Abstract
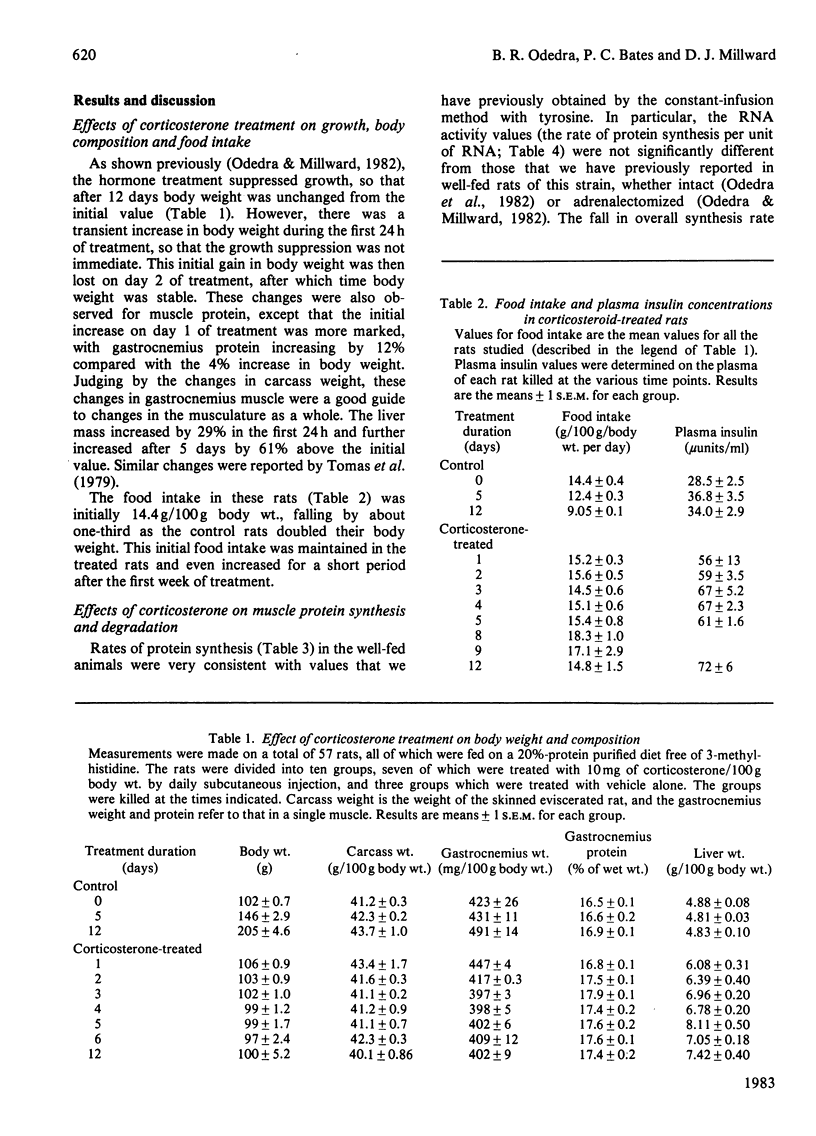
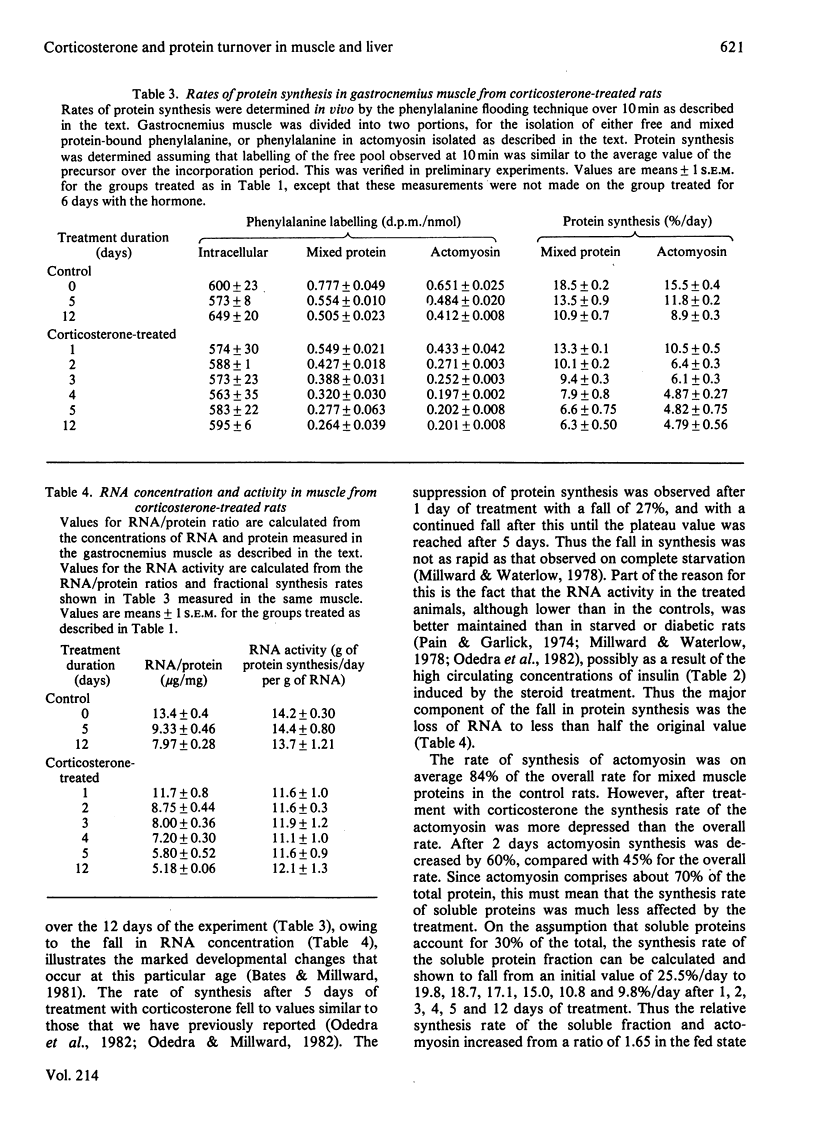
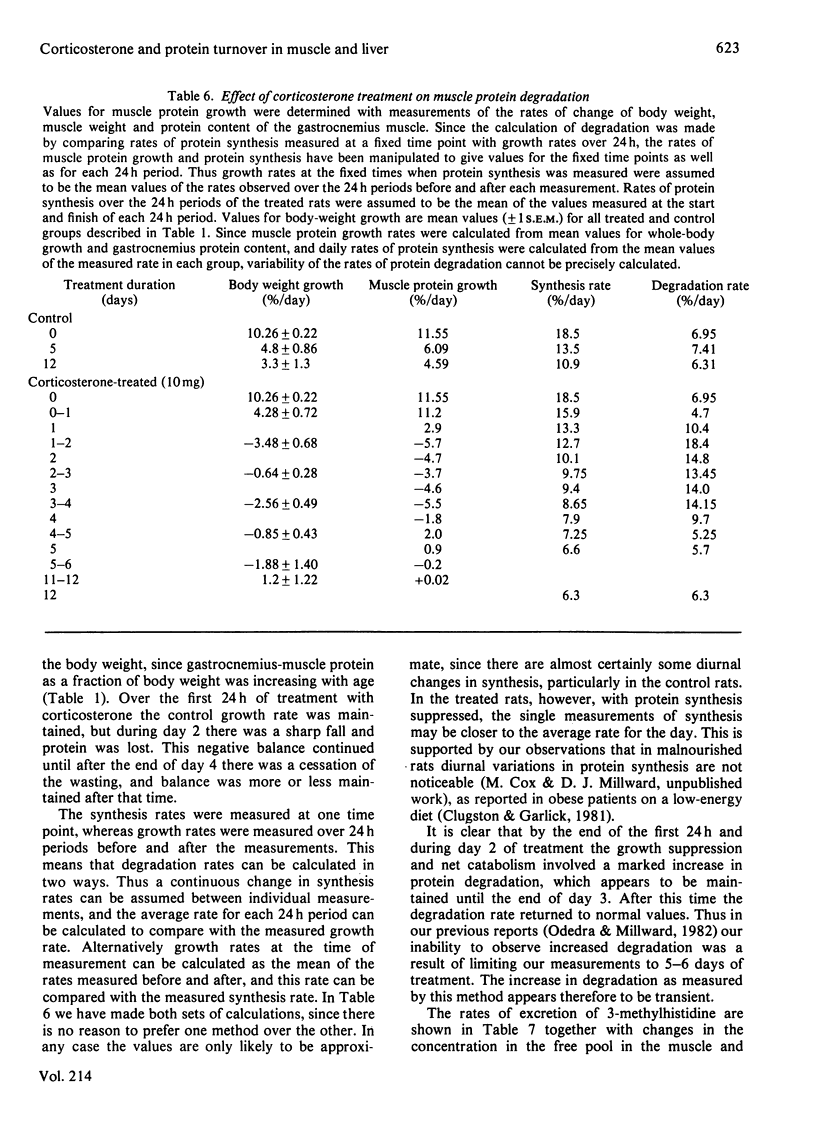
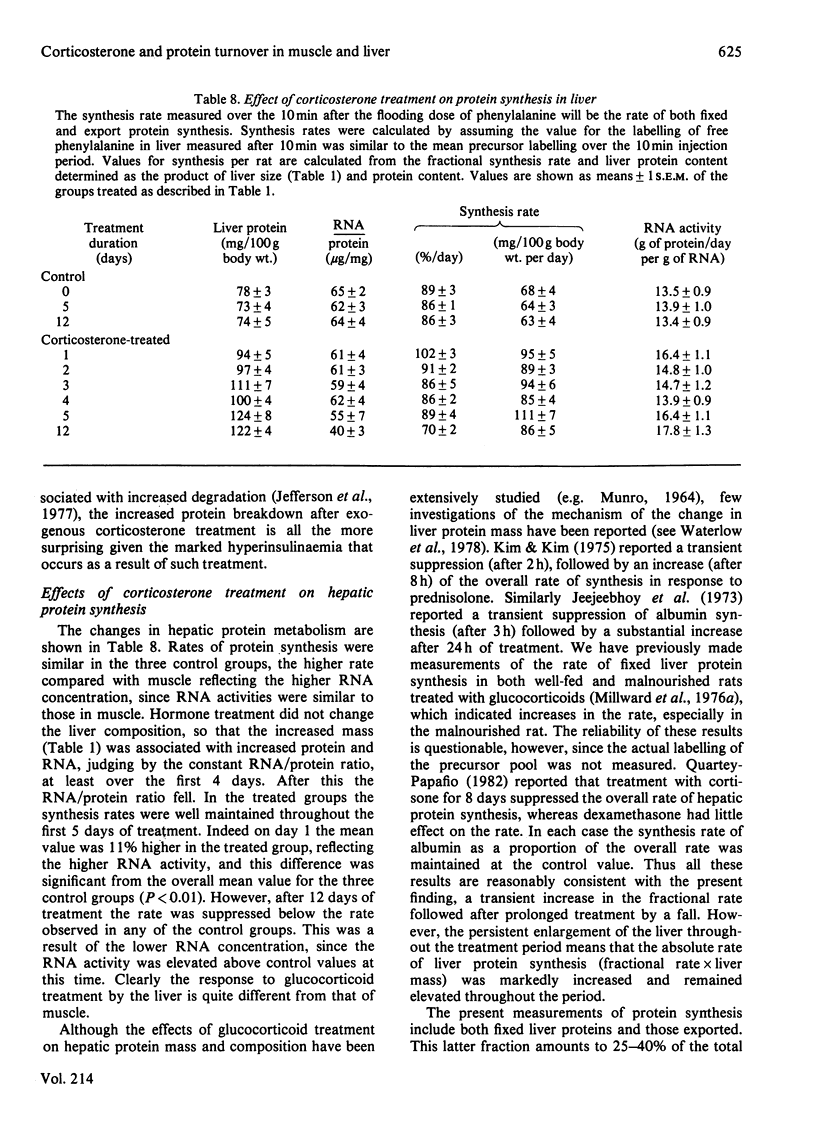
The time course of the response of protein synthesis in muscle and liver to catabolic doses of corticosterone (10 mg/day per 100 g body wt.) was studied in vivo in growing rats over a 12-day period. The rate of protein synthesis in muscle and liver and the rate of actomyosin synthesis in muscle were measured by the phenylalanine-flooding technique, and 3-methylhistidine (N tau-methylhistidine) synthesis was measured by injection of labelled histidine. 3-Methylhistidine concentrations in tissue free pools and urinary excretion were also measured to compare directly with the rate of muscle protein degradation determined as the difference between synthesis and growth each day during the treatment. The overall rate of protein synthesis in muscle fell gradually over the first 4 days, reaching a rate after 5 days that was 36% of the initial rate, and this lower rate was then maintained for the following week. This decrease in the overall rate was accompanied with changes in the relative rate of synthesis in muscle proteins, since during the first 4 days there was a disproportionate decrease in the rate of actomyosin synthesis, and specifically 3-methylhistidine synthesis. In the latter case the synthesis rate was decreased to only 4% of its initial rate after 4 days. These changes in protein synthesis in muscle were accompanied by a transient increase in the rate of protein degradation, which was more than doubled on days 2 and 3 of treatment but which returned to the original rate on day 5, and a similar pattern of response was indicated by urinary 3-methylhistidine excretion, which also exhibited a transient increase. Thus in this case 3-methylhistidine excretion and measured rates of protein degradation in muscle do correlate. The transient effects of the glucocorticoids on degradation compared with the sustained effect on synthesis suggest that these two responses are achieved by different mechanisms. The hepatic size and protein mass were increased by the treatment, and protein synthesis was well maintained until after 12 days, when the rate was suppressed. Although the fractional synthesis rate was transiently increased for 24 h, it is argued that the enlarged liver most likely reflects a decrease in protein degradation resulting from the increased amino acid supply to the liver. This would result from the cessation of muscle growth while dietary supply was maintained.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bates P. C., Grimble G. K., Sparrow M. P., Millward D. J. Myofibrillar protein turnover. Synthesis of protein-bound 3-methylhistidine, actin, myosin heavy chain and aldolase in rat skeletal muscle in the fed and starved states. Biochem J. 1983 Aug 15;214(2):593–605. doi: 10.1042/bj2140593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. C., Millward D. J. Characteristics of skeletal muscle growth and protein turnover in a fast-growing rat strain. Br J Nutr. 1981 Jul;46(1):7–13. doi: 10.1079/bjn19810004. [DOI] [PubMed] [Google Scholar]
- Bates P. C., Millward D. J. Myofibrillar protein turnover. Synthesis rates of myofibrillar and sarcoplasmic protein fractions in different muscles and the changes observed during postnatal development and in response to feeding and starvation. Biochem J. 1983 Aug 15;214(2):587–592. doi: 10.1042/bj2140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Bruce-Robertson A., Ho J., Sodtke U. The comparative effects of nutritional and hormonal factors on the synthesis of albumin, fibrinogen and transferrin. Ciba Found Symp. 1972;9:217–247. doi: 10.1002/9780470719923.ch12. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- John D. W., Miller L. L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969 Nov 25;244(22):6134–6142. [PubMed] [Google Scholar]
- Kelly F. J., Goldspink D. F. The differing responses of four muscle types to dexamethasone treatment in the rat. Biochem J. 1982 Oct 15;208(1):147–151. doi: 10.1042/bj2080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Kim Y. Glucocorticoid inhibition of protein synthesis in vivo and in vitro. J Biol Chem. 1975 Mar 25;250(6):2293–2298. [PubMed] [Google Scholar]
- Lunn P. G., Whitehead R. G., Baker B. A., Austin S. The effect of cortisone acetate on the course of development of experimental protein-energy malnutrition in rats. Br J Nutr. 1976 Nov;36(3):537–550. doi: 10.1079/bjn19760107. [DOI] [PubMed] [Google Scholar]
- Malatová Z., Ahlers I. Diurnal rhythm corticosterone in fasted rats. Endocrinol Exp. 1977 Dec;11(4):241–247. [PubMed] [Google Scholar]
- McGrath J. A., Goldspink D. F. Glucocorticoid action on protein synthesis and protein breakdown in isolated skeletal muscles. Biochem J. 1982 Sep 15;206(3):641–645. doi: 10.1042/bj2060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Bates P. C. 3-Methylhistidine turnover in the whole body, and the contribution of skeletal muscle and intestine to urinary 3-methylhistidine excretion in the adult rat. Biochem J. 1983 Aug 15;214(2):607–615. doi: 10.1042/bj2140607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Bates P. C., Grimble G. K., Brown J. G., Nathan M., Rennie M. J. Quantitative importance of non-skeletal-muscle sources of N tau-methylhistidine in urine. Biochem J. 1980 Jul 15;190(1):225–228. doi: 10.1042/bj1900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Nnanyelugo D. O., James W. P., Garlick P. J. Protein metabolism in skeletal muscle: the effect of feeding and fasting on muscle RNA, free amino acids and plasma insulin concentrations. Br J Nutr. 1974 Jul;32(1):127–142. doi: 10.1079/bjn19740063. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Waterlow J. C. Effect of nutrition on protein turnover in skeletal muscle. Fed Proc. 1978 Jul;37(9):2283–2290. [PubMed] [Google Scholar]
- Mortimore G. E., Schworer C. M. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature. 1977 Nov 10;270(5633):174–176. doi: 10.1038/270174a0. [DOI] [PubMed] [Google Scholar]
- Odedra B. R., Dalal S. S., Millward D. J. Muscle protein synthesis in the streptozotocin-diabetic rat. A possible role for corticosterone in the insensitivity to insulin infusion in vivo. Biochem J. 1982 Feb 15;202(2):363–368. doi: 10.1042/bj2020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odedra B. R., Millward D. J. Effect of corticosterone treatment on muscle protein turnover in adrenalectomized rats and diabetic rats maintained on insulin. Biochem J. 1982 Jun 15;204(3):663–672. doi: 10.1042/bj2040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Rennie M. J., Edwards R. H., Millward D. J., Wolman S. L., Halliday D., Matthews D. E. Effects of Duchenne muscular dystrophy on muscle protein synthesis. Nature. 1982 Mar 11;296(5853):165–167. doi: 10.1038/296165a0. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Millward D. J. 3-Methylhistidine excretion and the urinary 3-methylhistidine/creatinine ratio are poor indicators of skeletal muscle protein breakdown. Clin Sci (Lond) 1983 Sep;65(3):217–225. doi: 10.1042/cs0650217. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Rosochacki S., Quartey-Papafio P., Millward D. J. Intracellular free 3-methylhistidine concentration as an index of protein degradation [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):355–355. doi: 10.1042/bst0080355. [DOI] [PubMed] [Google Scholar]
- Richards J. R., Drury J. K. Energy exchanges and injury. Proc Nutr Soc. 1978 May;37(1):29–43. doi: 10.1079/pns19780006. [DOI] [PubMed] [Google Scholar]
- Santidrian S., Moreyra M., Munro H. N., Young V. R. Effect of corticosterone and its route of administration on muscle protein breakdown, measured in vivo by urinary excretion of N tau-methylhistidine in rats: response to different levels of dietary protein and energy. Metabolism. 1981 Aug;30(8):798–804. doi: 10.1016/0026-0495(81)90026-3. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Shoji S., Pennington R. J. The effect of cortisone on protein breakdown and synthesis in rat skeletal muscle. Mol Cell Endocrinol. 1977 Jan;6(3):159–169. doi: 10.1016/0303-7207(77)90082-x. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]


