Abstract
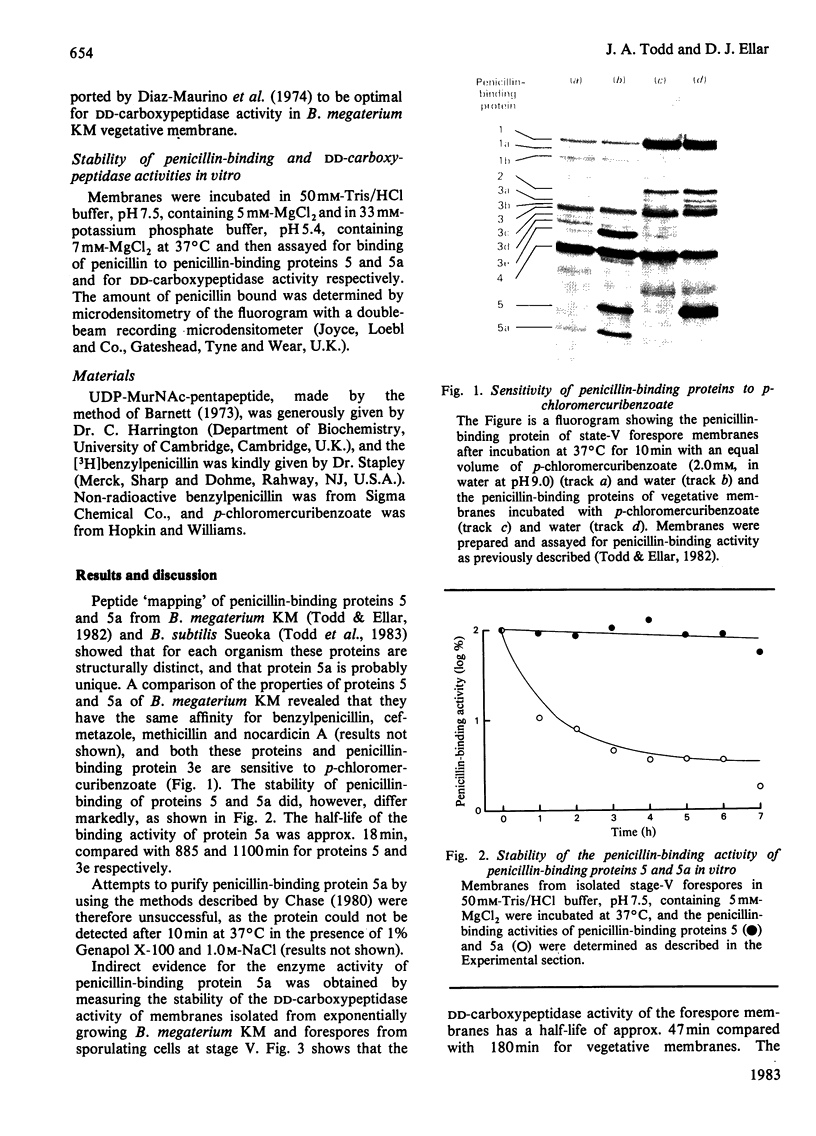
Measurement of the stabilities of DD-carboxypeptidase activity and the penicillin-binding activity of proteins 5 and 5a in membranes isolated from vegetative cells and stage-V forespores suggests that the unique sporulation-specific protein 5a may be a penicillin-sensitive DD-carboxypeptidase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett H. J. D-alanine carboxypeptidases of Bacillus stearothermophilus: solubilisation of particulate enzymes and mechanism of action of penicillin. Biochim Biophys Acta. 1973 Apr 28;304(2):332–352. doi: 10.1016/0304-4165(73)90252-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chase H. A. Purification of four penicillin-binding proteins from Bacillus megaterium. J Gen Microbiol. 1980 Mar;117(1):211–224. doi: 10.1099/00221287-117-1-211. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Dring G. J. Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex. Nature. 1975 Dec 4;258(5534):402–405. doi: 10.1038/258402a0. [DOI] [PubMed] [Google Scholar]
- Johnson K., Duez C., Frère J. M., Ghuysen J. M. Beta-lactamases (Actinomycetes species). Methods Enzymol. 1975;43:687–698. doi: 10.1016/0076-6879(75)43134-2. [DOI] [PubMed] [Google Scholar]
- Mauriño T., Nieto M., Perkins H. R. Membrane-bound DD-carboxypeptidases from Bacillus megaterium KM general properties, substrate specificity and sensitivity to penicillins, cephalosporins and peptide inhibitors of the activity at pH5. Biochem J. 1974 Nov;143(2):391–402. doi: 10.1042/bj1430391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd S. T., Chase H. A., Reynolds P. E. The separation and properties of two penicillin-binding proteins from Salmonella typhimurium. Eur J Biochem. 1977 Sep;78(2):521–523. doi: 10.1111/j.1432-1033.1977.tb11765.x. [DOI] [PubMed] [Google Scholar]
- Sowell M. O., Buchanan C. E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983 Mar;153(3):1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Johnstone K., Hagelberg E., Ellar D. J. Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem J. 1981 Jul 15;198(1):101–106. doi: 10.1042/bj1980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Ellar D. J. Alteration in the penicillin-binding profile of Bacillus megaterium during sporulation. Nature. 1982 Dec 16;300(5893):640–643. doi: 10.1038/300640a0. [DOI] [PubMed] [Google Scholar]