ABSTRACT
Aquatic ecosystems are often negatively affected by invasive species. However, biotic resistance by native species, either by competition or predation, can reduce the impacts of invasions by non‐native species. The Western Mosquitofish (Gambusia affinis) is one of the most impactful invasive species of freshwater fish and cause declines in native fish populations. Using two mesocosm experiments conducted in different years, we examined the ecological interactions between juveniles of the native fish, Bluegill Sunfish (Lepomis macrochirus), and adults of the invasive fish, G. affinis. We found evidence for interactions between L. macrochirus and G. affinis. However, interactions did not appear symmetric, with L. macrochirus generally more affected by intraspecific interactions than interspecific interactions whereas G. affinis was more affected by interspecific interactions than intraspecific interactions. The presence of either species of fish led to a decrease in the number of large zooplankton and a tendency for a decrease in the total number of zooplankton. Based on these results, native L. macrochirus appear to be able to reduce the ability of non‐native G. affinis to establish or maintain populations through both competition and predation (i.e., acting as an intraguild predator). The consistency of our results across both experiments, with their different designs and their occurring in different years, gives weight to these conclusions. The reduction of or prevention of establishment of populations of invasive G. affinis would likely benefit the aquatic communities of ponds with fish, especially small‐bodied native fish.
Keywords: biotic resistance, competition, Gambusia affinis, intraguild predation, invasive species, Lepomis macrochirus
Native Bluegill Sunfish had important competitive impacts on invasive Western Mosquitofish and also had intraguild predation effects that prevented successful recruitment of Western Mosquitofish. In contrast, Bluegill Sunfish were affected by intraspecific effects more than by interspecific effects from Western Mosquitofish. Our results suggest that native Bluegill Sunfish might provide a degree of biotic resistance to invasion by Western Mosquitofish in small temperate ponds.
1. Introduction
Invasive species are a significant and widespread threat to aquatic ecosystems (Cambray 2003; Havel et al. 2015; Gangloff, Edgar, and Wilson 2016). The introduction of non‐native species, especially fish, into freshwater ecosystems can have unpredictable and extensive impacts on the ecosystem and native species (Cambray 2003). Predation and competition are the two most important impacts of non‐native fish on native ecosystems (Van der Veer and Nentwig 2015), and more observations on the interactions between native and non‐native fishes, especially their competitive interactions, are needed (García‐Berthou 2007; Almeida and Grossman 2012).
Western Mosquitofish (Gambusia affinis) and Eastern Mosquitofish (G. holbrookii) are two of the most invasive species of freshwater fish (Copp et al. 2009). These two species of mosquitofish are hardy and aggressive, adapt easily to a variety of environments, and reach high population densities rapidly, making them effective invaders (Pyke 2008; Rehage et al. 2020). The invasion of Gambusia spp. appears to have led to the extirpation, population declines, of native fish, or shifts in the distributions of native fishes (Goren and Galil 2005; Habit et al. 2010; Schumann et al. 2016; Ennen et al. 2021). The negative effects of mosquitofish on native fishes can arise due to competition, aggressive interactions, or predation (e.g., Mills, Rader, and Belk 2004; Goldsworthy and Bettoli 2006; Sutton, Zeiber, and Fisher 2013; Wedderburn et al. 2020). In particular, the consumption of the eggs or young of other fishes by G. affinis appears to often explain their negative impacts on native fish populations and communities (e.g., Rogowski and Stockwell 2006; Ayala et al. 2007; Laha and Mattingly 2007; Schumann, Hoback, and Koupal 2015). Indeed, the presence of G. affinis reduced the ability of some native fishes to produce juveniles or successfully reproduce (Sutton, Zeiber, and Fisher 2009; Goodchild and Stockwell 2016). For example, the survivorship of juvenile Iotichthys phlegethontis was reduced by a third in the presence of G. affinis, and at high G. affinis density, young‐of‐year Iotichthys phlegethontis had 100% mortality (Mills, Rader, and Belk 2004).
Biotic resistance can lead to failed invasions by non‐native species (Zenni and Nuñez 2013). In particular, native predators can limit the ability of invasive species to invade (e.g., de Rivera et al. 2005; Verhelst et al. 2016; Flaherty and Lawton 2019). Indeed, native fish can reduce the ability of invasive species to invade communities through predation (e.g., Britton 2012; Hill 2016; see also Deacon, Fraser, and Farrell 2023; Gu et al. 2023) and biotic resistance to invasive species in freshwater habitats appears to be stronger from predation rather than competition (Alofs and Jackson 2014). Native intraguild predators may be especially effective at resisting invasive species (e.g., Tuckett et al. 2021; Deacon, Fraser, and Farrell 2023). For example, intraguild predation by native species that are large enough to consume mosquitofish may limit their successful invasion and allow co‐existence (e.g., Henkanaththegedara and Stockwell 2013, 2014). Native competitor fishes can also resist the invasion of mosquitofish. For example, native Galaxias maculatus may be able to resist invasive G. affinis in clear water conditions due to their better foraging efficiency relative to G. affinis relative to turbid water (Abrahams, Bassett, and Montgomery 2017). Interference or physical harassment of non‐native fish by native fish can also injure and reduce the growth of the invader (Schofield et al. 2021).
Within their native range, G. affinis often co‐occurs with Lepomis spp., including Bluegill Sunfish (L. macrochirus) and Longear Sunfish (L. megalotis; e.g., Gelwick et al. 2001; Parham 2009; Matthews and Marsh‐Matthews 2011; Fisher, Kelso, and Rutherford 2012). In addition, L. macrochirus and other Lepomis spp. can use the same ponds and waterbodies as G. affinis in areas where both taxa are introduced (Moyle and Nichols 1973; Lynch 1988). The co‐occurrence of Lepomis spp. and G. affinis can persist within their native ranges (Hargrave and Taylor 2010) and in the non‐native range of G. affinis (Lynch 1988; Burskey and Simon 2008).
Native Lepomis species, including L. macrochirus, might affect the ability of G. affinis to establish or maintain populations. Both fish species have been observed to exploit similar food resources, including zooplankton and aquatic macroinvertebrates (Hurlbert and Mulla 1981; Lazzaro et al. 1992; Rettig 2003; Rettig et al. 2023), and both species are known to have significant effects on zooplankton communities (Nowlin and Drenner 2000; Fryxell et al. 2015; Geyer, Smith, and Rettig 2016; Rettig and Smith 2021). This dietary overlap may result in competition between L. macrochirus and mosquitofish, especially juvenile L. macrochirus. Previous studies have demonstrated this potential interaction between Lepomis sp. and Gambusia sp. For example, Green Sunfish (Lepomis cyanellus) and G. affinis compete, with both species negatively affected by the presence of the other, and their presence reduces the abundance and richness of invertebrates (Blaustein 1991). The presence of L. cyanellus also causes a habitat shift in G. affinis (Blaustein 1991). The presence of juvenile L. macrochirus reduced the foraging efficiency of male G. affinis, potentially reducing resource use, but not of females, and also reduced the aggressive interactions of G. affinis (Clemmer and Rettig 2019). However, in their native ranges, dollar sunfish (Lepomis marginatus) had no effect on G. holbrooki (Schofield et al. 2014).
The presence of vegetation can sometimes mediate the interactions among fishes. Vegetation can reduce predation risk for prey fish (e.g., Werner and Hall 1988; Santos et al. 2009; Alexander et al. 2015), the trophic structure of fish communities (Carey et al. 2010), or the growth of the fish (e.g., Crowder and Cooper 1982; Carey et al. 2010). However, interactions of G.a ffinis with other fish, including L. cyanellus, are sometimes not affected by vegetation or habitat complexity (Simkins and Belk 2017). Lepomis macrochirus are associated with submerged vegetation in some lakes (Werner, Hall, and Werner 1978; Kraus and Jones 2012), especially juveniles (Collingsworth and Kohler 2010). Gambusia spp. can also be found in submerged vegetation (Kraus and Jones 2012). Thus, the presence of vegetation or habitat structure might influence the interactions between L. macrochirus and G. affinis.
Using two mesocosm experiments conducted in two different years, we examined the effects of juvenile L. macrochirus and adult G. affinis on the growth and survival of each other. Our first experiment was conducted in the presence of artificial vegetation and the resource base for the fishes was limited to zooplankton. In our second experiment, we manipulated the presence and absence of artificial vegetation to examine the potential influence of vegetation on interactions between these two species. We also used more realistic vegetation in this second experiment. In addition, we allowed colonization of the mesocosm by insects and amphibians throughout the second experiment to more closely mimic the resource base for the fishes that would be found in natural ponds. The differences between the experiments thus allow us to potentially generalize our results more than either experiment on its own.
Because mosquitofish are known to be aggressive competitors and have been observed to reduce growth and cause high mortality in juvenile populations of other fish species (Laha and Mattingly 2007; Mills, Rader, and Belk 2004), we predicted that L. macrochirus would experience reduced growth in the presence of mosquitofish due to competition over a shared zooplankton resource (and insects and amphibians in Experiment 2). Also, because juvenile L. macrochirus prefer vegetated habitat with sufficient cover to avoid other fish (Casterlin and Reynolds 1978), we predicted that L. macrochirus that were exposed to both G. affinis and vegetation in Experiment 2 would experience a smaller decline in growth than L. macrochirus in the presence of G. affinis only. In addition, given the potential for resource competition and aggressive interactions between G. affinis and juvenile L. macrochirus, we predicted that G. affinis would show lower individual growth rates and survivorship in the presence of L. macrochirus compared to intraspecific competition.
2. Materials and Methods
We conducted our two mesocosm experiments in two different years at the Experimental Pond Array facility at the Denison University Biological Reserve, Granville, Licking County, Ohio. Below we describe the establishment of these two unique but similar experiments.
2.1. Experiment 1 (2010)
We filled 42 mesocosms (1136 L capacity, 63.5 cm height, 175 cm diameter) with well water to a depth of 44 cm and covered each with fiberglass screening (1 mm mesh) to prevent colonization by insects and amphibians. On 17 May, we added 2.5 L of water from three small local ponds (Middleton, Ebaugh, and Olde Minnow) to each mesocosm. On 21 May, we added 1.25 L aliquots of zooplankton collected using 3 1‐m vertical tows of a 153 μm zooplankton net per 3.78 L from Ebaugh Pond. These three ponds differ in the fish community present, as well as their size and productivity, and therefore provide a variety of zooplankton communities (Rettig, Schuman, and McCloskey 2006). By using these three ponds, we hoped to provide a diverse zooplankton community in the mesocosms. We added an additional 1.25 L aliquot of zooplankton collected using 4 1‐m vertical tows of a 153 μm zooplankton net per 3.78 L from Olde Minnow Pond on 24 May. On 27 May, we added artificial vegetation (strands of plastic rope) to each mesocosm at a density that visually approximated the density observed in local ponds. Rope has been used successfully in previous experiments to simulate aquatic vegetation (Savino and Stein 1982). We added a final 1 L aliquot of zooplankton collected using 3 1.5‐m vertical tows of a 153 μm zooplankton net per 3.78 L from Middleton Pond to each mesocosm on 28 May. We did not include leaf litter that is often used in mesocosm experiments to provide nutrients or substrates for zooplankton and algae. However, other mesocosm experiments we have perfomed using similar methods (i.e., no leaf litter) found sufficient zooplankton and algal resources to support the fish densities used in our experiment, even during less productive months (e.g., Geyer, Smith, and Rettig 2016). The lack of the additional resources might have reduced the abundance of zooplankton and algae in the mesocosms and thus would increase the potential for competition; however, fish in similar mesocosm experiments rapidly lowered zooplankton abundances to very low levels even with leaf litter (Rettig and Smith 2021; Rettig, Teeters, and Smith 2021). In addition, it seems unlikely that the lack of leaf litter had a major effect on our results since both species showed positive changes in body size or recruitment, as well as high survivorship, in at least some treatments (see Results), suggesting that fish had access to sufficient zooplankton resources in the experiments.
We randomly assigned each mesocosm to one of the seven treatments (each replicated six times): control (no fish), low density G. affinis (4 fish; 2 males, and 2 females), high density G. affinis (8 fish; 4 males, and 4 females), low density juvenile L. macrochirus (4 fish), high density juvenile L. macrochirus (8 fish), low density mixed species (2 G. affinis [1 male, 1 female], 2 juvenile L. macrochirus), and high density mixed species (4 G. affinis [2 males, 2 females], 4 juvenile L. macrochirus). The total length ranged from approximately 25–30 mm for male G. affinis, 30–40 mm for female G. affinis, and 35–65 mm for juvenile L. macrochirus. These densities are in the range of densities of these species in local ponds (Rettig and Arrington, n.d.). In addition, previous mesocosm experiments involving L. macrochirus or G. affinis from these populations found that the densities (or lower) and body sizes used in our experiment resulted in reductions in zooplankton abundances and affected insect and amphibian abundance or colonization, while demonstrating relatively high survivorship (G. affinis, Christenson et al. 2014; Geyer, Smith, and Rettig 2016; Smith and Harmon 2019; Harmon and Smith 2021; Rettig and Smith 2021; L. macrochirus: Smith et al. 2016; Rettig, Teeters, and Smith 2021), suggesting these are reasonable densities for our experiments. This design allowed us to examine the effects of both intra‐ and interspecific competition. On 4 June, we began the experiment by adding the appropriate number and type of fish to each mesocosm. We collected L. macrochirus using a seine from Middleton Pond and captured G. affinis using dip nets from Olde Minnow Pond. We recorded the total length (TL) and mass of each G. affinis and L. macrochirus prior to stocking.
Using tube samplers (Rettig 2003; Aday et al. 2005; Chase et al. 2009), we collected zooplankton samples from three predetermined spots in each mesocosm on five sampling dates: 2 June, 9 June, 16 June, 30 June, and 14 July. Water collected from tube samplers (0.68 L) was filtered through a 63 μm mesh sieve and the filtered zooplankton was pooled for each mesocosm and preserved in acid Lugol's solution. We subsequently used a Nikon SMZ800 dissecting microscope (Nikon Instruments Inc., Melville, New York) to identify and count the zooplankton. We counted all zooplankton in the sample which included cladocerans (Alona, Bosmina, Ceriodaphnia, Chydorus, Scaphaloberus, and Simocephalus), copepods (both cyclopoid and calanoid), rotifers (Brachionus, Keratella, Lecane, Platyias), and ostracods. We divided the counts by the volume of water sampled to calculate the number of zooplankton per liter.
On 15 July, we removed all surviving fish from the mesocosms and recorded the number of fish and measured their TL and final mass. At the end of the experiment, we discovered that one of the low density, mixed fish mesocosms had inadvertently been stocked with too many L. macrochirus and so was excluded from all analyses. We calculated a survivorship/recruitment index (SRI) for G. affinis by dividing the number of individuals (including adults, juveniles, and larvae) present in each mesocosm at the end of the experiment by the initial number of adults introduced into each mesocosm to account for recruitment in the mesocosms (the juvenile L. macrochirus did not reproduce in the experiment). Newly recruited G. affinis were easy to distinguish from the adults that were stocked at the beginning of the experiment since the recruits were much smaller and had a different appearance compared to the adults (i.e., they were obviously fry). We also calculated a body condition index (BCI) by dividing body mass by TL and mass change by subtracting the mean initial mass in each mesocosm from the mean final mass of L. macrochirus.
We used two‐way analyses of variance to examine the effects of competition type (intraspecific vs. interspecific) and fish density (low vs. high) and their interaction on body size or growth, BCI, and survivorship (arcsin square root transformed) or SRI for each fish species. For analyses of count data (e.g., total number of G. affinis at the end of the experiment), we used a generalized linear model with a Poisson distribution and log link. We used a one‐way ANOVA to compare the numbers of total zooplankton (cladocerans, copepods, rotifers, ostracods)and large zooplankton (Scaphaloberus, Daphnia, Ceriodaphnia, and Simocephalus) in the initial sample (2 June) prior to the implementation of treatments. We used a one‐way repeated‐measures analysis of variance to examine the effects of experimental treatments (including the fishless control) on the total number of zooplankton (L−1) and the number of large zooplankton (L−1). We used JMP Pro 16.0 (SAS Institute, Cary, NC) for the statistical analyses. We used an α‐value of 0.05 for significance. Means are given ±1 S.E.
2.2. Experiment 2 (2012)
We filled 48 mesocosms (1136 L capacity, 63.5 cm height, 175 cm diameter) with well water to a depth of 44 cm and covered each with a fiberglass screen (1 mm mesh). We inoculated each tank with a 2 L aliquot of pond water from Olde Minnow Pond on 11 May. We collected zooplankton from Middleton Pond using vertical tows at a depth of 2 m with a 153 μm net and added a 370 mL aliquot (the equivalent of one vertical tow at 2 m depth) of zooplankton and pond water to each mesocosm on each of three consecutive days (22–24 May), as well as on 6 June.
We assigned each mesocosm to one of eight treatment combinations (each replicated six times) based on the manipulation of three variables: the presence or absence of vegetation, the presence or absence of juvenile L. macrochirus, and the presence or absence of adult G. affinis. On 13 June, we placed plastic aquarium plants and strands of yellow rope into the vegetation present treatments, to simulate vegetation. Artificial plants appear to support similar numbers of macroinvertebrates as natural plants (Gerrish and Bristow 1979), and G. affinis do not appear to differentiate between real and artificial plants (Casterlin and Reynolds 1977).
On 14 June, we started the experiment by adding two juvenile L. macrochirus (total length = 34–65 mm) to each L. macrochirus mesocosm and four adult G. affinis to each G. affinis mesocosm. Initially, we stocked two male (total length = 24–29 mm) and two female (total length = 31–41 mm) G. affinis but due to high male mortality early in the experiment and the limited availability of male G. affinis, some of the males were replaced with females. Male mortality may have been higher than females because they are smaller and we have noted that their gonopodium can be easily damaged during handling. We collected juvenile L. macrochirus by seining at Middleton Pond and G. affinis by dipnetting in Olde Minnow Pond. We recorded the TL and mass of each L. macrochirus and the TL of each G. affinis prior to stocking into the mesocosms. To allow invertebrate and amphibian colonization throughout the experiment, we did not cover mesocosms. By the end of the experiment, the mesocosms were colonized by Gray Treefrogs (Hyla versicolor) and a variety of insects (Corixidae, Dytiscidae, Gerridae, Hydrophilliidae, and Notonectidae).
We collected zooplankton samples from each tank on six sampling dates: 14, 18, 21, 28 June, 9, and 12 July using the same methods as in Experiment 1. We counted and identified zooplankton using the methods used in Experiment 1.
On 13 July, we removed and counted surviving fish from mesocosms and recorded the TL and final mass of each fish. We calculated SRI and BCI for each species as in Experiment 1. We calculated changes in TL or BM for L. macrochirus and G. affinis by subtracting initial mean TL or BM from the final TL or BM, respectively.
We used two‐way analyses of variance to examine the effects of competitor presence or absence and vegetation treatments and their interaction on each fish species, on change of total length, change of mass, change of BCI, and survivorship (arcsin square root transformed) for L. macrochirus and SRI, number of larvae, and change of total length for G. affinis. For analyses of count data (e.g., number of larval G. affinis), we used a generalized linear model with a Poisson distribution and log link. We used a three‐way ANOVA to compare the initial numbers of total zooplankton (cladocerans, copepods, rotifers, ostracods) and large zooplankton (Scaphaloberus, Daphnia, Ceriodaphnia, and Simocephalus) on the initial sample (2 June) prior to the implementation of treatments. We used a three‐way repeated‐measures analysis of variance to examine the effects of vegetation, L. macrochirus, and G. affinis treatments on the total number of zooplankton (L−1) and the number of large zooplankton (L−1). We used JMP Pro 16.0 (SAS Institute, Cary, NC) for the statistical analyses. We used an α‐value of 0.05 for significance. Means are given ±1 S.E.
3. Results
3.1. Experiment 1
3.1.1. Lepomis macrochirus
Lepomis macrochirus survivorship was not affected by competition type (Figure 1A; Table 1). Fish density had no effect on L. macrochirus survivorship (Figure 1A; Table 1). The interaction between competition type and fish density had no effect on the survivorship of L. macrochirus (Figure 1A; Table 1).
FIGURE 1.
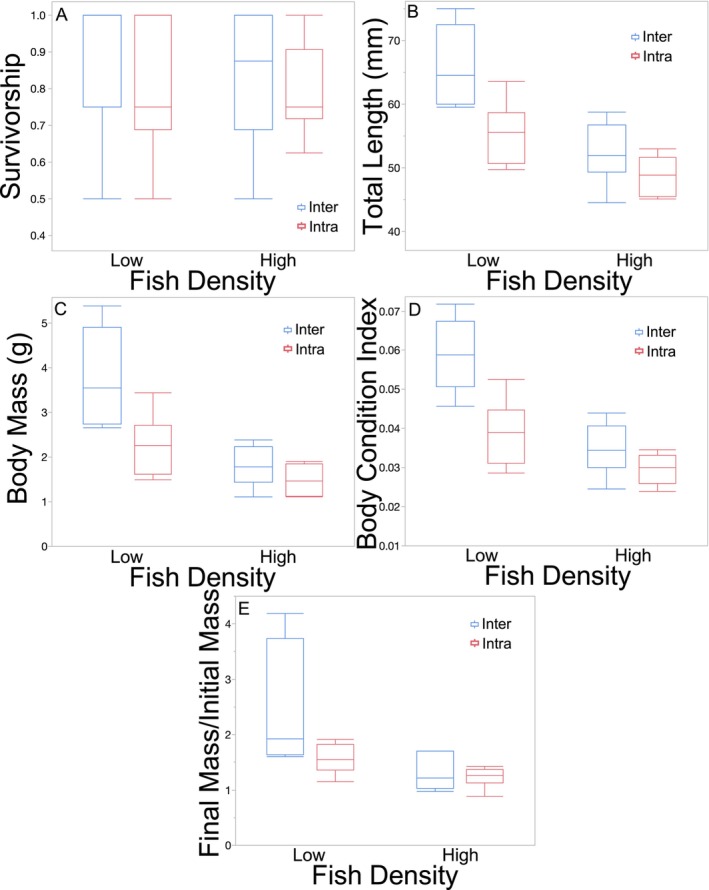
Boxplots with outliers of (A) survivorship, (B) total length, (C) body mass, (D) body condition index, and (E) body mass change of Lepomis macrochirus as a function of fish density and the presence (interspecific competition) or absence (intraspecific competition) of Gambusia affinis in Experiment 1.
TABLE 1.
Results of two‐way analyses of variance examining the effects of competition type (interspecific vs. intraspecific) and fish density (low or high) on the survivorship, total length (TL), mass, body condition index (BCI), and mass change of Lepomis macrochirus in Experiment 1.
| df | Survivorship | TL | Mass | BCI | Mass change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| Competition type | 1 | 1.89 | 0.19 | 11.79 | 0.0028 | 9.68 | 0.0058 | 16.94 | 0.0006 | 4.68 | 0.043 |
| Density | 1 | 0.51 | 0.48 | 24.18 | < 0.0001 | 22.12 | 0.0002 | 29.5 | < 0.0001 | 10.31 | 0.0046 |
| Competition type × Density | 1 | 0.17 | 0.68 | 2.85 | 0.11 | 4.12 | 0.057 | 5.92 | 0.025 | 3.42 | 0.08 |
| Error | 19 | ||||||||||
Mean L. macrochirus TL was 12% greater under interspecific competition than under intraspecific competition (Figure 1B; Table 1). Lepomis macrochirus in the high fish density mesocosms had a mean TL that was 10 mm smaller than those in low fish density mesocosms (Figure 1B; Table 1). The interaction between competition type and fish density was not significant (Figure 1B; Table 1).
Mean L. macrochirus mass was 44% greater in the interspecific competition treatment than the intraspecific competition treatment (Figure 1C; Table 1). Mean L. macrochirus mass in the high fish density mesocosms was almost 50% of the mean in low fish density mesocosms (Figure 1C; Table 1). There was a tendency for fish density to have a greater effect under interspecific competition than intraspecific competition (Figure 1C; Table 1).
Mean BCI of L. macrochirus was 35% greater under interspecific competition than intraspecific competition (Figure 1D; Table 1). Mean BCI was greater at low fish density than that at high fish density (Figure 1D; Table 1). The effect of fish density was greater under interspecific competition than intraspecific competition (Figure 1D; competition type*fish density interaction; Table 1).
Mean mass change of L. macrochirus was 34% greater under interspecific competition than under intraspecific competition (Figure 1E; Table 1). Mean mass change of L. macrochirus was almost 60% greater at low fish density than at high fish density (Figure 1E; Table 1). The interaction between competition type and fish density was not significant, but there was a tendency for the effect of competition type to be stronger at low density than at high density (Figure 1E; Table 1).
3.1.2. Gambusia affinis
The total number of G. affinis in a mesocosm at the end of the experiment was four times greater in intraspecific competition mesocosms than in interspecific competition mesocosms (Figure 2A; χ2 1 = 55.2, p < 0.0001). Fish density did not affect the total number of G. affinis in a mesocosm at the end of the experiment (Figure 2A; χ2 1 = 0.60, p = 0.44). The interaction of competition type and fish density was significant with the difference between intra‐ and interspecific competition mesocosms greater at low density (Figure 2A; χ2 1 = 6.52, p = 0.01).
FIGURE 2.
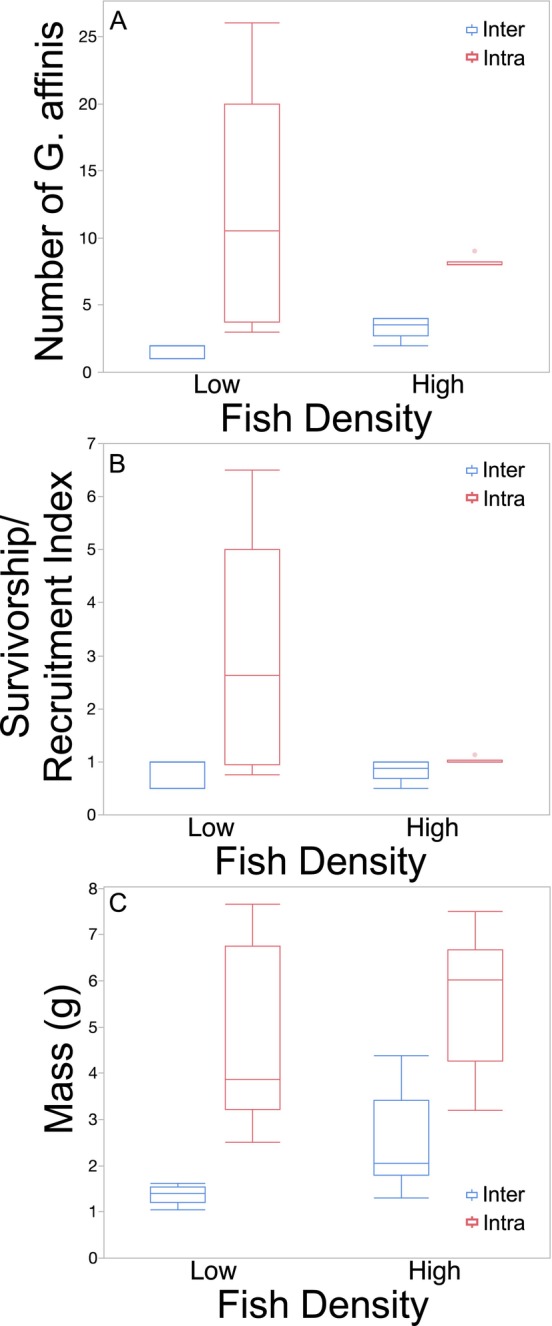
Boxplots with outliers of (A) number at end of experiment, (B) survivorship/recruitment index, and (C) total mass of Gambusia affinis as a function of fish density and the presence (interspecific competition) or absence (intraspecific competition) of Lepomis macrochirus in Experiment 1.
The mean SRI of G. affinis in the intraspecific competition mesocosms was more than two times the mean SRI in the interspecific competition mesocosms (Figure 2B; Table 2). The SRI tended to be higher at low fish density than high fish density, but this was not statistically significant (Figure 2B; Table 2). There was also a nearly significant tendency for the SRI to be low in the interspecific treatments (at both low and high fish density), low in the high fish density intraspecific treatments, and higher in the low density intraspecific treatments (Figure 2B; Table 2).
TABLE 2.
Results of two‐way analyses of variance examining the effects of competition type (interspecific vs. intraspecific) and fish density (low or high) on survivorship‐recruitment index (SRI) and total mass at the end of the experiment (mass) of G. affinis in Experiment 1.
| dfs | SRI | Mass | |||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Competition type | 1 | 5.22 | 0.034 | 30.09 | < 0.0001 |
| Density | 1 | 3.47 | 0.078 | 3.17 | 0.09 |
| Competition type × Density | 1 | 3.71 | 0.069 | 0.0093 | 0.92 |
| Error | 19 | ||||
The total mass of G. affinis in a mesocosm at the end of the experiment was almost 2.5 times greater in the intraspecific competition mesocosms than in the interspecific competition mesocosms (Figure 2C; Table 2). Total mass of G. affinis did not differ between low and high fish density (Figure 2C; Table 2). The interaction of competition type and fish density was not significant (Figure 2C; Table 2).
3.1.3. Zooplankton
For the initial zooplankton samples on 2 June, there were no differences among treatments in the mean total number of zooplankton per liter (F 6,34 = 0.22, p = 0.97). The overall mean (±1 S.E.) number of total zooplankton at the start of the experiment was 588.7 ± 47.4 individuals L−1. There was also no difference in the mean number of large zooplankton per liter among the treatments (F 6,34 = 0.44, p = 0.85). The overall mean (±1 S.E.) number of large zooplankton at the start of the experiment was 104.9 ± 12.8 individuals L−1.
Across the experiment, the total abundance of zooplankton did not differ among treatments (Table 3). The total abundance of zooplankton did not change over the course of the experiment (Figure 3A; Table 3). There was no interaction between time and treatment (Table 3). For large zooplankton, the control treatment (i.e., no L. macrochirus or G. affinis) had significantly more individuals per liter compared to all treatments with fish (Figure 3B; Table 3). The abundance of large zooplankton declined over the course of the experiment (Figure 3B; Table 3). The number of large zooplankton in the control mesocosms was greater for all sampling dates except the last sampling date, 14 July (Figure 3B; sampling date * treatment interaction; Table 3).
TABLE 3.
Results of repeated measures analyses of variance examining the effects of treatment combinations of fish species composition and density on the total number of zooplankton and the number of large zooplankton (Scaphaloberis, Daphnia, Ceriodaphnia, and Simocephalus) over the course of Experiment 1.
| dfs | Total zooplankton | Large zooplankton | |||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Treatment | 6,34 | 0.52 | 0.79 | 11.51 | < 0.0001 |
| Time | 3102 | 2.32 | 0.08 | 6.72 | 0.0003 |
| Time × Treatment | 18,102 | 0.79 | 0.70 | 1.77 | 0.040 |
FIGURE 3.
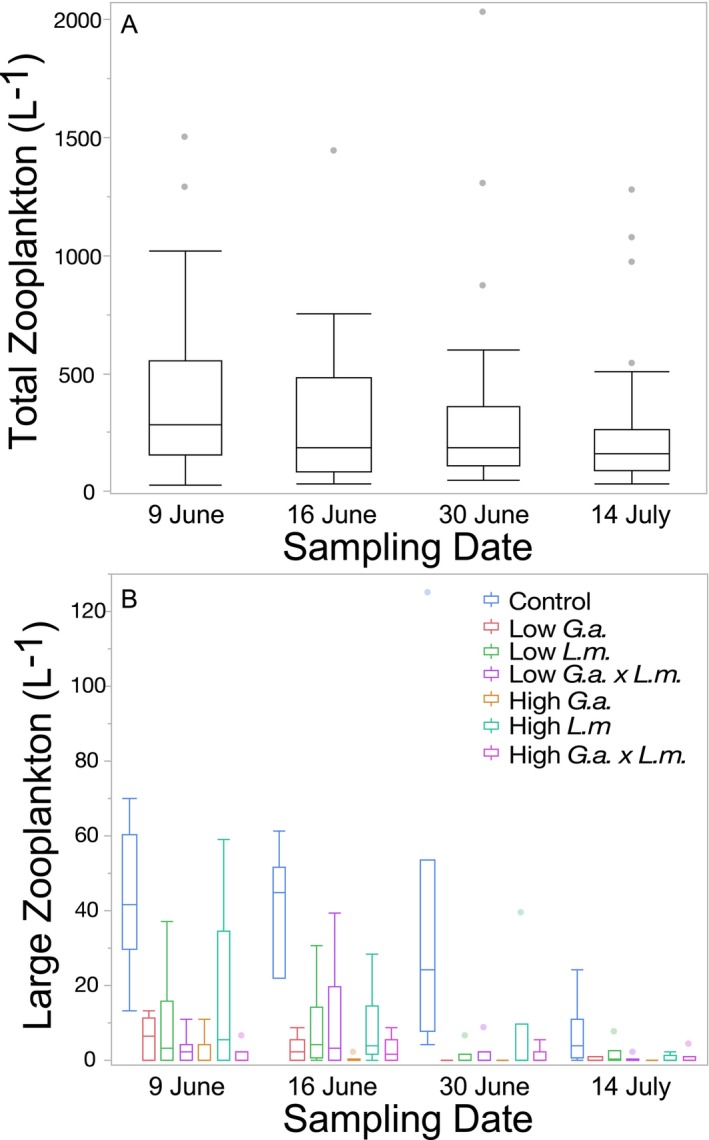
Boxplots with outliers of (A) total number of zooplankton per liter over the course of Experiment 1 and (B) the number of large zooplankton per liter in each treatment over the course of Experiment 1.
3.2. Experiment 2
3.2.1. Lepomis macrochirus
Survivorship of L. macrochirus was generally high (overall = 0.96 ± 0.029). Survivorship of L. macrochirus was not affected by G. affinis treatment (Figure 4A; Table 4), vegetation treatment (Figure 4A; Table 4), or their interaction (Figure 4A; Table 4).
FIGURE 4.

Boxplots with outliers of (A) survivorship, (B) growth in total length, (C) body mass change, and (D) change in body condition index (BCI) of Lepomis macrochirus as a function of the presence or absence of Gambusia affinis and the presence or absence of vegetation in Experiment 2.
TABLE 4.
Results of two‐way analyses of variance examining the effects of the presence or absence of Gambusia affinis and the presence or absence of vegetation on the survivorship, growth in total length (growth), mass change, and BCI change of Lepomis macrochirus in Experiment 2.
| dfs | Survivorship | Growth | Mass change | BCI change | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| G. affinis | 1 | 2.5 | 0.13 | 1.26 | 0.28 | 3.58 | 0.073 | 3.40 | 0.08 |
| Vegetation | 1 | 2.5 | 0.13 | 0.19 | 0.67 | 1.35 | 0.26 | 1.60 | 0.22 |
| G. affinis × Vegetation | 1 | 2.5 | 0.13 | 0.19 | 0.67 | 0.25 | 0.62 | 0.097 | 0.76 |
| Error | 20 | ||||||||
Mean TL growth of L. macrochirus was not affected by the presence or absence of G. affinis (Figure 4B; Table 4). Mean TL growth of L. macrochirus was not affected by vegetation (Figure 4B; Table 4). The interaction of vegetation and G. affinis presence was not significant (Figure 4B; Table 4).
Mean mass change for L. macrochirus did not differ between mesocosms with and without G. affinis (Figure 4C; Table 4). Mean mass change for L. macrochirus did not differ between mesocosms with and without vegetation (Figure 4C; Table 4). The interaction between vegetation and G. affinis presence was not significant (Figure 4C; Table 4).
The mean change in BCI was not affected by the presence or absence of G. affinis (Figure 4D; Table 4). The mean change in BCI was not affected by vegetation (Figure 4D; Table 4). The interaction between vegetation and G. affinis presence was not significant (Figure 4D; Table 4).
3.2.2. Gambusia affinis
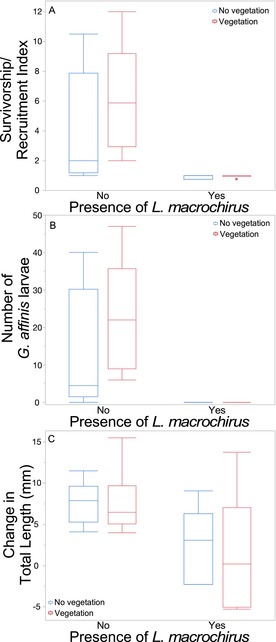
The SRI was over five times greater in mesocosms without L. macrochirus than in mesocosms with L. macrochirus (Figure 5A; Table 5). The SRI of G. affinis was not affected by vegetation (Figure 5A; Table 5). The interaction between vegetation and L. macrochirus was not significant (Figure 5A; Table 5).
FIGURE 5.
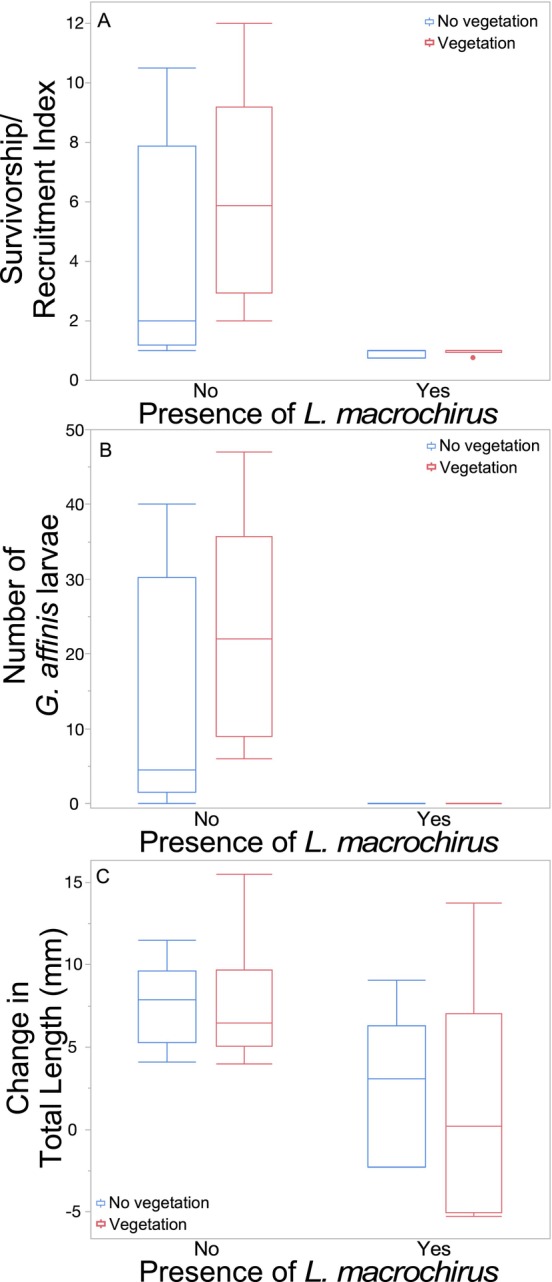
Boxplots with outliers of (A) survivorship/recruitment index, (B) number of larvae, and (C) total length change of Gambusia affinis as a function of the presence or absence of Lepomis macrochirus and the presence or absence of vegetation in Experiment 2.
TABLE 5.
Results of two‐way analyses of variance examining the effects of the presence or absence of Lepomis macrochirus and the presence or absence of vegetation on the survivorship‐recruitment index (SRI) and mean total length change (TL change) of Gambusia affinis in Experiment 2.
| df | SRI | TL change | |||
|---|---|---|---|---|---|
| F | p | F | p | ||
| L. macrochirus | 1 | 13.98 | 0.0013 | 7.94 | 0.01 |
| Vegetation | 1 | 1.07 | 0.31 | 0.11 | 0.74 |
| L. macrochirus × Vegetation | 1 | 0.99 | 0.33 | 0.09 | 0.77 |
| Error | 20 | ||||
There were significantly more G. affinis larvae in mesocosms without L. macrochirus than those with L. macrochirus, indeed no larvae were found in mesocosms with L. macrochirus (Figure 5B; χ2 1 = 283.4, p < 0.0001). The mean number of G. affinis larvae did not differ between mesocosms with and without vegetation (Figure 5B; χ2 1 < 0.001, p = 0.99). The interaction between vegetation and L. macrochirus presence was not significant (Figure 5B; χ2 1 < 0.001, p = 0.99).
Mean TL change in adult G. affinis was almost four times greater in mesocosms without L. macrochirus than in mesocosms with L. macrochirus (Figure 5C; Table 5). However, mean TL change was not affected by vegetation (Figure 5C; Table 5), and the interaction between vegetation and L. macrochirus was not significant (Figure 5C; Table 5).
3.2.3. Zooplankton
For the initial zooplankton samples on 14 June, there were no difference in mean total number of zooplankton per liter between the vegetation treatments (F 1,40 = 0.41, p = 0.53) or the G. affinis treatments (F 1,40 = 0.13, p = 0.72). The overall mean (±1 S.E.) number of total zooplankton at the start of the experiment was 209.7 ± 11.4 individuals L−1. There were more zooplankton in mesocosms assigned to the L. macrochirus present treatment than in mesocosms assigned to the L. macrochirus absent treatment (233.5 ± 17.8 [N = 24] vs. 185.8 ± 13.0 [N = 24]; F 1,40 = 4.24, p = 0.046). None of the interaction terms were significant (all p > 0.45). On 14 June, there were no differences in the mean number of large zooplankton per liter between the vegetation treatments (F 1,40 = 0.38, p = 0.54), the L. macrochirus treatments (F 1,40 = 1.78, p = 0.19), or the G. affinis treatments (F 1,40 = 3.25, p = 0.08). No interaction terms were significant (all p > 0.32). The overall mean (±1 S.E.) number of large zooplankton at the start of the experiment was 117.9 ± 8.8 individuals L−1.
The presence of L. macrochirus did not affect total zooplankton abundance (Figure 6A; Table 6), whereas the presence of G. affinis reduced the total abundance of zooplankton (Figure 6A; Table 6). The vegetation treatments did not affect total zooplankton abundance (Table 6). The interactions of vegetation and L. macrochirus (Table 6) and G. affinis (Table 6) were not significant. The interaction of L. macrochirus and G. affinis was also not significant (Table 6). The three‐way interaction between vegetation, L. macrochirus, and G. affinis was not significant (Table 6). The total abundance of zooplankton varied across the course of the experiment, generally decreasing for the first three sampling dates then peaking during the July 9 sampling date (Figure 6B; Table 6). There was a significant three‐way interaction between time, vegetation, and L. macrochirus, with more zooplankton found in mesocosms with L. macrochirus when there was vegetation present, but in mesocosms without L. macrochirus, there were more zooplankta when vegetation was present; this was only on the 9 July sampling date (Figure 6C; Table 6). No other interactions with time were significant (Table 6).
FIGURE 6.
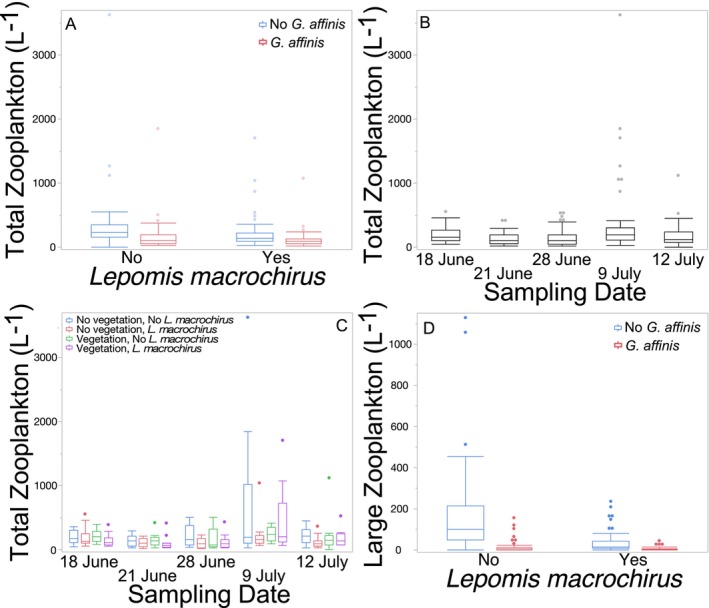
Boxplots with outliers of (A) total number of zooplankton per liter in the presence or absence of Lepomis macrochirus and the presence or absence of Gambusia affinis, (B) total number of zooplankton per liter over the course of the experiment, (C) total number of zooplankton per liter in the presence or absence of vegetation and the presence or absence of L. macrochirus over the course of the experiment, and (D) number of large zooplankton per liter in the presence or absence of L. macrochirus and the presence or absence of G. affinis in Experiment 2.
TABLE 6.
Results of three‐way repeated measures analyses of variance examining the effects of the presence or absence of Lepomis macrochirus (Lm), Gambusia affinis (Ga), and vegetation (Veg) on the number of total zooplankton and large zooplankton (Scaphaloberis, Daphnia, Ceriodaphnia, and Simocephalus) over the course of Experiment 2.
| dfs | Total Zooplankton | Large Zooplankton | |||
|---|---|---|---|---|---|
| F | p | F | p | ||
| L. macrochirus | 1,40 | 3.04 | 0.09 | 11.56 | 0.0015 |
| G. affinis | 1,40 | 12.70 | 0.001 | 31.04 | < 0.0001 |
| Vegetation | 1,40 | 0.25 | 0.62 | 0.07 | 0.80 |
| Lm × Veg | 1,40 | 2.82 | 0.10 | 0.32 | 0.57 |
| Ga × Veg | 1,40 | 0.05 | 0.83 | 0.02 | 0.90 |
| Lm × Ga | 1,40 | 0.47 | 0.50 | 10.10 | 0.0029 |
| Lm × Ga × Veg | 1,40 | 0.30 | 0.59 | 0.12 | 0.73 |
| Time | 4160 | 6.08 | 0.0001 | 0.70 | 0.59 |
| Lm × Time | 4160 | 0.20 | 0.94 | 0.64 | 0.63 |
| Ga × Time | 4160 | 0.56 | 0.69 | 0.40 | 0.81 |
| Veg × Time | 4160 | 0.38 | 0.82 | 0.57 | 0.68 |
| Lm × Veg × Time | 4160 | 2.76 | 0.03 | 0.43 | 0.79 |
| Ga × Veg × Time | 4160 | 0.58 | 0.68 | 0.54 | 0.71 |
| Lm × Ga × Time | 4160 | 0.19 | 0.94 | 0.80 | 0.52 |
| Lm × Ga × Veg × Time | 4160 | 0.69 | 0.60 | 0.43 | 0.79 |
There were fewer large zooplankton in mesocosms with L. macrochirus (Figure 6C; Table 6) and with G. affinis (Figure 6C; Table 6). There was also a significant interaction between L. macrochirus and G. affinis treatments, with G. affinis generally having a greater effect on zooplankton than L. macrochirus (Figure 6D; Table 6). Vegetation had no effect on the number of large zooplankton (Table 6). None of the two‐way or three‐way interactions involving vegetation were significant (Table 6). The abundance of large zooplankton remained relatively constant over the course of the experiment (Table 6), and no interactions with the sampling date were significant (Table 6).
4. Discussion
In our experiments, we found evidence for competitive interactions between L. macrochirus and G. affinis. However, competition did not appear symmetric, with L. macrochirus generally more affected by intraspecific competition than interspecific competition and G. affinis more affected by interspecific competition than intraspecific competition. Additionally, it appears that predation on G. affinis offspring by L. macrochirus and G. affinis (at high density) can impact recruitment of G. affinis. We also found evidence that zooplankton communities were reduced by the fish in the experiment relative to controls, especially the number of large zooplankton taxa, suggesting competition may occur over this potentially limiting resource.
Gambusia affinis were more strongly affected by interspecific interactions than intraspecific interactions, with lower growth rates and SRI with significantly less to virtually no recruitment observed in the interspecific mesocosms in both experiments (i.e., those with L. macrochirus). Since our SRI includes larvae produced by the end of the experiment, the observed decrease in G. affinis SRI indicates a reduction in G. affinis recruitment in mesocosms with L. macrochirus. Our results are consistent with L. macrochirus consuming the offspring of G. affinis in the mesocosms. Indeed, Lepomis spp. can be predators on G. affinis (e.g., Simkins and Belk 2017) as well as small or larval fish (Seaburg and Moyle 1964; Rettig and Mittelbach 2002; Andraso 2005; Carpenter and Mueller 2008), and piscivorous fish (e.g., Micropterus salmoides) can eliminate G. affinis from small ponds (Swingle 1949). In addition, the presence of a predator reduced reproduction of G. holbrooki (Mukherjee et al. 2014). The reduced growth rates of G. affinis in the presence of L. macrochirus also suggest that L. macrochirus competitively affects G. affinis likely due to their effects on the common food items, such as zooplankton, or through interference competition. Previous studies suggest that G. affinis can be negatively affected by competition with other fishes (Taylor, Trexler, and Loftus 2001; Rehage, Lopez, and Sih 2020). Indeed, in both our experiments, the zooplankton communities were affected by the presence of both L. macrochirus and G. affinis, with a noticeable effect of these fish on the number of large zooplankton taxa. The effects of juvenile L. macrochirus on zooplankton and macroinvertebrates communities in lakes have been shown to competitively affect other species of fish (Aday et al. 2005). Lepomis macrochirus can be competitively superior to other native freshwater fishes (Cooper, Wagner, and Krantz 1971), and there is some evidence that L. macrochirus can affect the foraging success of G. affinis via interference (Clemmer and Rettig 2019).
The greater interspecific impacts compared to intraspecific impacts on G. affinis, and the greater intraspecific impacts compared to interspecific impacts on L. macrochirus suggest that L. macrochirus have the potential to control G. affinis populations in ponds. Indeed, in a local pond, we have observed a decline and near extirpation of a population of G. affinis following the “invasion” of another native Centrarchid, L. megalotis (Rettig et al. 2024). However, in a nearby pond, long‐term coexistence of G. affinis and L. macrochirus has been observed in populations that are at low densities due to a winterkill event from which the pond and its fish populations have never fully recovered (Rettig et al. 2024). Our experiments and observations thus suggest that the presence of L. macrochirus may provide biotic resistance to invasive G. affinis in small ponds. In fact, native predatory fish have been shown to be able to control or suppress populations of non‐native fishes (Santos et al. 2009), including G. affinis (Howell et al. 2013). Additionally, the resistance of freshwater fish assemblages to invasion by non‐native fish species may increase with the presence of native fish that consume small or larval non‐native fish (Baltz and Moyle 1993), especially if they are intraguild predators (e.g., Tuckett et al. 2021; Deacon, Fraser, and Farrell 2023). The intraguild predator, Anablepsoides hartii, reduced the ability of another fish, Poecilia reticulata, to colonize, especially at low propagule sizes (Deacon, Fraser, and Farrell 2023).
It appears that L. macrochirus, and other Lepomis, likely serve as intraguild predators on G. affinis in our experiments. While we do not have any direct evidence of predation of L. macrochirus on G. affinis in local ponds or in our experiment, the circumstantial evidence is strong (e.g., the absence of larval G. affinis in mesocosms with L. macrochirus). In addition, several studies have demonstrated a similar effect of L. macrochirus, or other Lepomis, on other species of fish. L. macrochirus consumed the eggs and larvae of invasive Cyprinus carpio and significantly reduced their recruitment (Bajer et al. 2012; Silbernagel and Sorensen 2013; Poole and Bajer 2019). Green Sunfish (L. cyanellus) and Longear Sunfish (L. megalotis) consumed Red Shiner (Cyprinella lutrensis) and Bigeye Shiners (Notropis boops) and prevented the establishment of populations of C. lutrensis (Marsh‐Matthews, Mattews, and Franssen 2011; Marsh‐Matthews et al. 2013). Lepomis gulosus preyed upon juvenile and adult Heterandria formosa and appeared to reduce populations of H. formosa in nature (Richardson, Gunzburger, and Travis 2006).
We also observed some evidence that G. affinis may limit their own populations through cannibalism, at least at high density (and in mesocosms). Adult G. affinis do eat fish hatchlings (Swingle 1949) and can prey upon conspecific fry (Miura, Takahashi, and Stewart 1979; Lee, Simon, and Perry 2018; Rettig et al. 2018, 2023). However, the high incidence of cannibalism in G. affinis in mesocosm experiments may be due to the greater ease of capture in confined spaces in addition to increasing with density (Riesch et al. 2022).
In our experiments, L. macrochirus experienced competitive effects on growth, body size, and body condition, with intraspecific competition appearing stronger than interspecific competition in Experiment 1, and G. affinis had no effects on L. macrochirus in Experiment 2. In neither experiment did competition affect the survivorship of the juvenile L. macrochirus. It appears likely that these effects arose through the impacts of fish treatments on the zooplankton abundance and composition that we observed. Indeed, growth of L. macrochirus appears to be limited by zooplankton availability (Osenberg et al. 1988), and L. macrochirus experience reduced growth under conditions of high fish densities (Osenberg et al. 1988; Shoup et al. 2007).
Although predation on juvenile non‐native fishes by a native predatory fish can be reduced by habitat complexity (Santos et al. 2009), in Experiment 2, we found no effects of vegetation on either L. macrochirus or G. affinis, and no significant interactions between vegetation and density or competition type. Our results are consistent with previous studies which suggest that habitat complexity or vegetation does not always influence the impacts of competition or predation involving G. affinis. For example, the presence of refuge habitats did not influence the predation of L. cyanellus on G. affinis (Simkins and Belk 2017). The presence of refuge habitats almost doubled the survival of Fundulus julisia exposed to G. affinis in experimental conditions, but refuge habitat in the field did not increase the abundance of F. julisia in the presence of G. affinis (Westhoff, Watts, and Mattingly 2013). These results contrast with other studies that have found that the presence of habitat complexity reduces the effects of predatory fishes (e.g., Santos et al. 2009; Alexander et al. 2015). However, the influence of aquatic habitat complexity on intraguild predation relationships of fish can depend on the size of the fish involved, with larger predators being less successful at invading complex environments and smaller predators more successful at invading complex environments (Reichstein et al. 2013). Given that we were studying G. affinis, a relatively small fish, and juvenile L. macrochirus, it may be that the size of the fish contributed to the absence of an effect of vegetation in our experiment. Indeed, our results may have been different if adult L. macrochirus were used. However, juvenile L. macrochirus are found more often in areas of lake that had emergent vegetation than in open water areas (Stahr and Kaemingk 2017), so our use of juveniles in the experiment reflects natural distributions.
5. Conclusions
Our experiments found that juvenile L. macrochirus and G. affinis can compete with each other, with G. affinis being more affected by interspecific competition with L. macrochirus and L. macrochirus being more affected by intraspecific competition, presumably mediated by observed changes to the zooplankton community in the presence of fish. Thus, the competitive interaction is asymmetrical. In addition, it appears that juvenile L. macrochirus can prevent successful recruitment of G. affinis offspring into a population. The consistency of our results across both experiments, with their slightly different designs and their occurrence in different years, gives weight to these conclusions, especially given that Experiment 2 potentially had additional resources available in the form of colonizing macroinvertebrates and amphibians. The most important result of our experiments from a conservation standpoint is that L. macrochirus can potentially resist the invasion of invasive G. affinis through both competition and predation (i.e., acting as an intraguild predator). Through their negative effects on invasive G. affinis via consumption of their young and competition with adults, native L. macrochirus, and potentially other native Lepomis, appear to be able to reduce the likelihood of successful establishment of G. affinis populations (see Marsh‐Matthews, Mattews, and Franssen 2011; Silbernagel and Sorensen 2013; Poole and Bajer 2019; Schofield et al. 2021 for similar examples involving other species of fish). These effects will likely benefit the native aquatic communties of ponds with fish by reducing the densities of G. affinis and thereby limiting their negative effects on native fauna, especially small‐bodied native fish.
Author Contributions
Jessica E. Rettig: conceptualization (lead), formal analysis (supporting), investigation (equal), writing – review and editing (equal). Elizabeth P. Tristano: conceptualization (supporting), formal analysis (supporting), investigation (equal), writing – review and editing (equal). Anthony C. Burger: conceptualization (supporting), formal analysis (supporting), investigation (equal), writing – review and editing (equal). Geoffrey R. Smith: conceptualization (lead), formal analysis (lead), investigation (equal), writing – original draft (lead).
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank R. Jost, J. Harmon, M. Surace, and W. and L. Smith for assistance with the experiments. Funding was provided by the Anderson Endowment of Denison University and the Denison University Research Foundation. Fish were collected under permit from the Ohio Department of Natural Resources. These experiments were conducted with the approval of the Denison University IACUC (09‐007).
Funding: The authors received no specific funding for this work.
Data Availability Statement
All data used in the preparation of this paper are provided in Dryad (https://doi.org/10.5061/dryad.9w0vt4bq8). [https://datadryad.org/stash/share/yq1Oqr7V8Fq7ohqwTlTxIV4ebvGNN1ylzDK8KZ1hWXo].
References
- Abrahams, M. V. , Bassett D. K., and Montgomery J. C.. 2017. “Sensory Biology as a Risk Factor for Invasive Success and Native Fish Decline.” Transactions of the American Fisheries Society 146: 1238–1244. 10.1080/00028487.2017.1353545. [DOI] [Google Scholar]
- Aday, D. D. , Shoup D. E., Neviackas J. A., Kline J. L., and Wahl D. H.. 2005. “Prey Community Responses to Bluegill and Gizzard Shad Foraging: Implications for Growth of Juvenile Largemouth Bass.” Transactions of the American Fisheries Society 134: 1091–1102. 10.1577/T04-073.1. [DOI] [Google Scholar]
- Alexander, M. E. , Kaiser H., Weyl O. L. F., and Dick J. T. A.. 2015. “Habitat Simplification Increases the Impact of a Freshwater Invasive Fish.” Environmental Biology of Fishes 98: 477–486. 10.1007/s10641-014-0278-z. [DOI] [Google Scholar]
- Almeida, D. , and Grossman G. D.. 2012. “Utility of Direct Observational Methods for Assessing Competitive Interactions Between Non‐Native and Native Freshwater Fishes.” Fisheries Management and Ecology 19: 157–166. 10.1111/j.1365-2400.2012.00847.x. [DOI] [Google Scholar]
- Alofs, K. M. , and Jackson D. A.. 2014. “Meta‐Analysis Suggests Biotic Resistance in Freshwater Environments Is Driven by Consumption Rather Than Competition.” Ecology 95: 3259–3270. 10.1890/14-0060.1. [DOI] [Google Scholar]
- Andraso, G. M. 2005. “Summer Food Habits of Pumpkinseeds (Lepomis gibbosus) and Bluegills (Lepomis macrochirus) in Presque Isle Bay, Lake Erie.” Journal of Great Lakes Research 31: 397–404. 10.1016/S0380-1330(05)70271-9. [DOI] [Google Scholar]
- Ayala, J. R. , Rader R. B., Belk M. C., and Schaalje G. B.. 2007. “Ground‐Truthing the Impact of Invasive Species: Spatio‐Temporal Overlap Between Native Least Chub and Introduced Western Mosquitofish.” Biological Invasions 9: 857–869. 10.1007/S1053-006-9087-4. [DOI] [Google Scholar]
- Bajer, P. G. , Chizinski C. J., Silbernagel J. J., and Sorensen P. W.. 2012. “Variation in Native Micro‐Predator Abundance Explains Recruitment of a Mobile Invasive Fish, the Common Carp, in a Naturally Unstable Environment.” Biological Invasions 14: 1919–1929. 10.1007/S10530-012-0203-3. [DOI] [Google Scholar]
- Baltz, D. M. , and Moyle P. B.. 1993. “Invasion Resistance to Introduced Species by a Native Assemblage of California Stream Fishes.” Ecological Applications 3: 246–255. 10.2307/1941827. [DOI] [PubMed] [Google Scholar]
- Blaustein, L. 1991. “Negative Interactions Between Two Predatory Fishes in Rice Fields: Relevance to Biological Control.” Israel Journal of Zoology 37: 164. [Google Scholar]
- Britton, J. R. 2012. “Testing Strength and Biotic Resistance Against an Introduced Fish: Inter‐Specific Competition or Predation Through Facultative Piscivory?” PLoS One 7, no. 2: e313707. 10.1371/journal.pone.0031707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burskey, J. L. , and Simon T. P.. 2008. “Distribution of Fishes of the Muscatatuck National Wildlife Refuge, Indiana.” Proceedings of the Indiana Academy of Sciences 117: 63–70. [Google Scholar]
- Cambray, J. A. 2003. “Impact on Indigenous Species Diversity Caused by the Globalisation of Alien Recreational Freshwater Fisheries.” Hydrobiologia 500: 217–230. 10.1023/A:1024648719995. [DOI] [Google Scholar]
- Carey, M. P. , Maloney K. O., Chipps S. R., and Wahl D. H.. 2010. “Effects of Littoral Habitat Complexity and Sunfish Composition on Fish Production.” Ecology of Freshwater Fish 19: 466–476. 10.1111/j.1600-0633.2010.00433.x. [DOI] [Google Scholar]
- Carpenter, J. , and Mueller G. A.. 2008. “Small Nonnative Fishes as Predators of Larval Razorback Suckers.” Southwestern Naturalist 53: 236–242. 10.1894/0038-4909(2008)53[236:SNFAPO]2.0.CO;2. [DOI] [Google Scholar]
- Casterlin, M. E. , and Reynolds W. W.. 1977. “Aspects of Habitat Selection in the Mosquitofish Gambusia affinis .” Hydrobiologia 55: 125–127. 10.1007/BF00021053. [DOI] [Google Scholar]
- Casterlin, M. E. , and Reynolds W. W.. 1978. “Habitat Selection by Juvenile Bluegill Sunfish, Lepomis macrochirus .” Hydrobiologia 59: 75–79. 10.1007/BF00017607. [DOI] [Google Scholar]
- Chase, J. M. , Biro E. G., Ryberg W. A., and Smith K. G.. 2009. “Predators Temper the Relative Importance of Stochastic Processes in the Assembly of Prey Metacommunities.” Ecology Letters 12: 1210–1218. 10.1111/j.1461-0248.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- Christenson, T. A. , Horton M. E., Jackson B. C., Smith G. R., and Rettig J. E.. 2014. “Effects of Cutrine‐Plus® Algaecide and Predators on Wood Frog (Lithobates sylvaticus) Tadpole Survival and Growth.” Environmental Science and Pollution Research 21: 12472–12478. 10.1007/511356-014-3186-z. [DOI] [PubMed] [Google Scholar]
- Clemmer, J. H. , and Rettig J. E.. 2019. “Native Bluegill Influence the Foraging and Aggressive Behavior of Invasive Mosquitofish.” PeerJ 7: e6203. 10.7717/peerj.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingsworth, P. D. , and Kohler C. C.. 2010. “Abundance and Habitat Use of Juvenile Sunfish Among Different Macrophyte Stands.” Lake and Reservoir Management 26: 35–42. 10.1080/07370651003634380. [DOI] [Google Scholar]
- Cooper, E. L. , Wagner C. C., and Krantz G. E.. 1971. “Bluegills Dominate Production in a Mixed Population of Fishes.” Ecology 52: 280–290. 10.2307/1934586. [DOI] [Google Scholar]
- Copp, G. H. , Vilizzi L., Mumford J., Fenwick G. V., Godard M. J., and Gozlan R. E.. 2009. “Calibration of FISK, an Invasiveness Screening Tool for Nonnative Freshwater Fishes.” Risk Analysis 29: 457–467. 10.1111/j.1539-6924.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Crowder, L. B. , and Cooper W. E.. 1982. “Habitat Structural Complexity and the Interaction Between Bluegills and Their Prey.” Ecology 63: 1802–1813. 10.2307/194022. [DOI] [Google Scholar]
- de Rivera, C. E. , Ruiz G. M., Hines A. H., and Jivoff P.. 2005. “Biotic Resistance to Invasion: Native Predator Limits Abundance and Distribution of an Introduced Crab.” Ecology 86: 3364–3376. 10.1890/05-0479. [DOI] [Google Scholar]
- Deacon, A. E. , Fraser D. F., and Farrell A. D.. 2023. “Resistance From a Resident Heterospecific Affects Establishment Success of a Globally Invasive Freshwater Fish.” Freshwater Biology 68: 425–436. 10.1111/fwb.14035. [DOI] [Google Scholar]
- Ennen, J. R. , Kuhajda B. R., Fix S., et al. 2021. “Assessing the Success of Conservation Efforts for a North American Topminnow at Risk of Extinction From Spatially Variable Mosquitofish Invasions.” Freshwater Biology 66: 458–467. 10.1111/fwb.13652. [DOI] [Google Scholar]
- Fisher, J. C. , Kelso W. E., and Rutherford D. A.. 2012. “Macrophyte Mediated Predation on Hydrilla‐Dwelling Macroinvertebrates.” Fundamental and Applied Limnology 181: 25–38. 10.1127/1863-9135/2012/0174. [DOI] [Google Scholar]
- Flaherty, M. , and Lawton C.. 2019. “The Regional Demise of a Non‐native Invasive Species: The Decline of Grey Squirrels in Ireland.” Biological Invasions 21: 2401–2416. 10.1007/S10530-019-01987-x. [DOI] [Google Scholar]
- Fryxell, D. C. , Arnett H. A., Apgar T. M., Kinnison M. T., and Palkovacs E. P.. 2015. “Sex Ratio Variation Shapes the Ecological Effects of a Globally Introduced Freshwater Fish.” Proceedings of the Royal Society 282B: 20151970. 10.1098/rspb.2015.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff, M. M. , Edgar G. J., and Wilson B.. 2016. “Imperilled Species in Aquatic Ecosystems: Emerging Threats, Management and Future Prognoses.” Aquatic Conservation: Marine and Freshwater Ecosystems 26: 858–871. 10.1002/aqc.2707. [DOI] [Google Scholar]
- García‐Berthou, E. 2007. “The Characteristics of Invasive Fishes: What Has Been Learned So Far?” Journal of Fish Biology 71: 33–55. 10.1111/j.1095-8649.2007.01668.x. [DOI] [Google Scholar]
- Gelwick, F. P. , Akin S., Arrington D. A., and Winemiller K. O.. 2001. “Fish Assemblage Structure in Relation to Environmental Variation in a Texas Gulf Coastal Wetland.” Estuaries 24: 285–296. 10.2307/1352952. [DOI] [Google Scholar]
- Gerrish, N. , and Bristow J. M.. 1979. “Macroinvertebrate Associations With Aquatic Macrophytes and Artificial Substrates.” Journal of Great Lakes Research 5: 69–72. 10.1016/S0380-1330(79)72129-0. [DOI] [Google Scholar]
- Geyer, R. L. , Smith G. R., and Rettig J. E.. 2016. “Effects of Roundup Formulations, Nutrient Addition, and Western Mosquitofish (Gambusia affinis) on Aquatic Communities.” Environmental Science and Pollution Research 23: 11729–11739. 10.1007/s11356-016-6381-2. [DOI] [PubMed] [Google Scholar]
- Goldsworthy, C. A. , and Bettoli P. W.. 2006. “Growth, Body Condition, Reproduction and Survival of Stocked Barrens Topminnows, Fundulus julisia (Fundulidae).” American Midland Naturalist 156: 331–343. 10.1674/0003-0031(2006)156[331:GBCRAS]2.0.CO;2. [DOI] [Google Scholar]
- Goodchild, S. C. , and Stockwell C. A.. 2016. “An Experimental Test of Novel Ecological Communities of Imperiled and Invasive Species.” Transactions of the American Fisheries Society 145: 264–268. 10.1080/00028487.2015.1114520. [DOI] [Google Scholar]
- Goren, M. , and Galil B. S.. 2005. “A Review of Changes in the Fish Assemblages of Levantine Inlandand Marine Ecosystems Following the Introduction of Non‐Native Fishes.” Journal of Applied Ichthyology 21: 364–370. 10.1111/j.1439-0426-2005.00674.x. [DOI] [Google Scholar]
- Gu, D. , Jia T., Wei H., et al. 2023. “Biotic Resistance to Fish Invasions in Southern China: Evidence From Biomass, Habitat, and Fertility Limitation.” Ecological Applications 33: e2819. 10.1002/eap.2819. [DOI] [PubMed] [Google Scholar]
- Habit, E. , Piedra P., Ruzzante D. E., et al. 2010. “Changes in the Distribution of Native Fishes in Response to Introduced Species and Other Anthropogenic Effects.” Global Ecology and Biogeography 19: 697–710. 10.1111/j.1466-8238.2010.00541.x. [DOI] [Google Scholar]
- Hargrave, C. W. , and Taylor C. M.. 2010. “Spatial and Temporal Variation in Fishes of the Upper Red River Drainage (Oklahoma‐Texas).” Southwestern Naturalist 55: 149–159. 10.1894/GG-27.1. [DOI] [Google Scholar]
- Harmon, J. J. , and Smith G. R.. 2021. “Invasive Fish (Gambusia affinis) as an Ecological Filter for Macroinvertebrate Colonization of Experimental Ponds.” Freshwater Ecology 40: 151–161. 10.1086/713006. [DOI] [Google Scholar]
- Havel, J. E. , Kovalenko K. E., Mithomaz S., Amalfitano S., and Kats L. B.. 2015. “Aquatic Invasive Species: Challenges for the Future.” Hydrobiologia 750: 147–170. 10.1007/s10750-014-2166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkanaththegedara, S. M. , and Stockwell C. A.. 2013. “The Role of Gape‐Limitation in Intraguild Predation Between Endangered Mohave Tui Chub and Non‐native Western Mosquitofish.” Ecology of Freshwater Fish 22: 11–20. 10.1111/j.1600-0633.2012.00587.x. [DOI] [Google Scholar]
- Henkanaththegedara, S. M. , and Stockwell C. A.. 2014. “Intraguild Predation May Facilitate Coexistence of Native and Non‐Native Fish.” Journal of Applied Ecology 51: 1057–1065. 10.1111/1365-2664.12285. [DOI] [Google Scholar]
- Hill, J. E. 2016. “Collapse of a Reproducing Population of Non‐native African Jewelfish (Hemichromis Letourneuxi) in a Florida Lake.” NeoBiota 29: 35–52. 10.3897/neobiota.29.7213. [DOI] [Google Scholar]
- Howell, D. H. , Woodford D. J., Weyl O. L. F., and Froneman W.. 2013. “Population Dynamics of the Invasive Fish, Gambusia affinis, in Irrigation Impoundments in the Sundays River Valley, Eastern Cape, South Africa.” Water SA 39: 485–490. 10.4314/wsa.v39.4.6. [DOI] [Google Scholar]
- Hurlbert, S. H. , and Mulla M. S.. 1981. “Impacts of Mosquitofish (Gambusia affinis) Predation on Plankton Communities.” Hydrobiologia 83: 125–151. 10.1007/BF02187157. [DOI] [Google Scholar]
- Kraus, R. T. , and Jones R. C.. 2012. “Fish Abundances in Shoreline Habitats and Submerged Aquatic Vegetation in a Tidal Freshwater Embayment of the Potomac River.” Environmental Monitoring and Assessment 184: 3341–3357. 10.1007/s10661-011-2192-6. [DOI] [PubMed] [Google Scholar]
- Laha, M. , and Mattingly H. T.. 2007. “Ex Situ Evaluation of the Impacts of Invasive Mosquitofish on the Imperiled Barrens Topminnow.” Environmental Biology of Fishes 78: 1–11. 10.1007/s10641-006-9040-5. [DOI] [Google Scholar]
- Lazzaro, X. , Drenner R. W., Stein R. A., and Smith J. D.. 1992. “Planktivores and Plankton Dynamics: Effects of Fish Biomass and Planktivore Type.” Canadian Journal of Fisheries and Aquatic Sciences 49: 1466–1473. 10.1139/f92-161. [DOI] [Google Scholar]
- Lee, F. , Simon K. S., and Perry G. L. W.. 2018. “Prey Selectivity and Ontogenetic Diet Shift of the Globally Invasive Western Mosquitofish (Gambusia affinis) in Agriculturally Impacted Streams.” Ecology of Freshwater Fish 27: 822–833. 10.1111/eff.12395. [DOI] [Google Scholar]
- Lynch, J. D. 1988. “Habitat Utilization by an Introduced Fish, Gambusia affinis, in Nebraska (Actinopterygii: Poeciliidae).” Transactions of the Nebraska Academy of Sciences 16: 63–67. [Google Scholar]
- Marsh‐Matthews, E. , Mattews W. J., and Franssen N. R.. 2011. “Can a Highly Invasive Species Re‐Invade Its Native Community? The Paradox of the Red Shiner.” Biological Invasions 13: 2911–2924. 10.1007/s10530-011-9973-2. [DOI] [Google Scholar]
- Marsh‐Matthews, E. , Thompson J., Mattews W. J., Geheber A., Franssen N. R., and Backstedt J.. 2013. “Differential Survival of Two Minnow Species Under Experimental Sunfish Predation: Implications for Re‐Invasion of a Species Into Its Native Range.” Freshwater Biology 58: 1745–1754. 10.1111/fwb.12165. [DOI] [Google Scholar]
- Matthews, W. J. , and Marsh‐Matthews E.. 2011. “An Invasive Fish Species Within Its Native Range: Community Effects and Population Dynamics of Gambusia affinis in the Central United States.” Freshwater Biology 56: 2609–2619. 10.1111/j.1365-2427.2011.02691.x. [DOI] [Google Scholar]
- Mills, M. D. , Rader R. B., and Belk M. C.. 2004. “Complex Interactions Between Native and Invasive Fish: The Simultaneous Effects of Multiple Negative Interactions.” Oecologia 141: 713–721. 10.1007/s00442-004-1695-z. [DOI] [PubMed] [Google Scholar]
- Miura, T. , Takahashi R. M., and Stewart R. J.. 1979. “Habitat and Food Selection by the Mosquitofish, Gambusia affinis .” Proceedings and Papers of the Annual Conference of the California Mosquito and Vector Control Association 47: 46–50. [Google Scholar]
- Moyle, P. B. , and Nichols R. D.. 1973. “Ecology of Some Native and Introduced Fishes of the Sierra Nevada Foothills in Central California.” Copeia 1973: 478–490. 10.2307/1443113. [DOI] [Google Scholar]
- Mukherjee, S. , Heithaus M. R., Trexler J. C., Ray‐Mukherjee J., and Vaudo J.. 2014. “Perceived Risk of Predation Affects Reproductive Life‐History Traits in Gambusia holbrooki, but Not in Heterandria formosa .” PLoS One 9: e88832. 10.1371/journal.pone.0088832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlin, W. H. , and Drenner R. W.. 2000. “Context‐Dependent Effects of Bluegill in Experimental Mesocosm Communities.” Oecologia 122: 421–426. 10.1007/s004420050048. [DOI] [PubMed] [Google Scholar]
- Osenberg, C. W. , Werner E. E., Mittelbach G. G., and Hall D. J.. 1988. “Growth Patterns in Bluegill (Lepomis macrochirus) and Pumpkinseed (L. gibbosus) Sunfish: Environmental Variation and the Importance of Ontogenetic Niche Shifts.” Canadian Journal of Fisheries and Aquatic Sciences 45: 17–26. 10.1139/f88-003. [DOI] [Google Scholar]
- Parham, R. W. 2009. “Structure of Assemblages and Recent Distribution of Riverine Fishes in Oklahoma.” Southwestern Naturalist 54: 382–399. 10.1894/GG-28.1. [DOI] [Google Scholar]
- Poole, J. R. , and Bajer P. G.. 2019. “A Small Native Predator Reduces Reproductive Success of a Large Invasive Fish as Revealed by Whole‐Lake Experiments.” PLoS One 14: e0214009. 10.1371/Journal.pone.0214009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke, G. H. 2008. “Plague Minnow or Mosquito Fish? A Review of the Biology and Impacts of Introduced Gambusia Species.” Annual Review of Ecology, Evolution, and Systematics 39: 171–191. 10.1146/annurev.ecolsys.39.110707.173451. [DOI] [Google Scholar]
- Rehage, J. S. , Lopez L. K., and Sih A.. 2020. “A Comparison of the Establishment Success, Response to Competition, and Community Impact of Invasive and Non‐invasive Gambusia Species.” Biological Invasions 22: 509–522. 10.1007/s10530-019-02113-7. [DOI] [Google Scholar]
- Rehage, J. S. , Maurer E. F., Lopez L. K., and Sih A.. 2020. “A Closer Look at Invasiveness and Relatedness: Life Histories, Temperature, and Establishment Success of Four Congeners.” Ecosphere 11, no. 11: e03222. 10.1002/ecs2.3222. [DOI] [Google Scholar]
- Reichstein, B. , Schröder A., Persson L., and De Roos A. M.. 2013. “Habitat Complexity Does Not Promote Coexistence in a Size‐Structured Intraguild Predation System.” Journal of Animal Ecology 82: 55–83. 10.1111/j.1365-2656.2012.02032.x. [DOI] [PubMed] [Google Scholar]
- Rettig, J. E. 2003. “Zooplankton Responses to Predation by Larval Bluegill: An Enclosure Experiment.” Freshwater Biology 48: 636–648. 10.1046/j.1365-2427.2003.01035.x. [DOI] [Google Scholar]
- Rettig, J. E. , Burger A. C., Mills L. B., et al. 2024. “When the Invader Becomes the Invaded: Temporal Variation of Gambusia affinis and Centrarchid Sunfish in Two Small Ponds.” Northeastern Naturalist 31: 355–369. 10.1656/045.031.0310. [DOI] [Google Scholar]
- Rettig, J. E. , and Mittelbach G. G.. 2002. “Interactions Between Adult and Larval Bluegill Sunfish: Positive and Negative Effects.” Oecologia 130: 222–230. 10.1007/s004420100790. [DOI] [PubMed] [Google Scholar]
- Rettig, J. E. , Schuman L. S., and McCloskey J. K.. 2006. “Seasonal Patterns of Abundance: Do Zooplankton in Small Ponds Do the Same Thing Every Spring‐Summer?” Hydrobiologia 556: 193–207. 10.1007/s10750-005-1278-y. [DOI] [Google Scholar]
- Rettig, J. E. , and Smith G. R.. 2021. “Relative Strength of Top‐Down Effects of an Invasive Fish and Bottom‐Up Effects of Nutrient Addition in a Simple Aquatic Food Web.” Environmental Science and Pollution Research 28: 5845–5853. 10.1007/s11356-020-10933-7. [DOI] [PubMed] [Google Scholar]
- Rettig, J. E. , Smith G. R., Eng‐Surowiac G., Mirzashvili D., Smyk M., and Hollis J.. 2018. “Consumption of Invasive Western Mosquitofish Fry by Adult Conspecifics and Native Crayfish.” Northeastern Naturalist 25: 117–122. 10.1656/045.025.0109. [DOI] [Google Scholar]
- Rettig, J. E. , Surace M., Rose K. D., Baird A. J., Baker Z. D., and Smith G. R.. 2023. “Variation in the Diets of Western Mosquitofish (Gambusia affinis) in Two Ponds: Effects of Time and Coexistence With Centrarchid Fishes.” Animal Biology 73: 407–421. 10.1163/15707563-bja10118. [DOI] [Google Scholar]
- Rettig, J. E. , Teeters N. R., and Smith G. R.. 2021. “Effects of the Interaction of Bluegill and Two Species of Tadpoles on Experimental Zooplankton Communities.” American Midland Naturalist 186: 95–105. 10.1674/0003-0031-186.1.95. [DOI] [Google Scholar]
- Richardson, J. M. L. , Gunzburger M. S., and Travis J.. 2006. “Variation in Predator Pressure as a Mechanism Underlying Differences in Numerical Abundance Between Populations of the Poecilid Fish Heterandria formosa .” Oecologia 147: 596–605. 10.1007/s00442-005-0306-y. [DOI] [PubMed] [Google Scholar]
- Riesch, R. , Araújo M. S., Bumgarner S., et al. 2022. “Resource Competition Explains Rare Cannibalism in the Wild in Livebearing Fishes.” Ecology and Evolution 12: e8872. 10.1002/ece3.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski, D. L. , and Stockwell C. A.. 2006. “Assessment of Potential Impacts of Exotic Species on Populations of a Threatened Species, White Sands Pupfish, Cyprinodon tularosa .” Biological Invasions 8: 79–87. 10.1007/s10530-005-0238-9. [DOI] [Google Scholar]
- Santos, A. F. G. N. , Santos L. N., García‐Berthou E., and Hayashi C.. 2009. “Could Native Predators Help to Control Invasive Fishes? Microcosm Experiments With the Neotropical Characid, Brycon orbignyanus .” Ecology of Freshwater Fish 18: 491–499. 10.1111/j.1600-0633.2009.00366.x. [DOI] [Google Scholar]
- Savino, J. F. , and Stein R. A.. 1982. “Predator‐Prey Interaction Between Largemouth Bass and Bluegills as Influenced by Simulated, Submerged Vegetation.” Transactions of the American Fisheries Society 111: 255–266. . [DOI] [Google Scholar]
- Schofield, P. J. , Slone D. H., Gregoire D. R., and Loftus W. F.. 2014. “Effects of a Non‐Native Cichlid Fish (African Jewelfish, Hemichromis letourneuxi Sauvage 1880) on a Simulated Everglades Aquatic Community.” Hydrobiologia 722: 171–182. 10.1007/s10750-013-1697-0. [DOI] [Google Scholar]
- Schofield, P. J. , Tuckett Q. M., Slone D. H., Reaver K. M., and Hill J. E.. 2021. “Invasion Frustration: Can Biotic Resistance Explain the Small Geographic Range of Non‐native Croaking Gourami Trichopsis vittata (Cuvier, 1831) in Florida, USA?” Aquatic Invasions 16: 512–526. https://doi.org/10/3391/ai.2021.16.3.08. [Google Scholar]
- Schumann, D. A. , Hoback W. W., and Koupal K. D.. 2015. “Complex Interactions Between Native and Invasive Species: Investigating the Differential Displacement of Two Topminnows Native to Nebraska.” Aquatic Invasions 10: 339–346. 10.3391/ai.2015.10.3.09. [DOI] [Google Scholar]
- Schumann, D. A. , Schoenebeck C. W., Hoback W. W., and Koupal K. D.. 2016. “Fish Assemblage Structure and Single Species Occurrence: Valuable Insight Into Interspecific Interactions of an Unfamiliar Species.” American Midland Naturalist 176: 186–199. 10.1674/0003-0031-176.2.186. [DOI] [Google Scholar]
- Seaburg, K. G. , and Moyle J. B.. 1964. “Feeding Habits, Digestive Rates, and Growth of Some Minnesota Warmwater Fishes.” Transactions of the American Fisheries Society 93: 269–285. 10.1577/1548-8659(1964)93[269:FHDRAG]2.0.CO;2. [DOI] [Google Scholar]
- Shoup, D. E. , Callahan S. P., Wahl D. H., and Pierce C. L.. 2007. “Size‐Specific Growth of Bluegill, Largemouth Bass, and Channel Catfish in Relation to Prey Availability and Limnological Variables.” Journal of Fish Biology 70: 21–34. 10.1111/j.1095-8649.2006.01204.x. [DOI] [Google Scholar]
- Silbernagel, J. J. , and Sorensen P. W.. 2013. “Direct Field and Laboratory Evidence That a Combination of Egg and Larval Predation Controls Recruitment of Invasive Common Carp in Many Lakes of the Upper Mississippi River Basin.” Transactions of the American Fisheries Society 142: 1134–1140. 10.1080/00028487.2013.788889. [DOI] [Google Scholar]
- Simkins, R. M. , and Belk M. C.. 2017. “No Evidence of Nonlinear Effects of Predator Identity, Refuge Availability, or Body Size of Prey on Prey Mortality Rates.” Ecology and Evolution 7: 6119–6124. 10.1002/ece3.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. R. , Burgett A. A., Temple K. G., and Sparks K. A.. 2016. “Differential Effects of Bluegill Sunfish (Lepomis macrochirus) on Two Fish‐Tolerant Species of Tadpoles (Anaxyrus americanus and Lithobates catesbeianus).” Hydrobiologia 773: 77–86. 10.1007/s10750-016-2680-3. [DOI] [Google Scholar]
- Smith, G. R. , and Harmon J. J.. 2019. “Differential Oviposition and Offspring Success of Gray Treefrogs in the Presence of an Invasive Fish.” Ecosphere 10, no. 2: e20612. 10.1002/ecs2.2612. [DOI] [Google Scholar]
- Stahr, K. J. , and Kaemingk M. A.. 2017. “An Evaluation of Emergent Macrophytes and Use Among Groups of Aquatic Taxa.” Lake and Reservoir Management 33: 314–323. 10.1080/10402381.2017.1339747. [DOI] [Google Scholar]
- Sutton, T. M. , Zeiber R. A., and Fisher B. E.. 2009. “Mesocosm Evaluation of Western Mosquitofish Impacts on Northern Starhead Topminnows.” Proceedings of the Indiana Academy of Sciences 118: 88–95. [Google Scholar]
- Sutton, T. M. , Zeiber R. A., and Fisher B. E.. 2013. “Agonistic Behavioral Interactions Between Introduced Western Mosquitofish and Native Top Minnows.” Journal of Freshwater Ecology 28: 1–16. 10.1080/02705060.2012.688492. [DOI] [Google Scholar]
- Swingle, H. S. 1949. “Experiments With Combinations of Largemouth Black Bass, Bluegills, and Minnows in Ponds.” Transactions of the American Fisheries Society 76: 46–62. 10.1577/1548-8659(1946)76[46:EWCOLB]2.0.CO;2. [DOI] [Google Scholar]
- Taylor, R. C. , Trexler J. C., and Loftus W. F.. 2001. “Separating the Effects of Intra‐ and Interspecific Age‐Structured Interactions in an Experimental Fish Assemblage.” Oecologia 127: 143–152. 10.1007/s004420000575. [DOI] [PubMed] [Google Scholar]
- Tuckett, Q. M. , Deacon A. E., Fraser D., Lyons T. J., Lawson K. M., and Hill J. E.. 2021. “Unstable Intraguild Predation Causes Establishment Failure of a Globally Invasive Species.” Ecology 102: e03411. 10.1002/ecy.3411. [DOI] [PubMed] [Google Scholar]
- Van der Veer, G. , and Nentwig W.. 2015. “Environmental and Economic Impact Assessment of Alien and Invasive Fish Species in Europe Using the Generic Impact Scoring System.” Ecology of Freshwater Fish 24: 646–656. 10.1111/eff.12181. [DOI] [Google Scholar]
- Verhelst, P. , Boets P., Van Thuyne G., Verreycken H., Goethals P. L. M., and Mouton A. M.. 2016. “The Distribution of an Invasive Fish Species Is Highly Affected by the Presence of Native Fish Species: Evidence Based on Species Distribution Modelling.” Biological Invasions 18: 427–444. 10.1007/s10530-015-1016-y. [DOI] [Google Scholar]
- Wedderburn, S. D. , Whiterod N. S., Barnes T. C., and Shiel R. J.. 2020. “Ecological Aspects Related to Reintroductions to Avert the Extirpation of a Freshwater Fish From a Large Floddplain River.” Aquatic Ecology 54: 281–294. 10.1007/s10452-019-09742-z. [DOI] [Google Scholar]
- Werner, E. E. , and Hall D. J.. 1988. “Ontogenetic Habitat Shifts in Bluegill: The Foraging Rate‐Predation Risk Trade‐Off.” Ecology 69: 1352–1366. 10.2307/1941633. [DOI] [Google Scholar]
- Werner, E. E. , Hall D. J., and Werner M. D.. 1978. “Littoral Zone Fish Communities of Two Florida Lakes and a Comparison With Michigan Lakes.” Environmental Biology of Fishes 3: 163–172. 10.1007/BF00691940. [DOI] [Google Scholar]
- Westhoff, J. T. , Watts A. V., and Mattingly H. T.. 2013. “Efficacy of Artificial Refuge to Enhance Survival of Young Barrens Topminnows Exposed to Western Mosquitofish.” Aquatic Conservation: Marine and Freshwater Ecosystems 23: 65–76. 10.1002/aqc.2265. [DOI] [Google Scholar]
- Zenni, R. D. , and Nuñez M. A.. 2013. “The Elephant in the Room: The Role of Failed Invasions in Understanding Invasion Biology.” Oikos 122: 801–815. 10.1111/j.1600-0706.2012.00254.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in the preparation of this paper are provided in Dryad (https://doi.org/10.5061/dryad.9w0vt4bq8). [https://datadryad.org/stash/share/yq1Oqr7V8Fq7ohqwTlTxIV4ebvGNN1ylzDK8KZ1hWXo].


