Abstract
Recently, the California Department of Public Health issued an advisory related to the substantial rise in Coccidioidomycosis in California, which has been attributed in part to climate change and rapid housing development. Most cases are self-limiting, but some may spread to the meninges, resulting in coccidioidal meningitis (CM). Many providers mistakenly presume that CM is limited to patients who are immunocompromised. In this case series and literature review, we present 12 cases of CM in immunocompetent individuals seen at a single tertiary academic center between 1 January 2019 and 31 December 2023. All 12 cases developed complications, with 10 requiring ventriculoperitoneal shunting, 6 having spinal cord involvement (5 with cervical spine involvement), 4 having strokes, and 3 dying from complications related to CM. It is important to recognize CM as it may be life-threatening if not promptly diagnosed.
Keywords: climate change, coccidioidal meningitis, coccidioides, fungal meningitis, immunocompetence
Epidemiology
Coccidioidomycosis is a fungal infection caused by Coccidioides, a fungus found in soil endemic to areas in the southwestern United States, northern Mexico, and parts of Central and South America [1, 2]. Rapid housing development has led to an increase in the incidence of cases. From 1998 to 2011 in Arizona and California, incident cases rose from 5.3 per 100 000 to 42.6 per 100 000 [3]. Earlier this year, the California Department of Public Health issued a health advisory regarding the substantial increase in Coccidioidomycosis, citing 9280 cases, suspected to be due in part to “heavy rainfall in the winter of 2022–2023 following years of drought” [4]. Global warming has been further implicated in the expansion of Coccidioides spores into new territories such as Washington state because of warmer and drier climates [5].
Although a review of coccidioidomycosis in immunocompetent patients is presented elsewhere [6], we review the existing literature specific to coccidioidal meningitis (CM), present a chart review of our single-center demographics of 33 CM cases, and provide a case series of 12 immunocompetent individuals (9 males, 3 females) to help guide recognition and management of this potentially life-threatening condition.
METHODS
Literature Review
We conducted a search of the PUBMED database for articles published in English, available as full text, involving human participants, up until 1 June 2024, using the MESH terms “meningitis” AND “coccidioides,” excluding preprints. The entries were screened by 3 authors (R.R., J.D., and M.H.) for potential relevance.
Participant Identification
Our institution is an academic tertiary referral center located in Orange County, California, USA. A chart review tool (Slicer Dicer, Epic, Verona, USA) was used to search the electronic medical record for cases of CM using the International Classification of Diseases, revision 10, code for CM (ICD-10-CM: B38.4) between 1 January 2019 and 31 December 2023. Thirty-three cases were identified and 12 cases were directly seen by at least 1 of the physician coauthors and were determined to be immunocompetent at time of initial infection. Institutional review board approval was obtained for this study from the University of California, Irvine institutional review board (protocol # 3352).
Statistical Analyses
Fisher exact test analyses were used to determine if CM prevalence occurred at a greater-than-chance rate based on self-identified race or ethnicity compared to 2021 US census data for racial and ethnic demographics in Orange County, California, USA, from the American Community Survey [7].
RESULTS
Literature Review
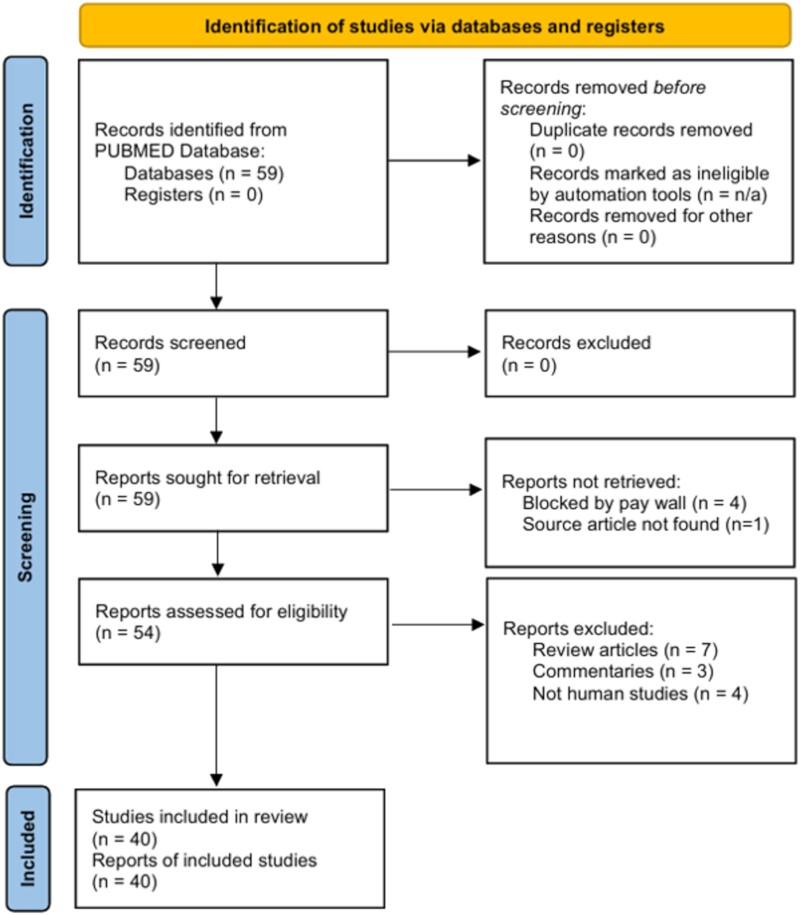
A total of 59 entries were identified (Figure 1). Forty articles were deemed to be relevant original articles; 18 articles reported at least 1 immunocompetent CM case, 15 did not report on immune status, and 7 had only immunocompromised cases. Supplementary Table 1 contains the complete study list. No studies directly compared immunocompetent cases to immunocompromised cases of CM. Twelve additional review articles are incorporated into the literature review to broaden the discussion of coccidiomycosis for context.
Figure 1.
Diagram of literature review search. This literature search does not meet all of the requirements of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. This format of this diagram is provided for the viewer's convenience. Source: Page MJ, et al. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
Literature Review: Epidemiology
A total of 89 immunocompetent CM cases were identified. The youngest case was age 23 days [8], most were between age 30 and 60 years, whereas the oldest was age 80 years [9]. A retrospective analysis from southern Arizona revealed that the demographics most affected were men (2:1 male: female ratio) of Asian, African, and Hispanic descent [10].
Literature Review: Clinical Presentations
It is estimated that 95% of pulmonary infections self-resolve, but if the fungus spreads to the central nervous system, it is universally fatal without treatment [1, 11]. Patients can present with symptoms as minor as a mild headache. More serious symptoms include global headaches lasting from weeks to months, cognitive decline, gait disturbances, diplopia, disorientation, lethargy, and/or stupor [1]. On physical examination, nuchal rigidity is uncommon [1]. Signs of pulmonary or extrapulmonary infection occur in only 1/3 to 2/3 of patients with CM and clinical presentations will depend on these additional sites of infection [1].
Literature Review: Diagnostic Work Up
CM diagnosis relies on cerebral spinal fluid (CSF) or biopsy [12]. In immunocompetent patients, detectable serum immunoglobulin M (IgM) antibody is typically seen within 1 to 3 weeks of symptom onset, followed shortly thereafter by IgG production [13]. Immunodiffusion (ID) and complement fixation (CF) remain the benchmarks, with the former test being more sensitive. A positive CF test for IgG antibody in CSF is considered diagnostic for CM in the setting of a compatible clinical syndrome [14]. High serum titers may “spill over” into CSF, resulting in false-positive ID results for CSF samples. Therefore, CF is the recommended diagnostic modality for CM diagnosis. A serum CF titer greater than 1:32 is concerning for dissemination of the disease [13, 14]. A CSF culture confirms a diagnosis of CM but is typically positive in only 25% of patients [15]. A cellular profile of CSF that would be suggestive of CM may include a lymphocytic, polymorphonuculear neutrophilic, or characteristically eosinophilic pleocytosis [12] with elevated protein, low glucose [1, 11, 12, 16], and elevated opening pressure. Diagnostic techniques that have not been thoroughly validated should be avoided, including measuring (1,3)-β-glucan in serum, antibody enzyme immunoassays, lateral flow tests [1, 11], and polymerase chain reaction for presence of fungal nucleic acid [17]. Hydrocephalus may be seen on noncontrast head computed tomography [18], whereas magnetic resonance imaging may show leptomeningeal enhancement and nodularity [1, 16].
Literature Review: Effective Treatments
Treatment was revolutionized in the 1990s when third-generation azoles were proven to be effective in managing CM [12]. Nonetheless, azoles are fungistatic agents and lifelong treatment is required. The most common primary treatment for CM is fluconazole at 400–1200 mg daily, but there is no consensus for the starting dose [12]. Azoles should also be avoided in patients in the first trimester of pregnancy because of teratogenicity [11, 12]. Adjunctive treatments include corticosteroids, which were associated with a significant reduction in secondary cerebrovascular events in a multicenter retrospective cohort [19]. A coccoides vaccine remains elusive [20, 21].
Chart Review Demographics
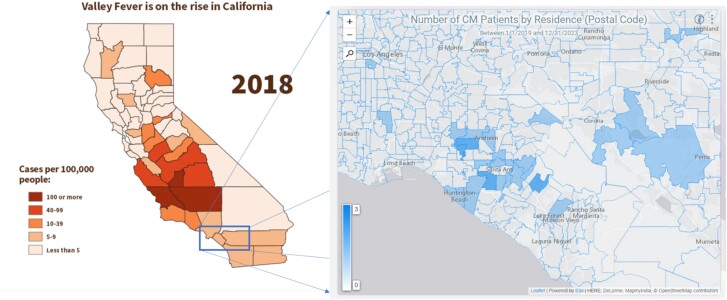
Demographic comparisons of the 33 cases identified through chart review and the 12 immunocompetent cases is provided in Table 1. The number of new cases remained stable across the 5-year period with an average of roughly 3 new cases/year (± 2) (Supplementary Figure 1). Fifteen cases (45%) occurred in patients who were between age 18 and 50 years and 18 were >50 (55%) with a median age of 53 (± 16) years. However, this represents the patients’ current age and the age of infection onset could not be determined from this method. Twenty-five patients were male and 8 were female (roughly 3:1). A total of 52% of patients self-identified as White, 19% as Other/Mixed race, 19% as Asian, 9% as Black, and 3% as Native Hawaiian or Pacific Islander (Supplementary Figure 2). Forty-eight percent identified as Hispanic and 52% as non-Hispanic (not pictured). Geographic distribution by postal code of residence for cases is presented in Figure 2.
Table 1.
Demographics of Coccidioides Meningitis Cases
| Total CM Cases (n = 33) (%) | Immunocompetent Subset (n = 12) (%) | |
|---|---|---|
| Age in y: mean (range) | 53 (24–86) | 34 (21–67) |
| Female | 8 (24) | 3 (25) |
| Male | 25 (76) | 9 (75) |
| Asian | 6 (19) | 2 (17) |
| Black | 3 (9) | 2 (17) |
| Native Hawaiian or Other Pacific Islander | 1 (3) | 1 (8) |
| Other Race or Mixed Race | 6 (19) | 2 (17) |
| Not stated | 1 (3) | 0 (0) |
| White | 17 (52) | 5 (42) |
| Hispanic | 16 (48) | 4 (33) |
| Non-Hispanic | 17 (52) | 8 (66) |
Abbreviation: CM, coccoides meningitis.
Figure 2.
Incidence of coccidioidomycosis reported by the California Department of Public Health in 2018 (left) compared to the geographic distribution of Coccidioides meningitis (CM) cases by postal code of residence in this case series (right). Shaded represent the postal codes in which patients which CM reside. The deeper intensity of shading represents a higher number of cases, ranging from 0 to 3. Source: Sondermeyer Cooksey GL, Nguyen A, Vugia D, Jain S. Regional analysis of coccidioidomycosis incidence—California, 2000–2018. MMWR Morb Mortal Wkly Rep. 2020;69(48):1817–1821. Published 2020 Dec 4. doi:10.15585/mmwr.mm6948a4.
Immunocompetent Cases
Twelve participants directly assessed by the physician authors were determined to be immunocompetent. All 12 had nonreactive HIV serum studies. A summary of these cases is provided in Table 2. The median age of symptom onset was 34 (± 15 years; range, 21–67) years. There were 9 males and 3 females. Two cases endorsed exposure to a construction site where excavation was ongoing. One had known celiac disease with no evidence of immunocompromise on diagnostic studies or history of treatment with immunomodulatory therapies. The 9 remaining cases had no clear predisposing condition or exposure risk.
Table 2.
Summary of Case Series
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Legal sex | M | M | F |
| Age of onset (y) | 21 | 25 | 25 |
| Time of onset before review (y) | 3 | 2 | 6 |
| Self-identified race | African American | Asian American | White |
| Self-identified ethnicity | Non-Hispanic | Non-Hispanic | Hispanic |
| Predisposing condition | None known | None known | None known |
| Possible source | Inland Empire, CA | Oregon | Fountain Valley, CA |
| Presenting symptoms | Headaches, progressive weakness | Headaches, diplopia, and left-sided weakness | Headaches, confusion, and vomiting |
| Classic hydrocephalus triad on presentation? | Yes | Yes | Yes |
| Initial examination findings | Diffuse, symmetric weakness affecting upper extremities > lower extremities, hyperreflexia with bilateral Hoffman and Babinski signs | Left-sided distal > proximal weakness, hyperreflexia, and ataxia | Unknown |
| Evidence of hydrocephalus on first scan? | Yes | Yes | Unknown |
| Time from symptom onset to first evidence of hydrocephalus on imaging (wk) | 6 | 3 | Unknown |
| HIV status | Negative | Negative | Negative |
| Method of establishing diagnosis | CSF and serum complement fixation | CSF antibody Serum complement fixation |
CSF fungal culture Serum complement fixation |
| Complications | Syringomyelia in C-spine with lumbar drain, superimposed bacterial infection of VPS | Hydrocephalus | Intradural lumbar spinal abscess, blurry vision, craniectomy, hydrocephalus, BLE weakness |
| C-spine involvement | Yes | Yes | No |
| Needed VPS? | Yes | Yes | Yes |
| Treatment | Fluconazole | Fluconazole | Fluconazole |
| Time to treatment (wk) | 6 | 12 | Unknown |
| Outcome | Return to full strength, near-total resolution of symptoms | Improved to near-baseline | Improved to near-baseline |
| Modified Rankin scale | 2 | 0 | 4 |
| Deceased? | No | No | No |
| Time from symptom onset to death | N/A | N/A | N/A |
| Case 4 | Case 5 | Case 6 | |
|---|---|---|---|
| Legal sex | M | M | F |
| Age of onset | 27 | 27 | 31 |
| Time of onset before review (y) | 2 | 22 | 8 |
| Self-identified race | Asian American | Other Race or Mixed Race | Native Hawaiian or Other Pacific Islander |
| Self-identified ethnicity | Non-Hispanic | Hispanic | Non-Hispanic |
| Predisposing condition | None known | None known | None known |
| Possible source | California central valley | Santa Ana, CA | Fountain Valley, CA |
| Presenting symptoms | Headaches, confusion, and vomiting | Neck pain, mild headache | Headaches, confusion, and vomiting |
| Classic hydrocephalus triad on presentation? | Yes | No | Yes |
| Initial examination findings | Right upper extremity weakness, blurred disc margins on fundoscopy | + Brudzinski sign, decreased breath sounds in RUL | Nonfocal headache |
| Evidence of hydrocephalus on first scan? | No | No | No |
| Time from symptom onset to first evidence of hydrocephalus on imaging (wk) | 5 | Unknown | 4 |
| HIV status | Negative | Negative | Negative |
| Method of establishing diagnosis | CSF fungal culture Serum immunodiffusion |
Serum complement fixation | CSF fungal culture Serum complement fixation |
| Complications | Hydrocephalus, strokes | Gait imbalance, hydrocephalus c/b tonsillar herniation with medulla compression, c-spine epidural abscess, propionibacterium acnes meningitis, ataxia, blurry vision, muscle atrophy, stroke c/b left hemiparesis | Bilateral retroorbital pain, diplopia, Parinaud syndrome, superior hemifield visual defects |
| C-spine involvement | No | Yes | No |
| Needed VPS? | No | Yes | Yes |
| Treatment | Fluconazole | Fluconazole, amphotericin | Amphotericin, fluconazole, voriconazole |
| Time to treatment (wk) | 3 | Recurrent across many years (nonadherent to fluconazole because of price) | Unknown |
| Outcome | Improved to near-baseline | New baseline of spasticity in BLEs, atrophic muscle bulk, and wide-based gait (patient now uses cane for ambulation) | Improved to near-baseline |
| Modified Rankin scale | 1 | 3 | 1 |
| Deceased? | No | No | No |
| Time from symptom onset to death | N/A | N/A | N/A |
| Case 7 | Case 8 | Case 9 | |
|---|---|---|---|
| Legal sex | M | M | M |
| Age of onset | 37 | 44 | 47 |
| Time of onset prior to review (y) | 7 | 8 | 6 |
| Self-identified race | Other Race or Mixed Race | African American | White |
| Self-identified ethnicity | Hispanic | Non-Hispanic | Hispanic |
| Predisposing condition | Exposure to excavation site | Exposure to excavation site | None known |
| Possible source | Lake Forest, CA | Sacramento, CA | Santa Ana, CA |
| Presenting symptoms | Headaches, dizziness, double vision | Intermittent headaches and left eye deficits | Headache, confusion, and seizures |
| Classic hydrocephalus triad on presentation? | No | No | Yes |
| Initial examination findings | Unknown | Left-sided vision loss and headache | Photosensitivity, headache, imbalance, speech difficulty, upper left maxillary abscess |
| Evidence of hydrocephalus on first scan? | Unknown | No | Unknown |
| Time from symptom onset to first evidence of hydrocephalus on imaging (wk) | Unknown | Unknown | Unknown |
| HIV status | neg | neg | neg |
| Method of establishing diagnosis | CSF fungal culture | Serum complement fixation | CSF and serum complement fixation |
| Complications | Abdominal pseudocyst adjacent to VPS catheter tip | Left optic nerve atrophy, possible left lacrimal neoplasm, left lower lobe scarring from inoculation pneumonia | Thalamic stroke, seizure disorder, communicating hydrocephalus, cerebritis, ptosis and diplopia of left eye, cranial nerve III palsy |
| C-spine involvement | No | No | No |
| Needed VPS? | Yes | No | Yes |
| Treatment | Voriconazole to fluconazole because of elevated LFTs | Voriconazole | Fluconazole (failed, transitioned to voriconazole now) |
| Time to treatment (wk) | Unknown | Unknown | Unknown |
| Outcome | Improved to near-baseline | unknown | Improved to near-baseline |
| Modified Rankin scale | 1 | 2 | 4 |
| Deceased? | No | No | No |
| Time from symptom onset to death | N/A | N/A | N/A |
| Case 10 | Case 11 | Case 12 | |
|---|---|---|---|
| Legal sex | M | M | F |
| Age of onset | 55 | 56 | 67 |
| Time of onset prior to review (y) | 11 | 4 | 3 |
| Self-identified race | White | White | White |
| Self-identified ethnicity | Non-Hispanic | Hispanic | Non-Hispanic |
| Predisposing condition | None known | None known | Celiac disease |
| Possible source | Tustin, CA | Bakersfield, CA | Arizona |
| Presenting symptoms | Progressive weakness involving all 4 limbs starting from the left side | Headaches, confusion, and vomiting | Headaches, fever, and confusion |
| Classic hydrocephalus triad on presentation? | No | Yes | Yes |
| Initial examination findings | Unknown | Encephalopathic without other pertinent findings | Obtunded, multidirectional nystagmus, localizing to noxious stimuli, bilateral Hoffman and Babinski signs |
| Evidence of hydrocephalus on first scan? | Unknown | Unknown | No |
| Time from symptom onset to first evidence of hydrocephalus on imaging (wk) | Unknown | Unknown | 7 |
| HIV Status | Negative | Negative | Negative |
| Method of establishing diagnosis | CSF and serum complement fixation | CSF immunodiffusion Serum complement fixation |
CSF complement fixation and immunodiffusion Brain biopsy |
| complications | Myelomalacia, Quadriparesis resulting in tracheostomy and ventilator dependency, arachnoid cyst (syrinx) formation (C3), bilateral blurry vision | Hydrocephalus, strokes, leptomeningeal spread | Hydrocephalus, strokes |
| C-spine involvement | Yes | Yes | No |
| Needed VPS? | Yes | Yes | Yes |
| Treatment | fluconazole | fluconazole | fluconazole |
| Time to treatment (wk) | Unknown | 20 | 12 |
| Outcome | Passed from complications of secondary infections in setting of quadriparesis | Passed from complications of basilar meningitis (recurrent strokes and hydrocephalus) | Passed from complications of basilar meningitis (recurrent strokes and hydrocephalus) |
| Modified Rankin scale | 6 | 6 | 6 |
| Deceased? | Yes | Yes | Yes |
| Time from symptom onset to death | 11 y | 18 mo | 5 mo |
Abbreviations: BLE, bilateral lower extremities; c/b, complicated by; C-spine, cervical spine; F, female; M, male; N/A, not applicable; RUL, right upper lung lobe; VPS, ventriculoperitoneal shunt; classic triad of hydrocephalus, headache, vomiting, fluctuating mental status.
Eight of 12 (67%) presented with the classic triad of hydrocephalus (headaches, confusion, and vomiting). Of the 4 without the classic triad, 2 presented with headache and oculomotor nerve deficits, 1 with neck pain, and 1 with progressive asymmetric weakness. Three of 7 had evidence of hydrocephalus on the first head computed tomography acquired. Two of 5 who underwent noncontrast head computed tomography scan at the time of having the classic triad did not show evidence of hydrocephalus. All 12 cases developed complications, with 10 requiring ventriculoperitoneal shunting, 6 having spinal cord involvement (5 with cervical spine involvement), 4 having strokes, and 3 dying from complications related to CM. All patients were treated with antifungal agents. Information on treatment initiation was available for 5 cases. The median time to treatment initiation was 12 weeks (± 6) but was 16 weeks for those with an age of symptom onset >50 compared to 6 weeks for those <50. The 3 cases with an age of symptom onset >50 passed away, whereas all others have survived. Two of these patients died in the subacute setting because of recurrent strokes secondary to basilar meningitis, whereas the third died from recurrent infections and medical complications secondary to quadriplegia from coccoides myelitis 11 years after the initial infection.
Table 3 provides a comparison of racial and ethnic demographics from US census data of Orange County to our total CM cases. Self-identified race or Hispanic ethnicity was not associated with a higher than chance probability of infection.
Table 3.
Fisher Exact Test Analyses of Prevalence of Coccidioides Meningitis (CM) by Racial and Ethnic Demographics for the Total Number of CM Cases (top) Compared to Census Data of Orange County (OC)
| White | Observed | Expected |
|---|---|---|
| White | 15 | 18 |
| Others | 18 | 15 |
| Fisher exact test P-value = .6075 |
| Asian | Observed | Expected |
|---|---|---|
| Asian | 6 | 7 |
| Others | 27 | 26 |
| Fisher exact test P-value = 1.0000 |
| Black | Observed | Expected |
|---|---|---|
| Black | 3 | 1 |
| Others | 30 | 32 |
| Fisher exact test P-value = .3545 |
| Other/Mixed race | Observed | Expected |
|---|---|---|
| Other | 6 | 3 |
| Others | 27 | 30 |
| Fisher exact test P-value = .3184 |
| Native Hawaiian or Pacific Islander | Observed | Expected |
|---|---|---|
| Native Hawaiian or Pacific Islander | 1 | 0 |
| Others | 32 | 33 |
| Fisher exact test P-value = .4848 |
| Hispanic | Observed | Expected |
|---|---|---|
| Hispanic | 16 | 11 |
| Others | 17 | 22 |
| Fisher exact test P-value = .2433 |
| Non-Hispanic | Observed | Expected |
|---|---|---|
| Non-Hispanic | 17 | 22 |
| Hispanic | 16 | 11 |
| Fisher exact test P-value = .2433 |
To correct for multiple comparisons, a P-value <.025 with adjustment of 6 degrees of freedom was considered significant. No comparisons were considered significant.
DISCUSSION
We note that immunocompetent cases had similar demographics to the total number of CM cases. In both groups, there was a 3:1 male: female ratio, with a majority of cases occurring in males between ages 20 and 70 years, similar to the male:female ratio of CM cases observed by others [10]. Unlike prior reports, we did not see a greater-than-chance prevalence of CM cases for those who identified as Asian or Hispanic [10]. The majority of patients presented with classic symptoms of hydrocephalus. Among our immunocompetent group, neuroimaging demonstrated signs of hydrocephalus 12 weeks after symptom onset on average. The nonspecific nature of initial presentation is unfortunate because CM has a number of serious complications, including quadriparesis from syringomyelia, refractory hydrocephalus, recurrent strokes, and death.
The time between symptom onset to initiation of treatment was largely dependent on timing of diagnosis. Although advanced age may be associated with poorer prognosis, it is important to note that the median time to initiation of treatment for those age <50 years was 6 weeks versus 16 weeks for those age >50 years. This highlights the importance of improving physician awareness of hydrocephalus and CM in elderly patients who present with nonfocal complaints because this 10-week delay may be a critical period in preventing the formation of life-threatening basilar meningitis. Fungal infections of immunocompetent individuals are not limited to Coccidioides. A large prospective cohort study has looked at cryptococcal infection of HIV-negative patients and noted significant sequelae of infection [22].
A strength of this investigation is that it is, to our knowledge, the first case series to review CM infections specifically in immunocompetent individuals. Three other case series were identified that had sizeable immunocompetent CM populations (≥10 cases) [10, 23, 24]. Demographic information on these subgroups was not reported, prohibiting comparisons to immunocompromised groups or to our data. Limitations include that this is a single-center study and the lack of an immunocompromised comparison group. Our institutional review board protocol restricted our review of cases not directly assessed by the physician authors to anonymized data. We cannot determine if the patients contracted the infection in the same place as their residence (Figure 2). Other healthcare networks exist in our county, and this may bias the demographics of the patients seen. Several cases presented for acute management and lacked sufficient records to determine their initial examination findings.
We exercise caution in interpreting our findings with regard to demographics. It remains possible that immunocompetent patients may have predisposing genetic conditions that were not identified. We emphasize that epidemiological investigations to understand differences in risk factors, pathogenesis, presenting symptoms, and response to treatment across diverse populations should remain a priority to ensure equitable care in the face of a growing prevalence of the disease. Future studies should assess for differences in clinical findings and disease progression between immunocompetent and immunocompromised patients with CM.
CONCLUSION
It is a common misconception that CM is an opportunistic infection. We discuss our single-center experience within the context of the existing literature to improve early diagnosis and management for common complications of this life-threatening disease. This is of particular importance as large-scale construction projects for housing development throughout the southwestern United States may lead to the release Cocci spores from the soil and to further infections with compounding concerns of more severe weather extremes brought on by global warming expanding the endemic territory of Coccidioides.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ravi Rajmohan, Department of Neurology, University of California, Irvine, California, USA.
Jacob Deyell, School of Medicine, University of California, Irvine, California, USA.
Mark Harris, School of Medicine, University of California, Irvine, California, USA.
Kevin Gramajo-Aponte, School of Medicine, University of California, Irvine, California, USA.
Gianna Fote, Department of Neurosurgery, University of California, Irvine, California, USA.
Jordan Davies, Department of Neurosurgery, University of California, Irvine, California, USA.
Nita Chen, Department of Neurology, University of California, Irvine, California, USA.
Catherine Diamond, Department of Infectious Diseases, University of California, Irvine, California, USA.
Xiaoying Lu, Department of Neurology, Loma Linda University, Loma Linda, California, USA.
Notes
Acknowledgments . Not applicable.
Author Contributions. R.R. provided conceptual framework for the manuscript, contributed to manuscript text and figure design, and critically revised the article. M.H. and J.S.D. drafted the article along with figure design. K.G.A., G.F., and J.D. obtained patient information and contributed to the text. N.C., C.D., and X.L. contributed to the conceptual framework and critically revised the manuscript. Neither chatbots nor artificial intelligence was used for any portion of this work.
Disclaimer. Ethics, approval, and consent to participate: The University of California, Irvine institutional review board approved this study (approval #3352). Written informed consent was obtained from all cases described within following reasonable attempts to contact the patient or next of kin as per the institutional review board's determination. Consent was waived by the institutional review board for circumstances in which the patient or next of kin could not be contacted despite all reasonable attempts.
Availability of data and materials. Anonymized data and materials will be made available upon reasonable request to the corresponding author within the stipulations of the institutional review board approval and applicable privacy laws.
Financial support. None.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jackson NR, Blair JE, Ampel NM. Central nervous system infections due to coccidioidomycosis. J Fungi (Basel) 2019; 5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stockamp NW, Thompson GR III. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–46. [DOI] [PubMed] [Google Scholar]
- 3. Krogstad P, Johnson R, Garcia-Lloret MI, Heidari A, Butte MJ. Host-pathogen interactions in coccidioidomycosis: prognostic clues and opportunities for novel therapies. Clin Ther 2019; 41:1939–1954.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. California Department of Public Health (CDPH) . Substantial Rise in Coccidioidomycosis in California: Recommendations for California Healthcare Providers. 1/18/2024. Available at: https://www.cdph.ca.gov/Programs/OPA/Pages/CAHAN/Substantial-Rise-in-Coccidioidomycosis-in-California-Recommendations-for-California-Healthcare-Providers.aspx. Accessed 5 January 2024.
- 5. Litvintseva AP, Marsden-Haug N, Hurst S, et al. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Infect Dis 2015; 60:e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galgiani JN, Kauffman CA. Coccidioidomycosis and histoplasmosis in immunocompetent persons. N Engl J Med 2024; 390:536–47. [DOI] [PubMed] [Google Scholar]
- 7. American Community Survey. ACS 5-year data estimates profiles ACS Demographic and Housing Estimates . 2021. Available at: https://data.census.gov/table/ACSDP5Y2021.DP05?q=DP05. Accessed 1 June 2024.
- 8. Nolt JD, Geertsma FR. Deep solitary brain mass in a four-month-old male with disseminated coccidioidomycosis: case report. Ann N Y Acad Sci 2007; 1111:385–94. [DOI] [PubMed] [Google Scholar]
- 9. Cárdenas G, Aristizábal S, Salinas C, et al. Coccidioidal meningitis in non-AIDS patients. A case series at a Mexican neurological referral center. Clin Neurol Neurosurg 2020; 196:106011. [DOI] [PubMed] [Google Scholar]
- 10. Drake KW, Adam RD. Coccidioidal meningitis and brain abscesses: analysis of 71 cases at a referral center. Neurology 2009; 73:1780–6. [DOI] [PubMed] [Google Scholar]
- 11. Bays DJ, Thompson GR III. Coccidioidomycosis. Infect Dis Clin North Am 2021; 35:453–69. [DOI] [PubMed] [Google Scholar]
- 12. Johnson R, Ho J, Fowler P, Heidari A. Coccidioidal meningitis: a review on diagnosis, treatment, and management of complications. Curr Neurol Neurosci Rep 2018; 18:19. [DOI] [PubMed] [Google Scholar]
- 13. McHardy IH, Barker B, Thompson GR III. Review of clinical and laboratory diagnostics for coccidioidomycosis. J Clin Microbiol 2023; 61:e0158122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McHardy IH, Dinh BN, Waldman S, et al. Coccidioidomycosis complement fixation titer trends in the age of antifungals. J Clin Microbiol 2018; 56:e01318–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crum NF. Coccidioidomycosis: a contemporary review. Infect Dis Ther 2022; 11:713–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singhi P, Saini AG. Fungal and parasitic CNS infections. Indian J Pediatr 2019; 86:83–90. [DOI] [PubMed] [Google Scholar]
- 17. Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010; 170:345–51. [DOI] [PubMed] [Google Scholar]
- 18. Orlowski HLP, McWilliams S, Mellnick VM, et al. Imaging spectrum of invasive fungal and fungal-like infections. Radiographics 2017; 37:1119–34. [DOI] [PubMed] [Google Scholar]
- 19. Thompson GR III, Blair JE, Wang S, et al. Adjunctive corticosteroid therapy in the treatment of coccidioidal meningitis. Clin Infect Dis 2017; 65:338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappagianis D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am Rev Respir Dis 1993; 148:656–60. [DOI] [PubMed] [Google Scholar]
- 21. Kirkland TN. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. J Fungi (Basel) 2016; 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marr KA, Sun Y, Spec A, et al. A multicenter, longitudinal cohort study of cryptococcosis in human immunodeficiency virus-negative people in the United States. Clin Infect Dis 2020; 70:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathisen G, Shelub A, Truong J, Wigen C. Coccidioidal meningitis: clinical presentation and management in the fluconazole era. Medicine (Baltimore) 2010; 89:251–84. [DOI] [PubMed] [Google Scholar]
- 24. Kassis C, Zaidi S, Kuberski T, et al. Role of coccidioides antigen testing in the cerebrospinal fluid for the diagnosis of coccidioidal meningitis. Clin Infect Dis 2015; 61:1521–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.