Abstract
Background
In adults with serious respiratory illness, breathlessness is prevalent and associated with reduced health-related quality of life. The aim of this review was to assess the impact of breathing techniques on breathlessness in adults with serious respiratory illness.
Methods
Electronic databases were searched to identify randomised controlled trials testing breathing techniques (techniques that aim to alter the respiratory pattern, excluding respiratory muscle training) in people with serious respiratory illness. The primary outcome was breathlessness and secondary outcomes were health-related quality of life and adverse events. Two authors independently screened for inclusion, evaluated risk of bias and extracted data.
Results
73 randomised controlled trials were included with 5479 participants, most with COPD or asthma. Breathing exercises (pursed lip and/or diaphragmatic breathing) reduced breathlessness measured by the modified Medical Research Council scale compared to usual care (mean difference (MD) −0.40 points, 95% CI −0.70– −0.11, eight studies, n=323), although the effect did not exceed the minimal important difference. Yoga breathing also improved modified Medical Research Council score compared to usual care (MD −1.05 points, 95% CI −2.45–0.35, three studies, n=175). Breathing techniques consistently improved health-related quality of life in people with COPD and asthma on multiple health-related quality of life measures in comparison to usual care, with effects that generally exceeded the minimal important difference. No adverse events related to breathing techniques were reported.
Conclusion
Breathing techniques may improve breathlessness, and consistently improve health-related quality of life, in people with serious respiratory illness. These findings support the use of breathing exercises in the care of people with serious respiratory illness.
Shareable abstract
Pursed lip breathing, diaphragmatic breathing and yoga breathing may reduce breathlessness and consistently improve quality of life in people with serious respiratory illness. These findings support the use of breathing techniques in clinical practice. https://bit.ly/4cM5APs
Introduction
People with serious respiratory illness experience a high symptom burden including chronic breathlessness, which is frequently distressing and contributes to poor health-related quality of life (HRQoL) [1]. Despite optimal pharmacological management, chronic breathlessness frequently persists [2] and may be underappreciated by health professionals [3]. New treatments to relieve breathlessness are a research priority for people with serious respiratory illness and their caregivers [4].
A variety of breathing techniques have been employed in the care of people with serious respiratory illness, including pursed lip breathing, diaphragmatic breathing and yoga breathing. Previous systematic reviews have not demonstrated consistent effects of breathing exercises on patient-centred outcomes such as breathlessness, and most have only examined a subset of available breathing techniques [5–12]. Breathing techniques are valued by patients [13, 14] and can be delivered in a wide range of healthcare and non-healthcare settings globally. Therefore, if effective, breathing techniques have the potential for widespread implementation.
This systematic review aimed to determine the efficacy and safety of breathing techniques in people with serious respiratory illness. The review was conducted as part of the evidence synthesis for the European Respiratory Society (ERS) Clinical Practice Guideline on symptom management for adults with serious respiratory illness.
Methods
The review protocol was developed a priori but was not published, due to the confidentiality requirements of the ERS Clinical Practice Guideline development process. Instead, the protocol was submitted to the European Respiratory Review editorial office in April 2023 to be held in confidence and made available to reviewers. The protocol can be found in the supplementary material.
Search strategy and study selection
Initial searches were conducted between July 2022 and November 2022 in MEDLINE (OVID), Embase (OVID), Cochrane Database of Systematic Reviews and CENTRAL (The Cochrane Library) to identify relevant systematic reviews. Systematic reviews that provided evidence for at least one of the outcomes of interest were used to identify relevant studies. Subsequently, searches were conducted in order to identify randomised controlled trials (RCTs) that had been published since the search date of the most recent relevant systematic review. Studies were included irrespective of date or language of publication. Reference lists of all primary studies and review articles were manually checked for additional references, and PubMed was searched for errata or retractions relating to included studies. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram documented the review process [15].
Types of studies
We included RCTs. Randomised crossover trials were only included if pre-crossover data were available because the intervention includes behavioural components, therefore carryover of intervention effects to the second period may occur.
Participants
Participants were aged ≥18 years with serious respiratory illness, defined as a respiratory condition that carries a high risk of mortality, negatively impacts quality of life and daily function, and/or is burdensome in symptoms, treatments or caregiver stress [16]. There was no restriction according to the underlying diagnosis of lung disease, except that patients with cancer were excluded.
Types of interventions
We included any type of breathing technique, either supervised or unsupervised, that aimed to alter the respiratory pattern. This could be achieved with or without external devices, and either during exercise or at rest. Eligible interventions included, but were not limited to, breathing exercises (e.g. pursed lip breathing, diaphragmatic breathing), specific breathing techniques (e.g. Buteyko, Papworth), breathing exercises with addition of biofeedback (e.g. feedback on breathing rate or pattern) or yoga breathing. Trials where breathing exercises were combined with another intervention (e.g. relaxation) were included provided 50% or more of the training consisted of breathing exercises. Trials of respiratory muscle training or airway clearance techniques were not included because these interventions aim to improve respiratory muscle strength or clear airway secretions, respectively, rather than alter the respiratory pattern.
Types of comparisons
We included two types of studies: 1) studies that reported the effects of breathing techniques compared with no breathing techniques or a sham intervention and 2) studies in which the intervention of interest was added to an intervention common to both groups (e.g. breathing techniques added to exercise training versus exercise training alone). We reported these two comparisons separately.
Outcomes
The primary outcome was breathlessness, assessed using any validated tool. This included measures at rest or during exercise, but exercise measures obtained before and after an intervention must have been recorded at iso-workload. Secondary outcomes were HRQoL (assessed using any validated tool) and adverse events (defined according to the investigators’ definition). Studies were not excluded if they did not report these outcomes.
Identification of studies
Titles and abstracts were screened for eligibility by two independent reviewers (A.T. Burge, A.M. Gadowski) with conflicts resolved by discussion and adjudication by a third reviewer (A.E. Holland) if required. Studies classified as “include” or “unsure” were obtained as full text. Full-text review was conducted by the same two reviewers to determine eligibility for inclusion.
Quality assessment
Risk of bias was assessed by two independent reviewers (A.T. Burge, A.M. Gadowski) with conflicts resolved by discussion and adjudication by a third reviewer (A.E. Holland) if required. Where included studies were identified from systematic reviews, we retrieved the published assessments of risk of bias. Methodological quality of the systematic reviews was assessed using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR 2) checklist [17]. For individual studies in the updated search, risk of bias was assessed using the Cochrane risk of bias tool 1 [18].
Data extraction
Data extraction was undertaken by two independent reviewers (A.T. Burge, A.M. Gadowski) with conflicts resolved by discussion and adjudication by a third reviewer (A.E. Holland) if required. For studies published in Chinese-language journals, a single reviewer (A. Jones) translated the methodology and extracted data. Custom-designed data collection forms were used to record study details (design, dates, setting, trial registration, funding source), participant inclusion and exclusion criteria, demographic characteristics (age, sex, diagnosis, disease severity, clinical stability), intervention details (type, duration, frequency, dose), care received by the comparison group and outcome data of interest. For crossover studies we extracted outcome data only for the first intervention period, prior to the crossover.
Data analysis and synthesis
We analysed dichotomous data as odds ratios (ORs). For continuous data, we used mean differences (MDs). Where available, we used the change from baseline; otherwise, the adjusted results or final score were used. Skewed data were narratively reported as medians and interquartile ranges (IQRs). Data were presented as a scale with a consistent direction of effect (e.g. HRQoL data). Attempts were made to contact study authors to clarify key study characteristics and obtain missing outcome data where possible.
Where trials were clinically heterogeneous, a narrative synthesis was performed. Meta-analyses (random-effects model) were undertaken only where interventions and/or participant features were clinically homogeneous. Where multiple arms were reported in a single trial, we included only the relevant arms. If two comparisons were combined in the same meta-analysis, we halved the control group to avoid double-counting. No subgroup analyses were undertaken. We used the I2 statistic to measure heterogeneity. We performed sensitivity analyses to examine the effects of methodological quality on the pooled estimate by removing studies that were at high or unclear risk of bias for the domains of blinding of outcome assessors and incomplete outcome data. Statistical analysis was conducted using RevMan version 5.4 [19].
Results
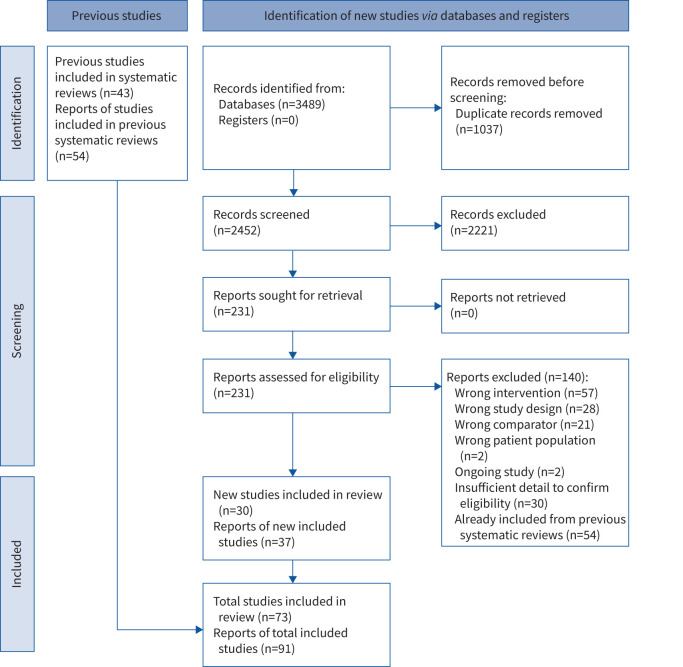
The search for systematic reviews identified 508 records, of which 74 were retrieved for full-text screening. Eight relevant systematic reviews were identified (supplementary table S1) containing 43 eligible RCTs. The search for additional RCTs identified 2452 records, of which 231 were screened in full text, with an additional 30 eligible RCTs identified (figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow of studies through the systematic review.
We included 73 RCTs (n=5479 participants) including people with COPD (37 RCTs), asthma (34 RCTs), interstitial lung disease (one RCT) and mixed COPD and asthma (one RCT) (supplementary tables S2 and S3). Most participants had moderate-to-severe lung disease. Three studies assessed interventions following hospital admission for an exacerbation [20–22]. Studies were undertaken in 17 countries, most commonly India (15 studies), China and the USA (13 studies each), Turkey (six studies), Iran and the UK (five studies each).
The most common intervention was breathing exercises (diaphragmatic breathing and/or pursed lip breathing, 43 studies) followed by yoga breathing (23 studies). Many studies did not provide details of the intervention, or used a unique intervention that could not be combined with others or replicated in practice. Five studies had two intervention groups that were compared with usual care [22–25] or placebo [21]. Five studies added breathing retraining with biofeedback to another intervention common to both groups (exercise training [26–29]; inhalation therapy [30]). Most interventions were 4–12 weeks in duration, but some were as long as 6 months (supplementary table S2).
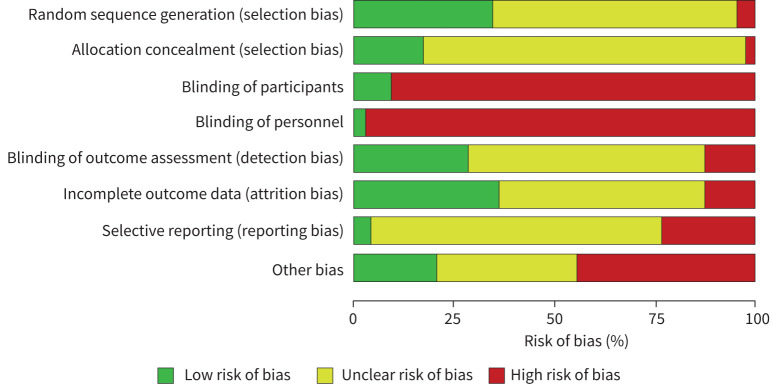
Quality assessment using the AMSTAR 2 checklist demonstrated that systematic reviews scored well for formulation of PICO questions, comprehensive searches and assessment of risk of bias (supplementary table S1). However, few reported a priori registration of a review protocol (three of eight reviews) or source of funding for included studies (two of eight studies), or accounted for risk of bias in interpretation of results (three of eight studies). Risk of bias was high or unclear for the majority of RCTs in the domains of random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data and selective reporting (figure 2, supplementary figure S1). As expected due to the nature of the intervention, few trials blinded participants or personnel. Certainty of evidence was affected by indirectness (limited data in conditions other than COPD and asthma), heterogeneity of interventions and heterogeneity of outcome measures and timepoints of measurement.
FIGURE 2.
Risk of bias for included randomised controlled trials. Judgements about each risk of bias item presented as percentages across all included studies.
Primary outcome: breathlessness
Descriptive results for the critical outcome breathlessness can be found in supplementary table S4.
Comparison: breathing exercises versus usual care
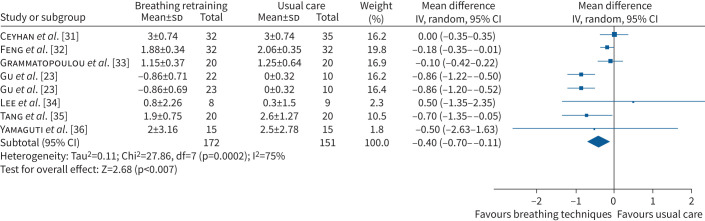
Breathing exercises reduced breathlessness measured by the modified Medical Research Council (mMRC) scale at 4–8 weeks compared to usual care (MD −0.40 points, 95% CI −0.70– −0.11 points, seven RCTs, n=323, I2=75%; figure 3) However, the lower end of the confidence interval did not include the minimal important difference (MID) of −1 point [37] and clinical significance is unclear. Sensitivity analysis with removal of trials that did not blind assessors (five RCTs) resolved heterogeneity but reduced the effect size (MD 0.07 points, 95% CI −1.33–1.47 points, three RCTs, n=87, I2=0%). An 8-week study that could not be combined in the meta-analysis (n=40 participants with COPD) reported improvements in mMRC score at the end of the intervention (mean 0.89 points versus 1.35 points in the usual care group) and after 4 weeks of follow-up (0.84 points versus 1.4 points) [38]. In participants with asthma, 6 months of breathing exercises tended to reduce mMRC score compared with usual care (MD −0.20 points, 95% CI −0.50–0.10 points, one RCT, n=40) [33]. In participants with COPD following hospitalisation for an exacerbation, 8 weeks of breathing exercises reduced mMRC score in the breathing exercises (breathing manoeuvre) group compared with usual care (MD −1.46 points, 95% CI −2.19– −0.73 points, one RCT, n=32) and in the breathing exercises (pursed lip breathing) group compared with usual care (MD −1.00 point, 95% CI −1.73– −0.27 points, one RCT, n=30) [22].
FIGURE 3.
Forest plot for breathlessness (modified Medical Research Council scale score) for breathing exercises versus usual care. IV: inverse variance.
Improvements in breathlessness in COPD were demonstrated using a range of other breathlessness measures. Daily balloon inflation reduced breathlessness on a visual analogue scale compared to usual care at 8 weeks (median of differences −9, 95% CI −18– −1, one RCT, n=22) [39]. Likewise, 10 weeks of breathing exercises reduced the Dyspnoea-12 physical subscale score (MD −11.42 points, 95% CI −14.18– −8.66 points, one RCT, n=77), emotional subscale score (MD −7.04 points, 95% CI −9.00– −5.08 points, one RCT, n=77) and total score (MD −17.45 points, 95% CI −21.75– −13.15 points, one RCT, n=77) compared with usual care [40]. 12 weeks of breathing exercises reduced the University of California San Diego Shortness of Breath Questionnaire score compared with usual care (MD −10 points, 95% CI −28.9–8.9 points, one RCT, n=19) [41].
Comparison: breathing exercises versus sham/placebo
In participants who had been hospitalised for a COPD exacerbation, a 4-week study demonstrated reductions in mMRC score in the breathing exercises group (pursed lip breathing and diaphragmatic breathing) compared with placebo (MD −1.49 points, 95% CI −1.67– −1.31 points, one RCT, n=59) and breathing exercises group (pursed lip breathing) compared with placebo (MD −0.66 points, 95% CI −0.81– −0.51 points, one RCT, n=59) [21].
Comparison: yoga breathing versus usual care
Yoga breathing reduced breathlessness measured with mMRC at 2–4 months compared to usual care, with the mean effect exceeding the MID of 1 point; however, this did not reach statistical significance (MD −1.05 points, 95% CI −2.45–0.35 points, three RCTs, n=175, I2=98%; supplementary figure S2). Sensitivity analysis could not be conducted to explore heterogeneity because all three RCTs were at high or unclear risk of bias for assessor blinding and/or incomplete outcome data. 12 weeks of yoga breathing tended to improve breathlessness in participants with COPD measured with the Chronic Respiratory Questionnaire (CRQ) dyspnoea domain (MD 0.32 points, 95% CI −1.08–1.72 points, one RCT, n=14) [42], but the mean effect was smaller than the MID and clinical significance is unclear.
Comparison: yoga breathing versus sham/placebo
No studies reported on yoga breathing versus sham/placebo with the outcome breathlessness.
Comparison: Buteyko technique versus usual care
No studies reported on the Buteyko technique versus usual care with the outcome breathlessness.
Comparison: Buteyko technique versus sham/placebo
No studies reported on the Buteyko technique versus sham/placebo with the outcome breathlessness.
Comparison: Papworth method versus usual care
No studies reported on the Papworth method versus usual care with the outcome breathlessness.
Comparison: breathing retraining with biofeedback added to exercise training versus exercise training
In participants with COPD, addition of breathing retraining with biofeedback to exercise training (4–12 weeks) improved the CRQ dyspnoea domain compared to exercise training alone (MD 0.30 points, 95% CI −0.02–0.62 points, four RCTs, n=251, I2=12%; supplementary figure S3). The upper end of the confidence interval included the MID of 0.5 points [37] although the mean effect did not exceed the MID. At 6-week follow-up, the MD in CRQ dyspnoea domain score was 0.52 (95% CI −0.18–1.22, one RCT, n=56) compared with exercise training alone [27]. Addition of breathing retraining did not improve Borg score at isotime on a constant work rate test compared with exercise training alone (MD −0.9 points, 95% CI −2.25–0.45 points, one RCT, n=33) [26].
Comparison: breathing exercises added to inhalation therapy versus inhalation therapy alone
No studies reported on breathing exercises added to inhalation therapy versus inhalation therapy alone with the outcome breathlessness.
Secondary outcome: health-related quality of life
Descriptive results for the outcome HRQoL can be found in supplementary table S5.
Comparison: breathing exercises versus usual care
Breathing exercises (4 weeks–4 months) improved HRQoL compared to usual care in participants with COPD, measured using the St George's Respiratory Questionnaire (SGRQ) total score (MD −9.44 points, 95% CI −16.47– −2.41 points, eight RCTs, n=452, I2=91%; supplementary figure S4.1), SGRQ symptoms domain (MD −8.61 points, 95% CI −16.33– −0.88 points, five RCTs, n=365, I2=91%; supplementary figure S4.2), SGRQ impact domain (MD −9.10 points, 95% CI −16.11– −2.08 points, five RCTs, n=365, I2=88%; supplementary figure S4.3) and SGRQ activities domain (MD −9.09 points, 95% CI −18.61–0.42 points, five RCTs, n=375, I2=93%; supplementary figure S4.4). The MD and lower end of the confidence interval for all domains exceeded the MID of −4 points [43]. Sensitivity analysis with removal of trials that did not blind assessors (five RCTs) reduced the effect size, but did not resolve heterogeneity (supplementary table S7). A single 6-month study involving participants with COPD that assessed the effect of Health Qigong Liuzijue demonstrated improvements in SGRQ total score and all domain scores [44]. In interstitial lung disease, breathing exercises improved SGRQ total score after 12 months compared with usual care (MD −8.53 points, 95% CI −13.81– −3.24 points, one RCT, n=59) [45].
Studies involving participants with COPD showed consistent improvements in HRQoL across a range of measures. Breathing exercises improved COPD Assessment Test (CAT) score compared to usual care after 4 weeks (median −5.5 points versus −3 points, one RCT, n=67, p=0.002) [31] and after 8 weeks (MD −3.20 points, 95% CI −7.08–0.68, one RCT, n=17) [34]. Similarly, 8 weeks of breathing exercises resulted in lower CAT scores at the end of the intervention period (intervention mean 5.11 points versus usual care mean 11.33 points, one RCT, 78 participants), which was maintained at 4-week follow-up (intervention mean 4.58 points versus usual care 11.7 points) [38]. Eight weeks of daily balloon inflation improved well-being rating on a visual analogue scale compared with usual care (median difference 9, 95% CI −3–21, n=22) [39].
For participants with asthma, HRQoL improved on disease-specific measures, but this was less consistent for generic HRQoL measures. Meta-analysis was not possible owing to differences in interventions, outcomes and follow-up periods. 12 weeks of breathing exercises improved the mini-Asthma Quality of Life Questionnaire (AQLQ) score compared to usual care (MD 0.56 points, 95% CI 0.28–0.85 points, n=193) and this was maintained at 6-month (MD 0.35 points, 95% CI 0.07–0.62 points, n=193) and 12-month follow-up (MD 0.38 points, 95% CI 0.12–0.65 points, n=193) [46]. Eight weeks of breathing retraining with biofeedback demonstrated improvements in mini-AQLQ activities subdomain score (MD 0.16 points, 95% CI 0.09–0.23 points, n=52) and emotion subdomain score (MD 0.76 points, 95% CI 0.55–0.97 points, n=52) compared with usual care; the MD in symptoms subdomain score was 0.11 points (95% CI −0.07–0.29 points, n=52) [47]. A 4-week study involving participants with asthma that assessed the effect of breathing exercises described no between-group difference in AQLQ score compared with usual care (data not presented, n=20) [48]. One 12-month study with a three-arm design involving participants with asthma demonstrated improvements in AQLQ total and domain scores after breathing exercises (self-guided) compared with usual care (n=523 participants) [24] and breathing exercises (face-to-face) compared with usual care (n=394 participants) [24]. However, studies that used the 36-Item Short Form Health Survey (SF36) reported no effect of breathing exercises compared to usual care at 12 weeks [41] or 6 months [33].
Comparison: breathing exercises versus sham/placebo
In participants with asthma, breathing exercises did not consistently improve HRQoL compared to a sham intervention. Four weeks of breathing exercises did not improve mini-AQLQ score compared with sham (MD −2.00 points, 95% CI −8.12–4.12 points, one RCT, n=90) [49]. A 12-week study (38 participants) demonstrated improvements in AQLQ domain scores compared with a sham intervention [50], but results are difficult to interpret because non-standard domains of AQLQ were used. A 28-week study demonstrated improvements in HRQoL that favoured breathing exercises over sham for AQLQ score (MD 0.14 points, 95% CI −0.11–0.38 points, 48 participants) and the seven-item Asthma Control Questionnaire score (MD 0.11 points, 95% CI −0.20–0.43 points, n=48) [51] but MDs were not clinically or statistically significant.
Comparison: yoga breathing versus usual care
Compared to usual care, yoga breathing improved HRQoL measured with the SGRQ total score (MD −7.42 points, 95% CI −13.02– −1.82 points, three studies, n=111, I2=45%; supplementary figure S5.1), SGRQ impact domain (MD −13.06 points, 95% CI −14.06– −12.05 points, three studies, n=111, I2=0%; supplementary figure S5.3) and SGRQ activities domain (MD −13.41 points, 95% CI −19.12– −7.16 points, three studies, n=111, I2=0%; supplementary figure S5.4); the MD for SGRQ symptom domain was −6.5 points (95% CI −14.93–1.94 points, three studies, n=111, I2=69%; supplementary figure S5.2). Sensitivity analysis with removal of one RCT that did not blind assessors reduced the effect size but did not resolve heterogeneity (MD −2.50 points, 95% CI −14.04–9.04 points, two RCTs, n=91, I2=73%). There was no effect of 12 weeks of yoga breathing on CAT score (MD −3.31 points, 95% CI −9.8–3.19 points, two studies, n=115, I2=85%; supplementary figure S6). Sensitivity analysis was not possible owing to the small number of studies. One additional RCT involving participants with COPD demonstrated no effect of yoga breathing on SF36 mental or physical component scores, or CRQ fatigue, emotion or mastery domain scores [42].
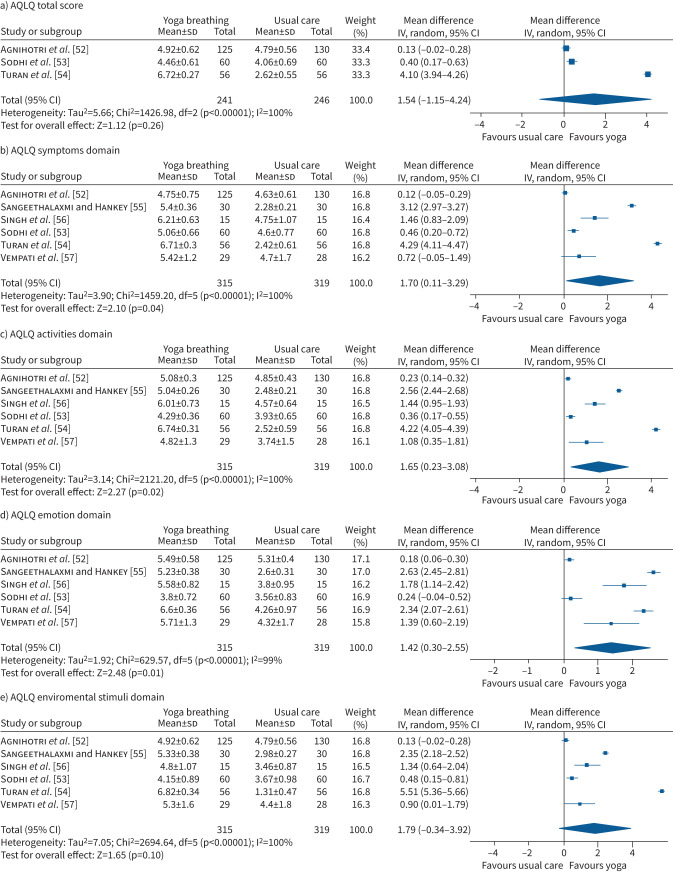
In people with asthma, improvements in HRQoL after 6–12 weeks favoured yoga breathing over usual care for AQLQ total score (MD 1.54 points, 95% CI −1.15–4.24 points, three studies, n=487, I2=100%; figure 4a). There were also improvements in AQLQ symptoms domain (MD 1.70 points, 95% CI 0.11–3.29 points, six studies, n=634, I2=100%; figure 4b), activities domain (MD 1.65 points, 95% CI 0.23–3.08 points, six studies, n=634, I2=100%; figure 4c) and emotion domain (MD 1.42 points, 95% CI 0.30–2.55 points, six studies, n=634, I2=99%; figure 4d). Improvements in the environment domain favoured yoga breathing (MD 1.79 points, 95% CI −0.34–3.92 points, six studies, n=634, I2=100%; figure 4e). Sensitivity analysis was not possible because all studies lacked assessor blinding and/or had incomplete outcome data. One additional study that could not be combined in the meta-analysis showed similar effects [25]. One study continued the intervention for 6 months [52], demonstrating sustained improvements in AQLQ total score and all domain scores.
FIGURE 4.
Forest plots for health-related quality of life (Asthma Quality of Life Questionnaire (AQLQ) total and domain scores) for yoga breathing versus usual care. IV: inverse variance.
Comparison: yoga breathing versus sham/placebo
In people with asthma, improvement in HRQoL measured using the AQLQ was found after 4 weeks of yoga breathing compared to a sham intervention for domains of symptoms (MD 0.96 points, 95% CI 0.46–1.46 points, one RCT, n=50), activities (MD 0.94 points, 95% CI 0.44–1.44 points, one RCT, n=50), emotion (MD 1.14 points, 95% CI 0.51–1.77 points, one RCT, n=50) and environment (MD 0.78 points, 95% CI 0.19–1.37 points, one RCT, n=50) [58]. However, a 16-week study involving participants with asthma demonstrated no effect on mini-AQLQ score (MD 0.22 points, 95% CI −0.57–1.01 points, one RCT, n=62) [59].
Comparison: Buteyko technique versus usual care
A single RCT that assessed the effects of 12 weeks of Buteyko technique in people with asthma demonstrated improvements in HRQoL measured with AQLQ total score compared to usual care (MD 0.97 points, 95% CI 0.48–1.46 points, n=79), as well as improvements in the domains of symptoms (MD 1.01 points, 95% CI 0.44–1.59 points, n=79), activities (MD 1.32 points, 95% CI 0.81–1.83 points, n=79) and environment (MD 0.91 points, 95% CI 0.13–1.69 points, n=79); the MD in the emotion domain score was 0.91 points (95% CI 0.13–1.69 points, n=79) [25].
Comparison: Buteyko technique versus sham/placebo
Four weeks of Buteyko technique compared to placebo in people with asthma improved HRQoL measured by AQLQ total score (MD −1.29 points, 95% CI −2.53– −0.05 points, n=36) and domains of symptoms (MD −1.53 points, 95% CI −3.06–0.00 points, n=36) and emotion (MD −1.59 points, 95% CI −3.04– −0.15 points, n=36); the MD in activities domain was −1.16 points (95% CI −2.54–0.22 points, n=36) and in the environment domain was −0.87 points (95% CI −2.18–0.44 points, n=36) [60]. A 6-month study involving participants with asthma demonstrated no differences between Buteyko technique and placebo for AQLQ total or subdomain scores, or any domain of the SF36, with the exception of improvement in the physical limitations domain (n=45 participants) [61].
Comparison: Papworth method versus usual care
A RCT that assessed the effects of 6 months of the Papworth method compared to usual care in people with asthma demonstrated improvements in SGRQ symptoms domain score at 6-month (MD −11.00 points, 95% CI −19.52– −2.48 points, n=78) and 12-month follow-up (MD −1.50 points, 95% CI −6.71–3.71 points, n=72). Improvements in the activities and impact domains and total score did not reach statistical significance [62]. A 6-week study involving participants with asthma (n=69) demonstrated improvements in SF36 domains that did not reach statistical significance [63].
Comparison: breathing retraining with biofeedback added to exercise training versus exercise training
Addition of breathing retraining with biofeedback to exercise training did not consistently improve HRQoL in participants with COPD compared to exercise training alone. After 4–12 weeks of training, the MD between groups in CRQ fatigue domain score was 0 points (95% CI −0.32–0.32 points, three RCTs, n=209, I2=9%; supplementary figure S7.1), in emotional function domain score was −0.12 points (95% CI −0.41–0.18 points, three RCTs, n=209, I2=0%; supplementary figure S7.2) and in the mastery domain score was 0.10 points (95% CI −0.21–0.40 points, three RCTs, n=209, I2=0%; supplementary figure S7.3). Two additional RCTs that could not be combined in meta-analysis reported similar results [27, 29].
Comparison: breathing exercises added to inhalation therapy versus inhalation therapy alone
No studies reported on breathing exercises added to inhalation therapy versus inhalation therapy alone with the outcome HRQoL.
Secondary outcome: adverse events
Descriptive results for the outcome adverse events can be found in supplementary table S6.
Comparison: breathing exercises versus usual care or sham/placebo
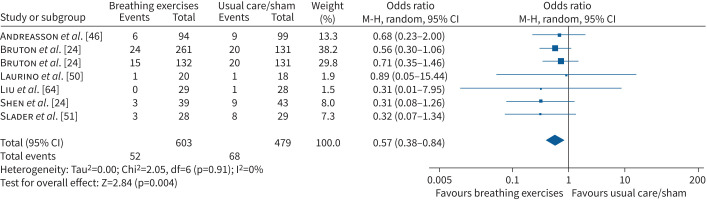
In 11 RCTs (1433 participants) that reported this outcome, there were no adverse events related to the intervention. In participants with asthma, there was no effect of breathing exercises over 6–12 months on the odds of a serious adverse event (OR 0.62, 95% CI 0.37–1.5, three studies, n=848, I2=0%) or an adverse event (OR 0.71, 95% CI 0.53–0.94, I2=0%) (supplementary figure S8). In participants with COPD, breathing exercises did not increase the odds of a hospital admission (OR 0.98, 95% CI 0.27–3.54, two studies, n=176, I2=0%; supplementary figure S9) or mortality (OR 0.74, 95% CI 0.18–3.06, three studies, n=216, I2=0%; supplementary figure S10). There was a reduction in the odds of an exacerbation with breathing exercises (12 weeks–12 months) compared to usual care or sham/placebo interventions (OR 0.57, 95% CI 0.38–0.84, six studies, n=1082, I2=0%; figure 5) but no effect on the odds of requiring oral corticosteroids (OR 0.45, 95% CI 0.08–2.76, one RCT, n=48) [65].
FIGURE 5.
Forest plots for adverse events (number of participants with exacerbation) for breathing exercises versus usual care or sham/placebo. M-H: Mantel-Haenszel.
Comparison: yoga breathing versus usual care or sham/placebo
Three studies in people with COPD reported that there were no adverse events related to yoga breathing [42, 59, 66]. There was no effect of 3–4 months of yoga breathing on the odds of developing an exacerbation (OR 0.67, 95% CI 0.30–1.47, two studies, n=147, I2=0%; supplementary figure S11). There was no effect of yoga breathing on the “number of attacks per week” (yoga breathing: mean±sd 0.38±0.48, n=30; usual care: mean±sd 0.58±0.53, n=30) [53] or the number of participants with “illness” (yoga breathing: one out of 36 participants; usual care: two out of 36 participants) [67].
Comparison: Buteyko technique versus usual care or sham/placebo
There was no effect of the Buteyko technique on the odds of an exacerbation (OR 0.59, 95% CI 0.17–1.99, one RCT, n=79) [25] or on the odds of a hospital admission due to asthma exacerbation (OR 0.31, 95% CI 0.01–8.28, one RCT, n=32) [60]. A 6-month study involving participants with asthma that assessed the effect of Buteyko technique compared to placebo reported a median of one exacerbation (IQR 0–1.75) in the intervention group and one exacerbation (IQR 0–2) in the placebo group (45 participants) [61].
Comparison: Papworth method versus usual care
A 6-month study involving participants with asthma that assessed the effect of the Papworth methods compared with usual care stated “no adverse events were reported by patients or GPs” (n=85 participants) [62].
Comparison: breathing exercises added to exercise training versus exercise training
Breathing retraining with biofeedback added to exercise training (8–12 weeks) did not alter the odds of a COPD exacerbation compared with exercise training alone (OR 0.26, 95% CI 0.04–1.64, three studies, n=231, I2=0%; supplementary figure S12).
Comparison: breathing exercises added to inhalation therapy versus inhalation therapy alone
One 4-week study involving participants with COPD that assessed the effect of breathing exercises added to tiotropium bromide and N-acetylcysteine reported a reduction in “adverse reactions” (OR 0.28, 95% CI 0.08–0.92, n=100) compared with the group receiving only tiotropium bromide and N-acetylcysteine [30].
Discussion
This systematic review has shown that breathing techniques probably improve breathlessness in people with COPD and asthma compared to usual care or sham treatment; however, there is some uncertainty regarding both the magnitude of the effect and its clinical significance. Breathing techniques consistently improved HRQoL in people with COPD and asthma in comparison to usual care, with effects that exceeded the MID for most analyses. There are few data in other serious respiratory illnesses. The breathing techniques with most evidence for benefit were breathing exercises (pursed lip breathing or diaphragmatic breathing) and yoga breathing, with less data to support the effects of Buteyko and the Papworth method. Adding breathing exercises to whole-body exercise training probably improved breathlessness in people with COPD, with no effect on HRQoL. The likelihood of adverse effects with breathing exercises is very low.
Based on the evidence presented in this systematic review, the recent ERS guideline on symptom management has made a conditional recommendation that breathing techniques be used to reduce symptoms in people with serious respiratory illness. This recommendation places a high value on consistent improvements in HRQoL for people with COPD and asthma, and a lower value on the uncertainty regarding their effect on breathlessness. It is possible that breathlessness measures used in the included RCTs were not sufficiently sensitive to detect change with breathing techniques (e.g. the mMRC scale). Furthermore, breathing techniques may have a greater impact on breathlessness in those with greater symptom burden, such as those near the end of life, a group who were not included in existing RCTs. In addition to the outcomes measured in this review, people with severe respiratory illness have reported a range of other benefits from breathing techniques, including increased confidence, reduced panic, increased disease mastery and greater control over breathing in daily life [13, 14, 68, 69]. An advantage of breathing techniques is that they can be taught by a variety of health professionals across many settings, with a modest time commitment for patient instruction and training, and low cost. Breathing techniques can also be included in an individualised treatment plan using existing models of care, such as pulmonary rehabilitation or multicomponent symptom management services. Breathing exercises are employed across a range of cultures and spiritual practices, which may enhance acceptability to patients and aid implementation. Health professionals should thoroughly understand the physiological rationale underpinning the chosen technique, such that appropriate instructions and supervision are targeted to individual patient needs.
The strength of our conclusions was limited by the low certainty of evidence. A lack of assessor blinding and intention-to-treat analysis were common, factors which may have a substantial impact in trials of non-pharmacological interventions. The majority of RCTs lacked a prospectively registered protocol, so reporting bias could not be excluded. The included trials were conducted over a 57-year period, with the earliest trial published in 1965 [70]. Although it should be acknowledged that clinical trial methodologies and reporting have changed substantially over that time, risk of bias was also high in many studies that were published more recently. The marked statistical heterogeneity evident in some meta-analyses could not be resolved by removal of trials at high risk of bias. This heterogeneity could reflect the wide variety of breathing techniques used, many of which were poorly described and may be difficult for health professionals and patients to replicate. There was variety in the intensity and duration of the intervention periods, which may have affected the magnitude and persistence of the observed effects. The Template for Intervention Description and Replication (TIDieR) checklist [71] should be used to enhance reporting of intervention components in future trials, along with consideration of emerging initiatives such as video abstracts or video supplements, to ensure that these non-pharmacological treatments can be effectively replicated and delivered. Trial reporting should adhere to the CONSORT statement for RCTs of non-pharmacological interventions [72].
Although current data support the implementation of breathing techniques in clinical practice, there are knowledge gaps that should be addressed in future research. Breathing exercises such as pursed lip breathing were developed for use in people with obstructive lung disease, with the proposed mechanism of reducing intrinsic positive end expiratory pressure and dynamic hyperinflation, and thus reducing symptoms [73, 74]. The physiological relevance and clinical efficacy of these breathing exercises in patients with restrictive lung disease remains to be tested. The potential of biofeedback to enhance breathing techniques should be more comprehensively explored [26–28]. Remote delivery of training for breathing techniques should be examined to enhance accessibility of this intervention, and long-term persistence of benefits should be documented, including measures of cost-effectiveness.
A strength of this review is the inclusion of RCTs from 17 countries across Asia, the Middle East, North and South America, Europe, the UK and Australia. This demonstrates the global interest in breathing techniques for people with respiratory illness, and enhances generalisability. We translated and extracted data from RCTs published in Chinese-language journals. We used a robust systematic review methodology, which included two reviewers independently conducting screening of citations, full-text review and data extraction. While our strategy of using existing systematic reviews to identify earlier trials increased research efficiency, it is possible that some earlier trials could have been missed using this strategy, although the use of eight systematic reviews (supplementary table S1) reduces this risk. Some studies were excluded because they contained insufficient detail to confirm eligibility and no registered protocol (figure 1), which may have excluded relevant data.
In conclusion, breathing techniques may improve breathlessness, and consistently improve HRQoL, in people with serious respiratory illness. Evidence is strongest for breathing exercises (pursed lip breathing and diaphragmatic breathing) and yoga breathing in people with COPD and asthma. These findings support the use of breathing exercises to reduce symptoms in people with serious respiratory illness.
Points for clinical practice
Pursed lip breathing, diaphragmatic breathing and yoga breathing may be useful to reduce breathlessness and enhance quality of life in people with serious respiratory illness.
Breathing techniques can be implemented by a variety of health professionals in many settings; however, health professionals should thoroughly understand the physiological rationale behind the technique, such that appropriate instructions and supervision can be delivered.
Questions for future research
Can breathing techniques reduce breathlessness in people with restrictive lung disease?
Can biofeedback enhance the efficacy of breathing exercises in people with serious respiratory illness?
Do the benefits of breathing techniques persist over time in people with serious respiratory illness?
Acknowledgments
We acknowledge the contribution of Jeanette Boyd from the European Lung Foundation, and extend our sincere thanks to the consumer partners who participated in the ERS Symptom Management Guideline Task Force: Phil Collis, Tessa Jelen, John Solheim and Chantal Vandendungen.
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Smallwood NE, Pascoe A, Wijsenbeek M, et al. Opioids for the palliation of symptoms in people with serious respiratory illness: a systematic review and meta-analysis. Eur Respir Rev 2024; 33: 230265. No. 2: Burge AT, Gadowski AM, Romero L, et al. The effect of graded exercise therapy on fatigue in people with serious respiratory illness: a systematic review. Eur Respir Rev 2024; 33: 240027.
Number 3 in the Series “Symptom management for advanced lung disease” Edited by Anne E. Holland, Magnus Ekström and Natasha E. Smallwood
This article has an editorial commentary: https://doi.org/10.1183/16000617.0205-2024
Conflicts of interest: L. Romero declares funding from the European Respiratory Society to design search strategies for this review. A.E. Holland declares authorship on one of the systematic reviews included in this study but no other conflicts of interest. All other authors declare no conflicts of interest.
Support statement: The European Respiratory Society funded a medical librarian (L. Romero) to design the search strategies for this review. Funding information for this article has been deposited with the Crossref Funder Registry.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0012-2024.SUPPLEMENT (865.7KB, pdf)
References
- 1.Janson C, Marks G, Buist S, et al. . The impact of COPD on health status: findings from the BOLD study. Eur Respir J 2013; 42: 1472–1483. doi: 10.1183/09031936.00153712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanania NA, O'Donnell DE. Activity-related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. Int J Chron Obstruct Pulmon Dis 2019; 14: 1127–1138. doi: 10.2147/COPD.S188141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage 2008; 36: 451–460. doi: 10.1016/j.jpainsymman.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 4.Tikellis G, Tong A, Lee JYT, et al. . Top 10 research priorities for people living with pulmonary fibrosis, their caregivers, healthcare professionals and researchers. Thorax 2021; 76: 575–581. doi: 10.1136/thoraxjnl-2020-215731 [DOI] [PubMed] [Google Scholar]
- 5.Burgess J, Ekanayake B, Lowe A, et al. . Systematic review of the effectiveness of breathing retraining in asthma management. Expert Rev Respir Med 2011; 5: 789–807. doi: 10.1586/ers.11.69 [DOI] [PubMed] [Google Scholar]
- 6.Hindelang M, Kirsch F, Leidl R. Effectiveness of non-pharmacological COPD management on health-related quality of life - a systematic review. Expert Rev Pharmacoecon Outcomes Res 2020; 20: 79–91. doi: 10.1080/14737167.2020.1734455 [DOI] [PubMed] [Google Scholar]
- 7.Holland A, Hill C, Jones A, et al. . Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 10: CD008250. 10.1002/14651858.CD008250.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayawardena R, Ranasinghe P, Ranawaka H, et al. . Exploring the therapeutic benefits of pranayama (yogic breathing): a systematic review. Int J Yoga 2020; 13: 99–110. doi: 10.4103/ijoy.IJOY_37_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santino T, Chaves G, Freitas D, et al. . Breathing exercises for adults with asthma. Cochrane Database Syst Rev 2020; 3: CD001277. doi: 10.1002/14651858.CD001277.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu S, Zhang D, He Q, et al. . Efficacy of Liuzijue Qigong in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med 2022; 65: 102809. doi: 10.1016/j.ctim.2022.102809 [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Zhong H, Mao C, et al. . Yoga for asthma. Cochrane Database Syst Rev 2016; 4: CD010346. 10.1002/14651858.CD010346.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Chen K, Tang W, et al. . Influence of Baduanjin on lung function, exercise capacity and quality of life in patients with mild chronic obstructive pulmonary disease. Medicine (Baltimore) 2020; 99: e22134. doi: 10.1097/MD.0000000000022134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arden-Close E, Teasdale E, Tonkin-Crine S, et al. . Patients’ perceptions of the potential of breathing training for asthma: a qualitative study. Prim Care Respir J 2013; 22: 449–453. doi: 10.4104/pcrj.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp ME, Henriques M, Biguet G, et al. . Experiences of hatha yogic exercises among patients with obstructive pulmonary diseases: a qualitative study. J Bodyw Mov Ther 2018; 22: 896–903. doi: 10.1016/j.jbmt.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Page M, McKenzie J, Bossuyt P, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley AS. Defining “serious illness”. J Palliat Med 2014; 17: 985. doi: 10.1089/jpm.2014.0164 [DOI] [PubMed] [Google Scholar]
- 17.Shea BJ, Reeves BC, Wells G, et al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Altman DG, Gotzsche PG, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cochrane Collaboration . Review Manager (RevMan). Version 5.4. London, The Cochrane Collaboration, 2020. Available at revman.cochrane.org [Google Scholar]
- 20.Dehkordi A, Ebrahimi-Dehkordi S, Banitalebi-Dehkordi F, et al. . The effect of teach-back training intervention of breathing exercise on the level of dyspnea, six-minutes walking test and FEV1/FVC ratio in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Expert Rev Respir Med 2021; 15: 161–169. doi: 10.1080/17476348.2020.1822740 [DOI] [PubMed] [Google Scholar]
- 21.Feng X, Zheng H, Jiang F, et al. . Rehabilitational effects of breathing training in patients with stable chronic obstructive pulmonary disease [Chinese]. Nurs J Chin People’s Liberation Army 2010; 27: 166–168. [Google Scholar]
- 22.Zhang Z, Chen R, Yang Q, et al. . Effects of respiratory training in relation to respiratory pathophysiology on respiratory muscle function and exercise tolerance in chronic obstructive pulmonary disease patients [Chinese]. J Clin Rehabil Tissue Eng Res 2008; 12: 3966–3971. [Google Scholar]
- 23.Gu W, Liang Z, Zhu C, et al. . Clinical outcome of a novel breathing training maneuver in stable COPD patients. Int J Clin Exp Med 2018; 11: 9802–9810. [Google Scholar]
- 24.Bruton A, Lee A, Yardley L, et al. . Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018; 6: 19–28. doi: 10.1016/S2213-2600(17)30474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prem V, Sahoo R, Adhikari P. Comparison of the effects of Buteyko and pranayama breathing techniques on quality of life in patients with asthma: a randomized controlled trial. Clin Rehabil 2013; 27: 133–141. doi: 10.1177/0269215512450521 [DOI] [PubMed] [Google Scholar]
- 26.Collins E, Langbein W, Fehr L, et al. . Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2008; 177: 844–852. doi: 10.1164/rccm.200703-477OC [DOI] [PubMed] [Google Scholar]
- 27.Collins E, O'Connell S, Jelinek C, et al. . Contrasting breathing retraining and helium-oxygen during exercise training in COPD: a randomized clinical trial. Am J Respir Crit Care Med 2014; 108: 297–306. 10.1016/j.rmed.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Collins E, Jelinek C, O'Connell S, et al. . The effect of breathing retraining using metronome-based acoustic feedback on exercise endurance in COPD: a randomized trial. Lung 2019; 197: 181–188. doi: 10.1007/s00408-019-00198-4 [DOI] [PubMed] [Google Scholar]
- 29.Van Gestel A, Kohler M, Steier J, et al. . The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration 2012; 83: 115–124. doi: 10.1159/000324449 [DOI] [PubMed] [Google Scholar]
- 30.Peng N, Chen M, Shou Z. Effect of tiotropium bromide, N-acetylcysteine and respiratory training on pulmonary function, activity tolerance and quality of life of patients with chronic obstructive pulmonary disease. Trop J Pharm Res 2022; 21: 649. doi: 10.4314/tjpr.v21i3.27 [DOI] [Google Scholar]
- 31.Ceyhan Y, Tekinsoy Kartin P. The effects of breathing exercises and inhaler training in patients with COPD on the severity of dyspnea and life quality: a randomized controlled trial. Trials 2022; 23: 707. doi: 10.1186/s13063-022-06603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y. The effects of respiratory function training on pulmonary function in patients with COPD [Chinese]. J Front Med 2016; 6: 356–358. [Google Scholar]
- 33.Grammatopoulou E, Skordilis E, Stavrou N, et al. . The effect of physiotherapy-based breathing retraining on asthma control. J Asthma 2011; 48: 593–601. doi: 10.3109/02770903.2011.587583 [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Park J, Lyu Y, et al. . The effect of lung-conduction exercise in chronic obstructive pulmonary disease: randomized, assessor-blind, multicenter pilot trial. Medicine (Baltimore) 2022; 101: e28629. doi: 10.1097/MD.0000000000028629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang W, Wang M, Lin W. The clinical applying study of a novel breathing training manoeuvre in patients with COPD [Chinese]. Int J Respiration (Guoji Huxi Zazhi) 2016; 36: 1458–1461. [Google Scholar]
- 36.Yamaguti W, Claudino R, Neto A, et al. . Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized trial. Arch Phys Med Rehabil 2012; 93: 571–577. 10.1016/j.apmr.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 37.De Torres JP, Pinto-Plata V, Ingenito E, et al. . Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002; 121: 1092–1098. doi: 10.1378/chest.121.4.1092 [DOI] [PubMed] [Google Scholar]
- 38.Lin F, Yeh M, Lai Y, et al. . Two-month breathing-based walking improves anxiety, depression, dyspnoea and quality of life in chronic obstructive pulmonary disease: a randomised controlled study. J Clin Nurs 2019; 28: 3632–3640. doi: 10.1111/jocn.14960 [DOI] [PubMed] [Google Scholar]
- 39.Chauhan A, McLindon J, Dillon P, et al. . Regular balloon inflation for patients with chronic bronchitis: a randomised controlled trial. BMJ 1992; 304: 1668–1669. doi: 10.1136/bmj.304.6843.1668-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nese A, Baglama S. The effect of progressive muscle relaxation and deep breathing exercises on dyspnea and fatigue symptoms of COPD patients: a randomized controlled study. Holist Nurs Pract 2022; 36: E18–E26. doi: 10.1097/HNP.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 41.Nield M, Soo Hoo G, Roper J, et al. . Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27: 237–244. doi: 10.1097/01.HCR.0000281770.82652.cb [DOI] [PubMed] [Google Scholar]
- 42.Donesky D, Melendez M, Nguyen H, et al. . A responder analysis of the effects of yoga for individuals with COPD: who benefits and how? Int J Yoga Therap 2012; 22: 23–35. doi: 10.17761/ijyt.22.1.84352754221r1774 [DOI] [PubMed] [Google Scholar]
- 43.Jones PW. St George's respiratory questionnaire: MCID. COPD 2005; 2: 75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 44.Li P, Liu J, Lu Y, et al. . Effects of long-term home-based Liuzijue exercise combined with clinical guidance in elderly patients with chronic obstructive pulmonary disease. Clin Interv Aging 2018; 13: 1391–1399. doi: 10.2147/CIA.S169671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen L, Zhang Y, Su Y, et al. . New pulmonary rehabilitation exercise for pulmonary fibrosis to improve the pulmonary function and quality of life of patients with idiopathic pulmonary fibrosis: a randomized control trial. Ann Palliat Med 2021; 10: 7289. doi: 10.21037/apm-21-71 [DOI] [PubMed] [Google Scholar]
- 46.Andreasson K, Skou S, Ulrik C, et al. . Breathing exercises for patients with asthma in specialist care: a multicenter randomized clinical trial. Ann Am Thorac Soc 2022; 19: 1498–1506. doi: 10.1513/AnnalsATS.202111-1228OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georga G, Chrousos G, Artemiadis A, et al. . The effect of stress management incorporating progressive muscle relaxation and biofeedback-assisted relaxation breathing on patients with asthma: a randomised controlled trial. Advan Integ Med 2019; 6: 73–77. 10.1016/j.aimed.2018.09.001. [DOI] [Google Scholar]
- 48.Baskan B, Alptekin K, Pur Ozyigit L, et al. . The impact of breathing exercises on quality of life and anxiety in asthma patients. Gazz Med Ital 2022; 181: 59–65. 10.23736/S0393-3660.19.04293-1. [DOI] [Google Scholar]
- 49.Coulson E, Carpenter L, Georgia T, et al. . Breathing exercises in older adults with asthma: a blinded, randomized, placebo-controlled trial. J Asthma 2022; 59: 1438–1444. doi: 10.1080/02770903.2021.1936015 [DOI] [PubMed] [Google Scholar]
- 50.Laurino R, Barnabe V, Saraiva-Romanholo B, et al. . Respiratory rehabilitation: a physiotherapy approach to the control of asthma symptoms and anxiety. Clinics (Sao Paulo) 2012; 67: 1291–1297. doi: 10.6061/clinics/2012(11)12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slader C, Redel H, Spencer L, et al. . Double blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax 2006; 61: 651–656. doi: 10.1136/thx.2005.054767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agnihotri S, Kant S, Mishra S, et al. . Assessment of significance of yoga on quality of life in asthma patients: a randomized controlled study. Ayu 2017; 38: 28–32. doi: 10.4103/ayu.AYU_3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sodhi C, Singh S, Bery A. Assessment of the quality of life in patients with bronchial asthma, before and after yoga: a randomised trial. Iran J Allergy Asthma Immunol 2014; 13: 55–60. [PubMed] [Google Scholar]
- 54.Turan G, Tan M. The effect of yoga on respiratory functions, symptom control and life quality of asthma patients: a randomized controlled study. Complement Ther Clin Pract 2020; 38: 101070. 10.1016/j.ctcp.2019.101070 [DOI] [PubMed] [Google Scholar]
- 55.Sangeethalaxmi MJ, Hankey A. Impact of yoga breathing and relaxation as an add-on therapy on quality of life, anxiety, depression and pulmonary function in young adults with bronchial asthma: a randomized controlled trial. J Ayurveda Integr Med 2023; 14: 100546. 10.1016/j.jaim.2022.100546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S, Soni R, Singh K, et al. . Effect of yoga practices on pulmonary function tests including transfer factor of lung for carbon monoxide (TLCO) in asthma patients. Indian J Physiol Pharmacol 2012; 56: 63–68. [PubMed] [Google Scholar]
- 57.Vempati R, Bijlani R, Deepak K. The efficacy of a comprehensive lifestyle modification programme based on yoga in the management of bronchial asthma: a randomized controlled trial. BMC Pulm Med 2009; 30: 37. 10.1186/1471-2466-9-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuce G, Tasci S. The effect of pranayama breathing technique on asthma control, pulmonary function and quality of life: a single-blind, randomized, controlled trial. Complement Ther Clin Pract 2020; 38: 101081. doi: 10.1016/j.ctcp.2019.101081 [DOI] [PubMed] [Google Scholar]
- 59.Sabina A, Williams A, Wall H, et al. . Yoga intervention for adults with mild-to-moderate asthma: a pilot study. Ann Allergy Asthma Immunol 2005; 94: 543–548. doi: 10.1016/S1081-1206(10)61131-3 [DOI] [PubMed] [Google Scholar]
- 60.Opat A, Cohen M, Bailey M, et al. . A clinical trial of the Buteyko breathing technique in asthma as taught by a video. J Asthma 2000; 37: 557–564. doi: 10.3109/02770900009090810 [DOI] [PubMed] [Google Scholar]
- 61.Cooper S, Oborne J, Newton S, et al. . Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax 2003; 58: 674–679. doi: 10.1136/thorax.58.8.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holloway E, West R. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007; 62: 1039–1042. doi: 10.1136/thx.2006.076430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pourdowlat G, Hejrati R, Lookzadeh S. The effectiveness of relaxation training in the quality of life and anxiety of patients with asthma. Adv Respir Med 2019; 87: 146–151. doi: 10.5603/ARM.2019.0024 [DOI] [PubMed] [Google Scholar]
- 64.Liu F, Cai H, Tang Q, et al. . Effects of an animated diagram and video-based online breathing program for dyspnea in patients with stable COPD. Patient Prefer Adherence 2013; 7: 905–913. 10.2147/PPA.S43305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehrer P, Vaschillo E, Vaschillo B, et al. . Biofeedback treatment for asthma. Chest 2004; 126: 352–361. doi: 10.1378/chest.126.2.352 [DOI] [PubMed] [Google Scholar]
- 66.Kaminsky D, Guntupalli K, Lippmann J, et al. . Effect of yoga breathing (pranayama) on exercise tolerance in patients with chronic obstructive pulmonary disease: a randomized, controlled trial. J Altern Complement Med 2017; 23: 696–704. doi: 10.1089/acm.2017.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranjita R, Badhai S, Hankey A, et al. . A randomized controlled study on assessment of health status, depression, and anxiety in coal miners with chronic obstructive pulmonary disease following yoga training. Int J Yoga 2016; 9: 137–144. doi: 10.4103/0973-6131.183714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arden-Close E, Yardley L, Kirby S, et al. . Patients’ experiences of breathing retraining for asthma: a qualitative process analysis of participants in the intervention arms of the BREATHE trial. NPJ Prim Care Respir Med 2017; 27: 56. doi: 10.1038/s41533-017-0055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts S, Schreuder F, Watson T, et al. . Do COPD patients taught pursed lips breathing (PLB) for dyspnoea management continue to use the technique long-term? A mixed methodological study. Physiotherapy 2017; 103: 465–470. doi: 10.1016/j.physio.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 70.Saunders K, White J. Controlled trial of breathing exercises. Br Med J 1965; 2: 680–682. doi: 10.1136/bmj.2.5463.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann TC, Glasziou PP, Boutron I, et al. . Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 72.Boutron I, Altman DG, Moher D, et al. . CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017; 167: 40–47. doi: 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 73.Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101: 75–78. doi: 10.1378/chest.101.1.75 [DOI] [PubMed] [Google Scholar]
- 74.Visser FJ, Ramlal S, Dekhuijzen PN, et al. . Pursed-lips breathing improves inspiratory capacity in chronic obstructive pulmonary disease. Respiration 2011; 81: 372–378. doi: 10.1159/000319036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0012-2024.SUPPLEMENT (865.7KB, pdf)