Abstract
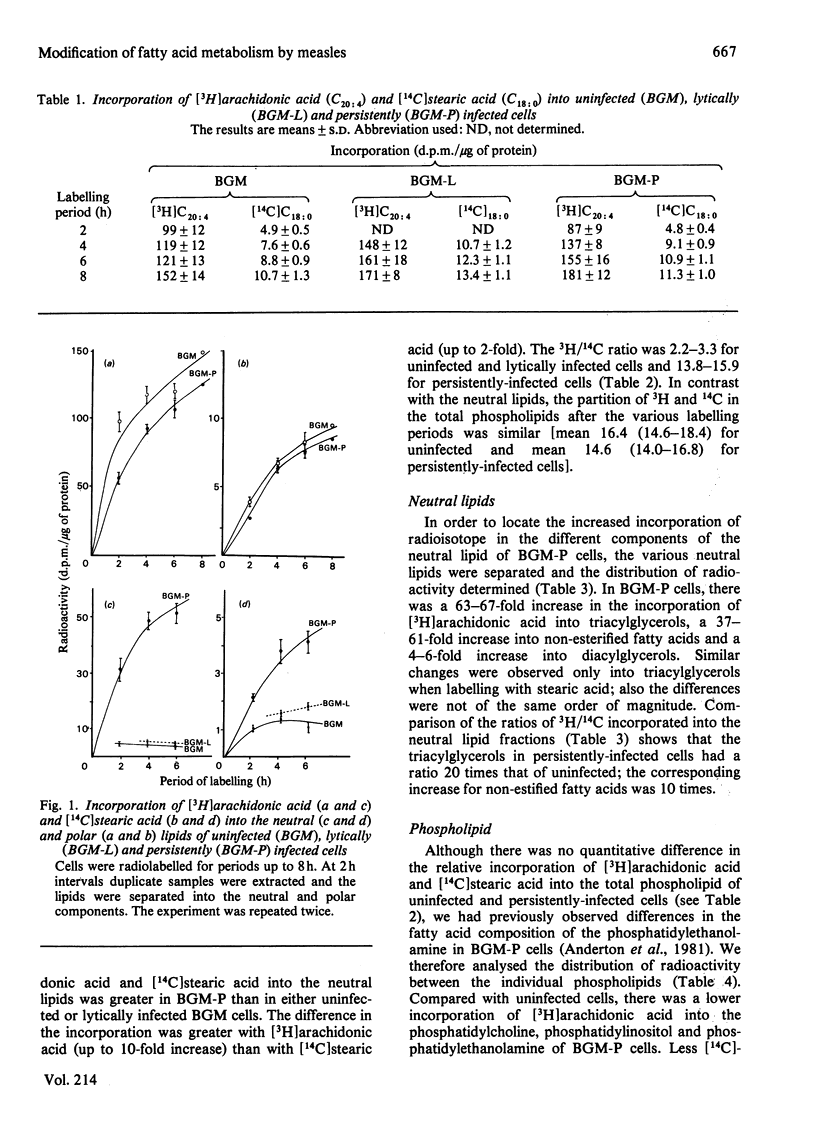
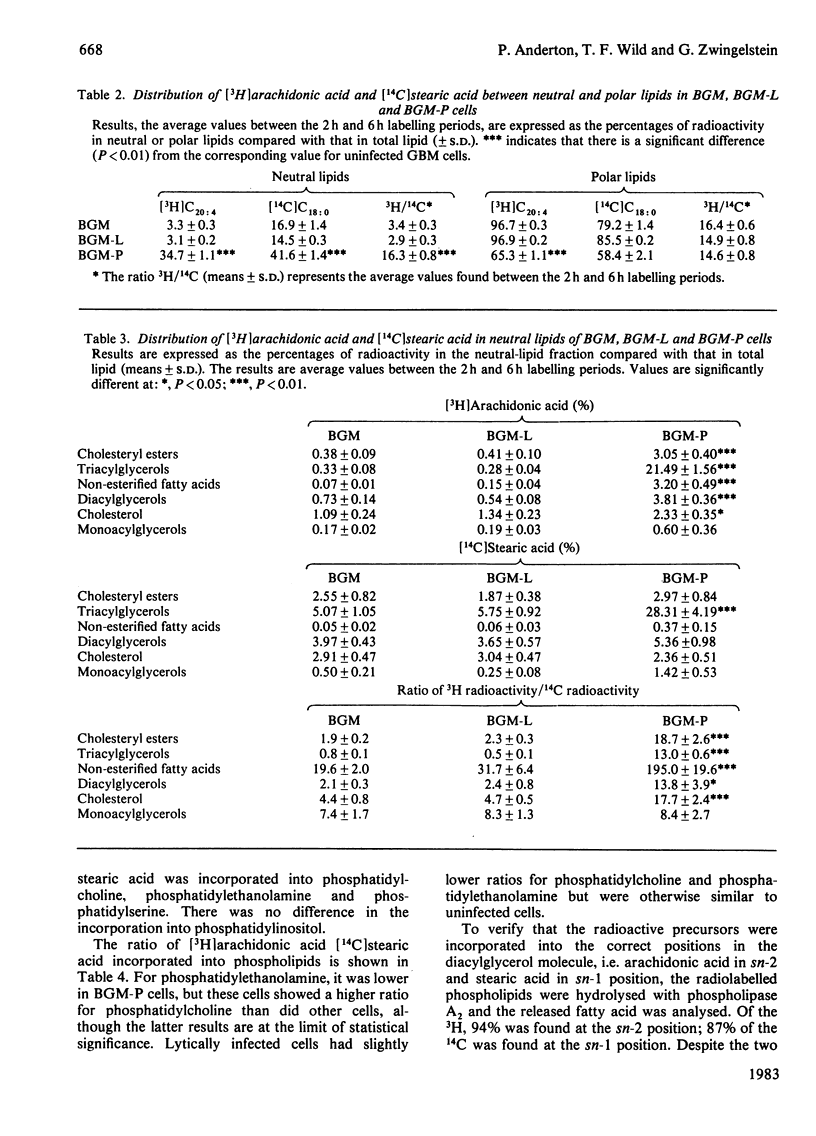
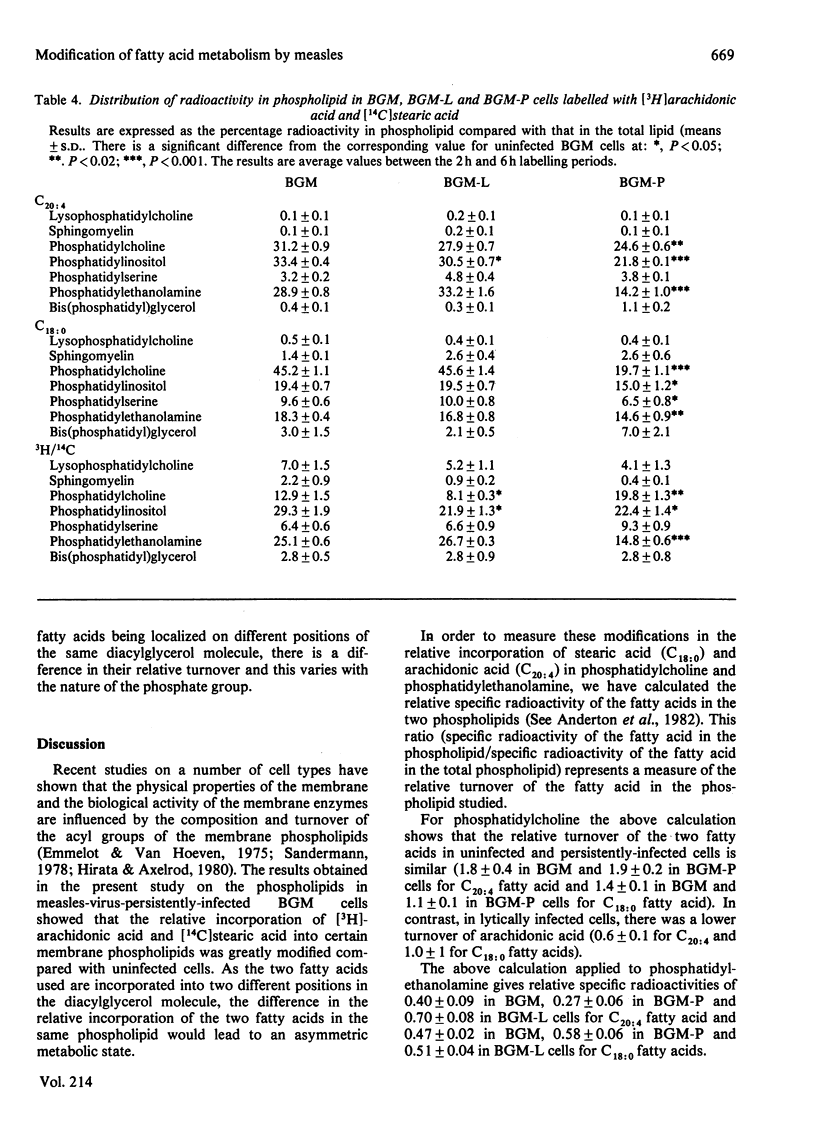
In BGM cells chronically infected with measles virus, although the composition of the phospholipids is unaltered, the fatty acid composition is modified. Uninfected, lytic and persistently infected cells were labelled with [3H]arachidonic acid and [14C]stearic acid and their metabolic fate analysed. No difference in the total incorporation was observed in the different systems. However, the [14C]stearic acid and [3H]arachidonic acid were incorporated up to 2-fold and 13-fold respectively greater into the neutral lipid of persistently infected compared with that of uninfected cells. Both radioactive fatty acids were specifically accumulated in the triacylglycerol and non-esterified fatty acids fractions. Lytically infected cells were similar to uninfected cells. Although there was no significant difference in the incorporation of radioactivity into the total phospholipid in either system, there was a large decrease in [3H]arachidonic acid incorporated into phosphatidylethanolamine and to a lesser extent phosphatidylcholine and phosphatidylinositol in persistently infected cells. [14C]Stearic acid incorporation was also reduced in phosphatidylcholine and phosphatidylethanolamine fractions of persistently infected cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton P., Wild T. F., Zwingelstein G. Accumulation of radiolabelled fatty acids in the neutral lipid fraction of measles virus persistently infected BGM cells. Biochem Biophys Res Commun. 1983 Apr 15;112(1):29–34. doi: 10.1016/0006-291x(83)91792-8. [DOI] [PubMed] [Google Scholar]
- Anderton P., Wild T. F., Zwingelstein G. Modification of the fatty acid composition of phospholipid in measles virus-persistently infected cells. Biochem Biophys Res Commun. 1981 Nov 16;103(1):285–291. doi: 10.1016/0006-291x(81)91691-0. [DOI] [PubMed] [Google Scholar]
- Anderton P., Wild T. F., Zwingelstein G. Phospholipids in a measles virus persistent infection: modification of fatty acid metabolism and fatty acid composition of released virus. J Gen Virol. 1982 Oct;62(Pt 2):249–258. doi: 10.1099/0022-1317-62-2-249. [DOI] [PubMed] [Google Scholar]
- Arvidson G. A. Biosynthesis of phosphatidylcholines in rat liver. Eur J Biochem. 1968 Aug;5(3):415–421. doi: 10.1111/j.1432-1033.1968.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Emmelot P., Van Hoeven R. P. Phospholipid unsaturation and plasma membrane organization. Chem Phys Lipids. 1975 May;14(3):236–246. doi: 10.1016/0009-3084(75)90005-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Haverkate F., van Deenen L. L. Isolation and chemical characterization of phosphatidyl glycerol from spinach leaves. Biochim Biophys Acta. 1965 Jul 7;106(1):78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic synthesis and rapid translocation of phosphatidylcholine by two methyltransferases in erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 May;75(5):2348–2352. doi: 10.1073/pnas.75.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Münzel P., Koschel K. Alteration in phospholipid methylation and impairment of signal transmission in persistently paramyxovirus-infected C6 rat glioma cells. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3692–3696. doi: 10.1073/pnas.79.12.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Skipski V. P. Thin-layer chromatography of neutral glycosphingolipids. Methods Enzymol. 1975;35:396–425. doi: 10.1016/0076-6879(75)35178-1. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Baringer J. R. Persistent, slow and latent viral infections. Prog Med Virol. 1982;28:1–43. [PubMed] [Google Scholar]
- Vance D. E., Burke D. C. Inhibition of 3-sn-phosphatidylcholine biosynthesis in baby-hamster kidney-21 cells infected with Semliki Forest virus. Eur J Biochem. 1974 Apr 1;43(2):327–336. doi: 10.1111/j.1432-1033.1974.tb03416.x. [DOI] [PubMed] [Google Scholar]
- Wild T. F., Dugre R. Establishment and characterization of a subacute sclerosing panencephalitis (measles) virus persistent infection in BGM cells. J Gen Virol. 1978 Apr;39(1):113–124. doi: 10.1099/0022-1317-39-1-113. [DOI] [PubMed] [Google Scholar]


