ABSTRACT
The guinea fowl (Numida meleagris) holds significant agricultural importance in Ghana, particularly in the Northern, Upper East and Upper West Regions. Despite their economic and cultural significance, guinea fowls face a potential threat from avian influenza, a global concern for its adverse impact on poultry populations. This study assessed the seroprevalence of the virus in mature guinea fowls in the Upper East Region. A cross‐sectional survey was conducted in three districts within the Upper East Region from April to June 2023. Blood samples were collected from 397 guinea fowls that are over 4 weeks old, and seroprevalence was determined using ID Screen Influenza A Antibody Competition Enzyme‐linked immunosorbent assay (ELISA). The study analysed demographic factors such as sex, age and source of birds, employing statistical methods to establish associations. Among the sampled guinea fowls, 24.7% tested positive for avian influenza antibodies, whereas 75.3% were seronegative. Age did not show statistically significant associations with seroprevalence, but intriguing patterns were observed. Adult guinea fowls exhibited higher seroprevalence (23.7%) compared to growers (1.0%). The source of birds showed no significant association, but birds from slaughter points demonstrated higher seroprevalence (11.6%) compared to households (5.0%) and live bird markets (8.1%). In conclusion, the study underscores the importance of monitoring avian influenza in guinea fowls to implement effective control measures. The presence of antibodies suggests guinea fowls may contribute to virus transmission in the Upper East Region. The study recommends ongoing nationwide surveillance to assess the true prevalence of avian influenza in guinea fowls across Ghana.
Keywords: avian influenza, biosecurity measures, guinea fowls, seroprevalence, Upper East Region
Guinea fowls hold significant agricultural importance in the Upper East Region but face threats from avian influenza. Cross‐sectional survey conducted in three districts from April to June 2023. Sampled 397 guinea fowls (aged >4 weeks). Test for avian influenza using ID Screen Influenza A virus Antibody Competition ELISA. It was observed that 24.7% tested positive for avian influenza antibodies. Sex, age and source of birds did not show statistically significant associations with seroprevalence of avian influenza.

1. Introduction
The guinea fowl species, scientifically known as Numida meleagris, holds significant importance within the agricultural landscape of Ghana especially in the Northern, Upper East and Upper West Regions (Agbolosu et al. 2015; Issaka and Yeboah 2016). These birds play a crucial role in supporting local economies and are highly regarded for their remarkable versatility, which enables them to fulfil several roles encompassing economic, nutritional and cultural dimensions (Issaka and Yeboah 2016; Teye and Adam 2000). In Africa, guinea fowls are still raised as free‐range scavenging birds and have seen little improvement (Dougnon et al. 2012; Aryee et al. 2019). Guinea fowls are easier to manage by resource poor farmers with hardly any access to formal veterinary services because they are resistant to most poultry diseases at the adult stage (Sayila 2009). The economic well‐being of communities in this particular location is closely interconnected with the health and overall welfare of the bird species inhabiting it (Charostad et al., 2023). Nonetheless, the current state of economic growth is confronted with an imminent peril in the shape of avian influenza, a viral disease acknowledged internationally for its devastating consequences on chicken populations (Charostad et al. 2023). The presence of this menace poses a significant risk not only to the well‐being of animals but also to the economic stability of the poultry farming industry (Naggujja et al. 2020). In the agricultural economy of Ghana, poultry farming plays a crucial role. Therefore, it is essential to comprehend the dynamics of avian influenza, specifically in relation to guinea fowls. This understanding is necessary to effectively prevent prospective outbreaks and protect the economic interests associated with their breeding.
The avian influenza, also referred to as bird flu caused by avian influenza virus, has caused significant disruptions in chicken populations on a global scale, resulting in large economic repercussions and presenting considerable obstacles to ensuring food security (Charostad et al. 2023). The nation of Ghana has seen the adverse consequences of avian influenza outbreaks within its poultry sector (Awuni et al. 2019). The consequences have a broader scope than just economic aspects, affecting the livelihoods of several individuals who rely on the chicken industry (Charostad et al. 2023). The importance of this threat, on a global scale as well as within the specific context of Ghana, highlights the need for research endeavours that focus on understanding the unique dynamics of avian influenza in guinea fowls. These birds are an essential component of Ghana's agricultural environment.
Although there has been prior investigation into avian influenza in Ghana (Suu‐Ire et al. 2019; Tasiame et al. 2020; Shaban et al. 2022, there remains a significant knowledge vacuum regarding the frequency of the virus primarily in guinea fowls, notably in the Upper East Region. Prior research has frequently adopted a more comprehensive perspective, incorporating other avian species, but failing to delve into the distinctive attributes and susceptibilities specific to guinea fowls. This study therefore aimed at addressing the existing knowledge gap by specifically investigating the seroprevalence of avian influenza among mature guinea fowls residing in the Upper East Region. The comprehension of seroprevalence patterns in guinea fowls facilitates the formulation of focused approaches for disease control, consequently promoting the long‐term viability of the poultry industry in the Upper East Region. In addition, the findings of this study have the potential to contribute to evidence‐based policy interventions aimed at facilitating the sustained coexistence of bird species in the face of the avian influenza threat in Ghana.
2. Materials and Methods
2.1. Study Area
The study was conducted in three districts the Upper East Region of Ghana: Bongo, Bolgatanga and Bolgatanga East. The region shares borders with Republic of Burkina Faso to the north, the Republic of Togo to the east, the Northeast Region of Ghana to the south and the Upper West Region of Ghana to the west. The region occupies a land mass of 8842 square kilometres (2.7% the total landmass of Ghana).
2.2. Study Design
A cross‐sectional survey was conducted from April to June 2023 in the study area to determine the seroprevalence of influenza A virus in guinea fowls. The three districts with high guinea fowl population within the live bird markets were chosen purposively. The live bird markets and slaughter points were purposively chosen. The households were randomly selected, and the management systems in the households were mainly semi‐intensive system. The households kept a mixture of guinea fowls and indigenous chicken. The inclusion criteria were all guinea fowls of both sexes, growers, adults apparently healthy and those showing signs of ill health.
2.3. Sample Size Determination
The sample size (n) was estimated using the formula of (Thrusfield 2007) using a 50% expected prevalence, 95% confidence level and 5% precision. The number of samples required was 385 guinea fowls; however, a total of 397 samples were collected.
2.4. Sample Collection
Blood samples were collected from guinea fowls that were over 4 weeks of age. A 21‐ga 5 mL syringe was used to collect blood from the brachial vein into appropriately labelled gel and clot activator tubes. The samples were transported on ice at 4°C to the laboratory. Once blood samples were fully clotted, they were centrifuged at 3000 rpm for 5 min. The serum was aliquoted into plain labelled cryotubes and stored at −20°C till serology was performed.
2.5. Serological Technique
Sera samples were screened for the presence of antibodies to influenza A virus using ID Screen Influenza A Antibody Competition Multi‐species obtained from ID‐vet Innovative Diagnostics, Grabels, France. The test is a competitive ELISA for the detection of influenza A antibodies in serum of avian species. It detects antibodies against all influenza A subtypes and antigenic variants using a monoclonal antibody against a highly conserved epitope of the influenza A virus nucleoprotein. A 0.9 µL of dilution buffer 2 was placed in each well. A 10 µL of positive control was added to each wells A1 and B1. A 10 µL of negative control was added to wells C1 and D1. A volume of 10 µL of the sera was added to the remaining wells. The plates were covered and incubated at 37°C (+2°C) for 60 min ± 5 min. The cells were emptied, and each well was washed five times with approximately 300 µL of wash solution. A volume of 10 µL of conjugate 1× was added to each well. The plate was covered and incubated at 21°C (+5°C) for 30 min ± 5 min. The wells were emptied and each well washed three times with approximately 300 µL of wash solution. A volume of 50 µL of the substrate solution was added to each well. The plate was covered and incubated at 21°C (+2°C) for 10 min ± 1 min in the dark. A volume of 50 µL of the stop was added to each well to stop the reaction. Optical density was read using ELISA reader at 450 nm.
2.6. Validity of Test
Validation of the ELISA test is helpful in checking if all test procedures were conducted and determine whether the positive and negative controls obtained were valid or not. According to the manufacturer, the test is valid when the mean value of the negative control optical density. Optical density (ODNC) is greater than 0.700, and the mean value of the positive control optical density (ODPC) is less than 30% of the ODNC. All the ELISA microplates tested were found to be valid.
2.7. Data Analysis
The data obtained were entered into Microsoft Office 365 Excel spreadsheet and analysed using StataCorp. 2019 (Stata Statistical Software: Release 16, College Station, TX: StataCorp LLC). Chi‐square was used to measure the strength of association among sex, age groups, sampling sites and seroprevalence of avian influenza. Significant values of p < 0.05 at 95% confidence interval were considered to be statistically significant.
3. Results
3.1. Animal Demography
The guinea fowl demography analysis reveals insightful information about the surveyed population. In terms of sex distribution, the study indicates a slightly higher prevalence of male animals, constituting 60.2%, compared to females, which make up 39.8% of the sample. Age‐wise, the majority of the animals fall into the adult (6 months and above) category, comprising an overwhelming 95.2%, whereas the grower (4 weeks to 6 months) category represents a smaller proportion at 4.8%. The origin or source of these animals varies significantly, with 20.2% sourced from households, 25.7% from live bird markets and the majority, 54.2%, obtained from slaughter points as seen in Table 1. The slaughtered guinea fowls were from Bolgatanga in the Bolgatanga district, Zorko and Bongo townships that are within the Bongo district.
TABLE 1.
Guinea fowl demographic characteristics.
| Animal parameters | Categories | Frequency (n) | Percentage |
|---|---|---|---|
| Sex | Female | 158 | 39.8 |
| Male | 239 | 60.2 | |
| Age Groups | Adult | 378 | 95.2 |
| Grower | 19 | 4.8 | |
| Source of birds | Household | 80 | 20.2 |
| Live bird market | 102 | 25.7 | |
| Slaughter point | 215 | 54.2 |
3.2. Seroprevalence of Avian Influenza
The findings in Figure 1 are indicative of the seroprevalence of avian influenza in guinea fowls of which 24.7% of the guinea fowls sampled were seropositive for avian influenza, whereas 75.3% were seronegative for avian influenza. The seroprevalence based on the three districts where the guinea fowls were sampled was 18.8% from Bolga East district, 24.2% from Bolgatanga district and 26.9% from Bongo district (Table 2).
FIGURE 1.
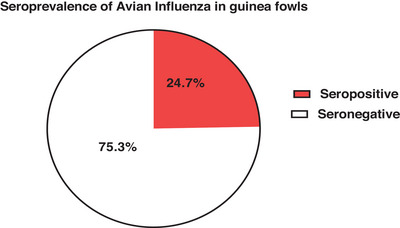
Seroprevalence of avian influenza in guinea fowls.
TABLE 2.
Seroprevalence of avian influenza in guinea fowls based on districts.
| District | No. positive | No. negative |
|---|---|---|
| Bolga East | 3 (18.8%) | 13 (81.2%) |
| Bolgatanga | 57 (24.2%) | 179 (75.8%) |
| Bongo | 39 (26.9%) | 106 (73.1%) |
3.3. Animal Characteristics and Seroprevalence of Avian Influenza
The association between animal demography and the seroprevalence of avian influenza has been explored, revealing interesting patterns within the studied population. Considering the sex and the source of the birds, there is no significant association between sex and prevalence in households (χ 2 = 0.9503, p value = 0.329642) as 21.8% of males tested positive and 32% of females tested positive. There is also statistically no significant association between sex and prevalence at the slaughter points (χ 2 = 0.1005, p value = 0.75128); 20.8% of males tested positive, whereas in females, 22.7% tested positive. There is, however, a statistically significant association between sex and the prevalence at the live bird markets (χ 2 = 27.0968, p value < 0.0001) as 14.9% of males tested positive and 65.7% of females tested positive (Table 3).
TABLE 3.
Association between sex and source seroprevalence of avian influenza.
| Source of birds | Sex | Avian influenza seroprevalence | Chi‐square value | p value | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Household | Male | 43 (78.2%) | 12 (21.8%) | 0.9503 | 0.329642ns |
| Female | 17 (68%) | 8 (32%) | |||
| Slaughter point | Male | 118 (79.2%) | 31 (20.8%) | 0.1005 | 0.75128ns |
| Female | 51 (77.3%) | 15 (22.7%) | |||
| Live bird market | Male | 57 (85.1%) | 10 (14.9%) | 27.0968 | < 0.0001 |
| Female | 12 (34.3%) | 23 (65.7%) | |||
Note: “ns”—no significant differences at 5% significance level (i.e., p > 0.05).
When considering age groups, no significant association is observed (χ 2 = 0.142, p value = 0.707). Among adults, 71.5% tested negative, and 23.7% tested positive, whereas for growers, 3.8% were negative, and 1.0% were positive (Table 4). Examining the source of birds, there is also no significant association (χ 2 = 3.109, p value = 0.156). For birds sourced from households, 15.1% tested negative, and 5.0% tested positive. In live bird markets, 17.6% were negative, and 8.1% were positive, whereas at slaughter points, 42.6% tested negative, and 11.6% tested positive (Table 4).
TABLE 4.
Association between animal demography and seroprevalence of avian influenza.
| Animal parameters | Categories | Avian influenza seroprevalence | Chi‐square value | p value | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Age groups | Adult | 284 (71.5%) | 94 (23.7%) | 0.142 | 0.707ns |
| Grower | 15 (3.8%) | 4 (1.0%) | |||
| Source of birds | Household | 60 (15.1%) | 20 (5.0%) | 3.709 | 0.156ns |
| Live bird market | 70 (17.6%) | 32 (8.1%) | |||
| Slaughter point | 169 (42.6%) | 46 (11.6%) | |||
Note: “ns”—no significant differences at 5% significance level (i.e., p > 0.05).
4. Discussion
This current study focused on the seroprevalence of avian influenza in adult guinea fowls in the Upper East Region of Ghana. The findings showed a 24.7% seroprevalence of avian influenza among the sampled guinea fowls, which are considered wild birds in nature. The seroprevalence of 24.7% aligns with existing literature that highlights the endemic nature of avian influenza in many bird populations, including domestic poultry and wild birds (Daodu et al. 2020; Akanbi et al. 2020). Several studies have emphasized the role of wild birds, including guinea fowls, as reservoirs and vectors of avian influenza viruses. However, this finding does not corroborate the findings of Suu‐Ire et al. (2019) who indicated that avian influenza virus antibody were neither detected in sera samples collected from wild birds in southern part of Ghana. The differences in the seroprevalence could be as a result of geographical differences. Additionally, differences in sampling methodologies, diagnostic techniques and study populations could contribute to variations in reported seroprevalence rates. The prevalence of avian influenza can vary across different regions and is influenced by factors such as bird species, ecological conditions and farming practices as earlier indicated by Liang et al. (2021).
The guinea fowls found to be seropositive for avian influenza implies a previous exposure or infection with the virus. On the other hand, 75.3% of the sampled guinea fowls were seronegative, suggesting that a significant portion of the population had not been exposed to avian influenza or had not developed antibodies. This finding has important implications for both avian health and public health, as avian influenza, also known as bird flu, is a viral infection that can have serious consequences for poultry populations and poses a potential threat to human health. In addition, the antibodies detected may be as a result of natural infection because the vaccination of guinea fowls against avian influenza is not a normal practice in Ghana.
It is noteworthy that the seroprevalence of avian influenza in guinea fowls (24.7%) appears to be higher than the rates reported in some studies involving other poultry species, such as ducks (6.25%) and geese (12.5%) (Chinyere et al. 2020). This discrepancy may be attributed to species‐specific variations in susceptibility, immune response, or contact patterns with other bird species. These comparative insights underscore the need for a nuanced understanding of avian influenza dynamics in different avian species. Although guinea fowls may exhibit a higher seroprevalence compared to some other bird species, their potential role as carriers or transmitters of specific avian influenza virus strains should not be underestimated.
Analysing the data by sex and source prevalence, there is a suggestive but not statistically significant association between sex and birds sampled from households and slaughter points. There was however a significant association between sex and prevalence in live bird markets. The higher seroprevalence at the live bird market was in females (65.7%) compared to males (14.9%) though the number of males sampled was almost twice that of females, which hints at a potential gender‐related difference in birds sold at the markets. The previous study by Navid et al. (2023) found variations in avian influenza prevalence between male and female poultry, with female birds showing higher seropositivity though no significance difference existed. These findings suggest a potential hormonal or physiological influence on susceptibility to avian influenza with regards to the sex of the bird; however, this may need further investigation.
Regarding the seroprevalence and the age groups, no significant association was observed in the current study. However, the higher seroprevalence among adults (23.7%) compared to growers (1.0%) raises questions about the potential role of age‐related immunity or exposure in avian influenza dynamics. The lack of statistical significance (p value < 0.05) might be influenced by factors such as sample size or variations in the study population. In agreement, other studies, such as that by Carozone (2022), identified age as not a significant factor influencing avian influenza prevalence. However, research by Lee et al. (2023) found out that age is a determining factor in the susceptibility of bird species such as ducks to avian influenza. These different findings emphasize the need for further exploration of age‐related patterns.
Examining the source of birds also provides valuable insights in the seroprevalence of avian influenza in terms of location of the bird. The absence of a statistically significant association (p value < 0.05) suggests that the source of birds may not be a primary determinant of avian influenza seroprevalence in the studied population. However, the higher seroprevalence in birds sourced from slaughter points (11.6%) compared to those from households (5.0%) and live bird markets (8.1%) warrants attention. This finding justifies the findings from other studies by Khan et al. (2018), Hassan et al. (2020), Le et al. (2022), Shaban et al. (2022), Modirihamedan et al. (2021) and Islam et al. (2023) that highlighted the role of live bird markets as potential hotspots for avian influenza transmission, as well as recognized live bird markets as critical habitats for the persistence, propagation, replication and spread of avian influenza virus.
The seroprevalence data obtained from this study underscore the importance of monitoring and surveillance programmes to track the prevalence and distribution of avian influenza in bird populations. Understanding the dynamics of avian influenza in guinea fowls is crucial for implementing effective control measures and preventing the spread of the virus to domestic poultry and potentially to humans. Additionally, exploring the factors contributing to the seroprevalence, such as environmental conditions, contact with other bird species and biosecurity measures, could provide valuable insights for designing targeted intervention strategies.
5. Conclusion
In conclusion, this investigation reveals the presence of antibodies to avian influenza A virus in guinea fowls within the Upper East Region of Ghana. The findings suggest that guinea fowls may contribute to the transmission of avian influenza virus in the region. Consequently, we recommend the implementation of stringent biosecurity measures to minimize interactions among guinea fowls, local birds and commercial chickens as a crucial control strategy against avian influenza. As a next step, it is advisable to isolate the circulating avian influenza virus and determine the neuraminidase and fusion protein subtype for further characterization. Additionally, we stress the importance of regularly conducting nationwide active surveillance of avian influenza virus in both previously affected and unaffected areas to stay informed about the true prevalence of this disease in guinea fowls across Ghana.
Author Contributions
A.A.T., S.A.M.J. and B.O.E. were responsible for conceptualizing the study, designing the methodology and supervision. A.A.T. was responsible for the investigation data curation, formal analyses of the data and drafting the original manuscript. P.M.A. was responsible for laboratory analysis. A.A.T., S.A.M.J., B.O.E. and D.A.A. contributed to data analysis, as well as reviewing and editing the manuscript.
Ethics Statement
Ethical approval for the study was obtained from the Veterinary Services Directorate of the Upper East Region dated 10/01/2023 with reference number MOFA/VSD/UER/SRA/23/10/02.
Consent
Consent was obtained from the farmers and traders, and participation was voluntary for samples to be collected from their flock.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70106.
Funding: This research was funded by the Pan African University Life and Earth Sciences Institute (Including Health and Agriculture), Ibadan—Nigeria.
Data Availability Statement
The data substantiating the conclusions of this research are accessible and may be acquired from the corresponding author, Emikpe Obukowho Benjamin, upon a reasonable inquiry. Researchers or individuals seeking access to the data for further research or validation may contact the appropriate author to discuss the specifics of the request.
References
- Agbolosu, A. A. , Ahunu B. K., Aboagye G. S., Naazie A., and Kayang B. B.. 2015. “Variation in Some Qualitative Traits of the Indigenous Guinea Fowls in Northern Ghana.” Global Journal of Animal Scientific Research 2: 396–401. [Google Scholar]
- Akanbi, O. B. , Taiwo V. O., Obishakin E. T., Ekong P. S., Barde I. J., and Meseko C. A.. 2020. “Features of Highly Pathogenic Avian Influenza (HPAI) H5N1 in Domestic Poultry.” In Viruses and Viral Infections in Developing Countries. London: IntechOpen. [Google Scholar]
- Aryee, Z. G. A. S. , Addison Y. D., and Eric A.. 2019. “Effects of Strain and Non‐Genetic Factors on the Egg Qualities and Carcass Characteristics of Indigenous Guinea Fowl (Numida meleagris).” International Journal of Applied Agricultural Sciences 5: 39–44. [Google Scholar]
- Awuni, J. A. , Bianco A., Dogbey O. J., et al. 2019. “Avian Influenza H9N2 Subtype in Ghana: Virus Characterization and Evidence of Co‐Infection.” Avian Pathology 48, no. 5: 470–476. [DOI] [PubMed] [Google Scholar]
- Carozone, N. S. 2022. “Prevalence of Influenza a Virus in Chicken and Determination of Risk Factors of Its Presence and Spread in Uasin Gishu County, Kenya.” Doctoral diss., University of Eldoret. [Google Scholar]
- Charostad, J. , Rukerd M. R. Z., Mahmoudvand S., et al. 2023. “A Comprehensive Review of Highly Pathogenic Avian Influenza (HPAI) H5N1: An Imminent Threat at Doorstep.” Travel Medicine and Infectious Disease 55: 102638. [DOI] [PubMed] [Google Scholar]
- Chinyere, C. N. , Okwor E. C., Meseko C. A., et al. 2020. “Sero‐Detection of Avian Influenza A/H7 in Nigerian Live‐Bird Markets in Plateau State.” Nigerian Veterinary Journal 41, no. 2: 161–174. [Google Scholar]
- Daodu, O. B. , Jegede H. O., Aiyedun J. O., et al. 2020. “Surveillance for Avian Influenza Virus in Captive Wild Birds and Indigenous Chickens in Nigeria.” Tropical Animal Health and Production 52: 2387–2393. [DOI] [PubMed] [Google Scholar]
- Dougnon, T. J. , Tobada P., Djossa B. A., Davito F. E., and Youssao I.. 2012. “Effects of Powdered Peanut (Arachis hypogea) on the Sex Reversing in Guinea Fowl and the Parameters of Production and Reproduction in the Fowl “Numida meleagris”.” International Journal of Advanced Biological Research 2, no. 2: 209–214. [Google Scholar]
- Hassan, M. M. , Islam A., Hasan R. B., et al. 2020. “Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl‐Chicken Interface in Wetlands.” Pathogens 9, no. 11: 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Islam S., Flora M. S., et al. 2023. “Epidemiology and Molecular Characterization of Avian Influenza A Viruses H5N1 and H3N8 Subtypes in Poultry Farms and Live Bird Markets in Bangladesh.” Scientific Reports 13, no. 1: 7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaka, B. Y. , and Yeboah R. N.. 2016. “Socio‐Economic Attributes of Guinea Fowl Production in Two Districts in Northern Ghana.” African Journal of Agricultural Research 11, no. 14: 1209–1217. [Google Scholar]
- Khan, S. U. , Gurley E. S., Gerloff N., et al. 2018. “Avian Influenza Surveillance in Domestic Waterfowl and Environment of Live Bird Markets in Bangladesh, 2007–2012.” Scientific Reports 8, no. 1: 9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotey, E. N. , Asante I. A., Adusei‐Poku M., et al. 2022. “Phylogenetic and Genetic Characterization of Influenza A H9N2 Viruses Isolated From Backyard Poultry in Selected Farms in Ghana.” Veterinary Medicine and Science 8, no. 4: 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, K. T. , Isoda N., Nguyen L. T., et al. 2022. “Risk Profile of Low Pathogenicity Avian Influenza Virus Infections in Farms in Southern Vietnam.” Journal of Veterinary Medical Science 84, no. 6: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Cho A. Y., Kim D. H., et al. 2023. “Live Recombinant Newcastle Disease Virus Vectored Vaccine Expressing the Haemagglutinin of H9N2 Avian Influenza Virus Suppresses Viral Replication in Chickens.” Avian Pathology 52, no. 2: 100–107. [DOI] [PubMed] [Google Scholar]
- Liang, Y. , Krog J. S., Ryt‐Hansen P., et al. 2021. “Molecular Characterization of Highly Pathogenic Avian Influenza Viruses H5N6 Detected in Denmark in 2018–2019.” Viruses 13, no. 6: 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirihamedan, A. , Aghajantabar S., King J., et al. 2021. “Wild Bird Trade at Live Poultry Markets Potentiates Risks of Avian Influenza Virus Introduction in Iran.” Infection Ecology & Epidemiology 11, no. 1: 1992083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggujja, J. , Njiru N., Msoffe P., et al. 2020. Tanzania and Ghana Poultry Sector Policy Review. The U.S. Government's Global Hunger and Food Security Initiative. Feed the Future Innovation Lab for Genomics to Improve Poultry. Nairobi, Kenya: ILRI. [Google Scholar]
- Navid, M. T. , Awais M. M., Anwar M. I., and Akhtar M.. 2023. “Seroprevalence of Avian Influenza Viruses in Asymptomatic Backyard Poultry Birds in District Multan, Pakistan.” Pakistan Journal of Zoology 55: 299–305. [Google Scholar]
- Sayila, A. 2009. “Guinea Fowl Farming Becomes Popular in Botswana.” World Poultry 25, no. 10: 30–31. [Google Scholar]
- Shaban, S. , Kyei F., Awuni J., et al. 2022. “Dynamics of Influenza A (Avian Influenza) Virus in Poultry in the Greater Accra Region of Ghana Amongst the Production Levels.” Journal of Immunoassay and Immunochemistry 43, no. 1: 1952426. [DOI] [PubMed] [Google Scholar]
- Suu‐Ire, R. , Awuni J., Benia P., and Kia G.. 2019. “Highly Pathogenic Avian Influenza H5N1 (HPAI/H5N1) Virus Search From Wild Birds in Ghana.” Folia Veterinaria 63, no. 3: 66–71. [Google Scholar]
- Tasiame, W. , Johnson S., Burimuah V., et al. 2020. “Outbreak of Highly Pathogenic Avian Influenza in Ghana, 2015: Degree of Losses and Outcomes of Time‐Course Outbreak Management.” Epidemiology & Infection 148: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teye, G. A. , and Adam M.. 2000. “Constraints to Guinea fowl production in northern Ghana: A case study of the Damongo area.” Ghana Journal of Agricultural Science 33, no. 2: 153–157. [Google Scholar]
- Thrusfield, M. 2007. Veterinary Epidemiology. 3rd edition, 46–65. London: Blackwell Science Ltd. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data substantiating the conclusions of this research are accessible and may be acquired from the corresponding author, Emikpe Obukowho Benjamin, upon a reasonable inquiry. Researchers or individuals seeking access to the data for further research or validation may contact the appropriate author to discuss the specifics of the request.


