonism to activation by lipolytic hormones. FEBS Lett. 1972 Feb 1;20(2):191–194. doi: 10.1016/0014-5793(72)80791-9. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Rawal S., Houslay M. D. Guanine nucleotides can activate the insulin-stimulated phosphodiesterase in liver plasma membranes. FEBS Lett. 1983 Apr 5;154(1):87–91. doi: 10.1016/0014-5793(83)80880-1. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Houslay M. D., Dipple I., Elliott K. R. Guanosine 5'-triphosphate and guanosine 5'-[beta gamma-imido]triphosphate effect a collision coupling mechanism between the glucagon receptor and catalytic unit of adenylate cyclase. Biochem J. 1980 Mar 15;186(3):649–658. doi: 10.1042/bj1860649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Palmer R. W. Changes in the form of Arrhenius plots of the activity of glucagon-stimulated adenylate cyclase and other hamster liver plasma-membrane enzymes occurring on hibernation. Biochem J. 1978 Sep 15;174(3):909–919. doi: 10.1042/bj1740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. Dual control of adenylate cyclase. Nature. 1983 May 12;303(5913):133–133. doi: 10.1038/303133a0. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Illiano G., Cuatrecasas P. Modulation of adenylate cyclase activity in liver and fat cell membranes by insulin. Science. 1972 Feb 25;175(4024):906–908. doi: 10.1126/science.175.4024.906. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Swartz T. L., Birnbaumer L. Coupling of glucagon receptor to adenylyl cyclase. Requirement of a receptor-related guanyl nucleotide binding site for coupling of receptor to the enzyme. J Biol Chem. 1979 Feb 25;254(4):1119–1123. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Inhibition of adenylate cyclase by hormones and neurotransmitters. Adv Cyclic Nucleotide Res. 1981;14:173–187. [PubMed] [Google Scholar]
- Johnson M. E., Das N. M., Butcher F. R., Fain J. N. The regulation of gluconeogenesis in isolated rat liver cells by glucagon, insulin, dibutyryl cyclic adenosine monophosphate, and fatty acids. J Biol Chem. 1972 May 25;247(10):3229–3235. [PubMed] [Google Scholar]
- Johnson R. A., Pilkis S. J., Hamet P. Liver membrane adenylate cyclase. Synergistic effects of anions on fluoride, glucagon, and guanyl nucleotide stimulation. J Biol Chem. 1975 Aug 25;250(16):6599–6607. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kiss Z. Inhibition of the glucagon stimulated adenylate cyclase activity by insulin. FEBS Lett. 1978 Aug 1;92(1):29–32. doi: 10.1016/0014-5793(78)80714-5. [DOI] [PubMed] [Google Scholar]
- Londos C., Lad P. M., Nielsen T. B., Rodbell M. Solubilization and conversion of hepatic adenylate cyclase to a form requiring MnATP as substrate. J Supramol Struct. 1979;10(1):31–37. doi: 10.1002/jss.400100104. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Insulin trigger, cyclic AMP-dependent activation and phosphorylation of a plasma membrane cyclic AMP phosphodiesterase. Nature. 1980 Aug 28;286(5776):904–906. doi: 10.1038/286904a0. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Exton J. H., Blackmore P. F. Angiotensin II inhibits hepatic cAMP accumulation induced by glucagon and epinephrine and their metabolic effects. FEBS Lett. 1983 Mar 7;153(1):77–80. doi: 10.1016/0014-5793(83)80122-7. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Claus T. H., Johnson R. A., Park C. R. Hormonal control of cyclic 3':5'-AMP levels and gluconeogenesis in isolated hepatocytes from fed rats. J Biol Chem. 1975 Aug 25;250(16):6328–6336. [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Torres H. N., Flawià M. M., Hernaez L., Cuatrecasas P. Effects of insulin on the adenylyl cyclase activity of isolated fat cell membranes. J Membr Biol. 1978 Sep 29;43(1):1–18. doi: 10.1007/BF01869039. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Moss J. Mechanism of action of choleragen. J Supramol Struct. 1978;8(4):473–488. doi: 10.1002/jss.400080410. [DOI] [PubMed] [Google Scholar]
- van Heyningen S. Cholera toxin. Biol Rev Camb Philos Soc. 1977 Nov;52(4):509–509. doi: 10.1111/j.1469-185x.1977.tb00858.x. [DOI] [PubMed] [Google Scholar]



