Abstract
Background:
Interferon-beta (IFN-β) still plays a fundamental role in immunomodulation of people with multiple sclerosis (MS) with low disease activity and in clinically isolated syndrome (CIS). In 2014, pegylated (PEG) interferon was licensed by the European Medicines Agency (EMA) for relapsing-remitting MS (RRMS), enabling a lower dosing frequency.
Objectives:
Our retrospective study compares laboratory findings and adverse events between subcutaneous (sc.) PEG-IFN-β-1a and IFN-β-1a in RRMS and CIS patients.
Design:
Patients with CIS or RRMS fulfilling the revised McDonald criteria from 2017 visiting the neurology department of the University Medical Center of the Johannes Gutenberg University Mainz from 2010 to 2019 and treated with sc. PEG-IFN-β-1a or sc. IFN-β-1a (n = 202) were screened for eligibility. Patients who underwent regular laboratory controls in-house were included in our analysis (n = 128).
Methods:
We evaluate disease progression through clinical examination, relapse history, and magnetic resonance imaging (MRI) disease activity (gadolinium-enhancing or new T2 lesions). Relevant laboratory findings such as leukopenia (leukocyte count < 3.5/nl) and neutropenia (neutrophil count <43% of lymphocytes or <1500/µl) were assessed. Telephone interviews evaluated the side effects of the respective medication. A subgroup of patients was analyzed regarding neutrophil quantities and qualities.
Results:
Patients treated with sc. PEG-IFN-β-1a had significantly lower leukocyte counts (p = 0.046) and higher incidences of leukopenia (p = 0.006) and neutropenia (p = 0.03) compared to sc. IFN-β-1a. Clinical and MRI disease activity showed no significant differences, but people treated with sc. PEG-IFN-β-1a reported more common adverse events such as joint/muscle pain, injection-site reaction, and infections. No serious adverse events were reported.
Conclusion:
Treatment with sc. PEG-IFN-β-1a compared to unpegylated sc. IFN-β resulted in a significantly greater reduction in leukocyte and neutrophil levels with a higher incidence of side effects. We suggest mandatory monitoring of differential blood counts before and during treatment.
Keywords: interferon, leukopenia, multiple sclerosis, neutropenia, risk factors, safety
Plain language summary
Possible risk factors for the occurrence of leukopenia in patients with multiple sclerosis treated with interferon beta
In our study, we compared two medications used to treat multiple sclerosis (MS): pegylated interferon-beta-1a (PEG-IFN-beta-1a) and interferon-beta-1a (IFN-beta-1a). The pegylated form needs to be taken less often. We looked at patients’ medical history, physical exams, lab results, and MRI scans to see how these drugs affected them. We also asked about side effects during phone interviews. We found that PEG-IFN-beta-1a caused lower levels of certain blood cells, like leukocytes, and more side effects such as skin rashes and infections compared to IFN-beta-1a. However, there were no differences in disease activity as seen in clinical exams and MRI scans. We recommend regular blood tests for patients using these medications to monitor their health.
Introduction
Despite a rapidly evolving therapeutic landscape with a growing number of disease-modifying therapies (DMT), platform therapies (interferons and glatiramer acetate) still remain a valid therapeutic option in clinically isolated syndrome (CIS) and multiple sclerosis (MS). This, for example, is reflected in the nationwide longitudinal cohort study of individuals with newly diagnosed MS from the German National MS cohort, in which 392 out of 809 patients (48%) received platform therapies 2 years after diagnosis. 1 Currently, the use of platform therapies is especially considered (i) for patients with mild disease symptoms and good prognostic markers, (ii) as a deescalating strategy in elderly patients, or (iii) for MS management in pregnancy. Interferon-beta (IFN-β) preparations, which modulate the immune system via pleiotropic effects, are a group of DMT with one of the longest histories of use in people with mild relapsing-remitting MS (RRMS). 2 It is hypothesized that IFN-β stabilizes the blood–brain barrier, thereby preventing the migration of leukocytes into the central nervous system. 3 Additionally, IFN-β binding to the cellular IFN-α/β receptor triggers a complex cellular cascade and is involved in antigen processing and presentation through transcriptional modulation of chemokines and cytokines. 4 Furthermore, IFN-β was shown to have a direct effect on inflammatory T-cells by modulating their survival, proliferation, and differentiation.3,5
The two main forms of IFN-β used in the treatment of RRMS are the subcutaneously (sc.) applied IFN-β-1b and IFN-β-1a, which can be injected either sc. or intramuscularly (im.). 3 The difference between these two IFN-β lies in the amino acid sequence and their glycosylation status.6,7 According to the European Medicines Agency (EMA), sc. IFN-β-1a (Rebif®, Merck, Darmstadt, Germany) was the third IFN-β therapy developed, receiving approval for relapsing MS in 1998. The PRISM approval study showed that the application of sc. IFN-β-1a three times a week reduces relapse rates significantly, compared to placebo.5,8 It has the same amino acid sequence as human IFN-β and a similar mode of action as IFN-β-1b.5,9
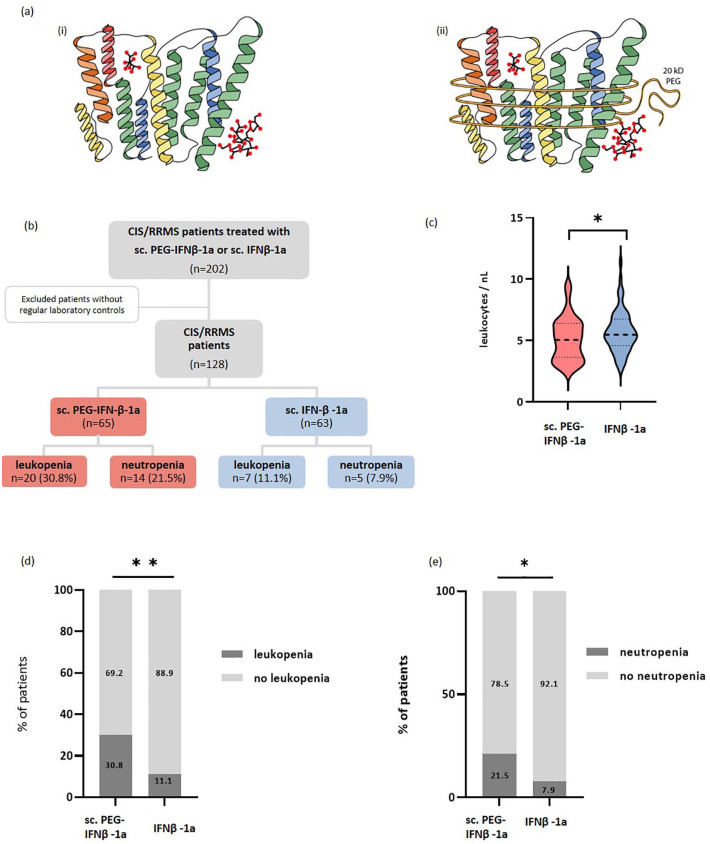
The addition of a polyethylene glycol molecule to IFN-β-1a, known as pegylation, enlarges the molecule (Figure 1(a)), reducing glomerular filtration and leading to a prolonged half-life and lower drug exposure. Therefore, application of sc. pegylated (PEG)-IFN-β-1a is only necessary every 2 weeks (125 μg).10,11 The ADVANCE trial showed that RRMS patients treated with sc. PEG-IFN-β-1a had a significant reduction in the annual relapse rate, risk of disability progression, and number of T2 lesions compared to placebo after 48 weeks. 12 With less frequent dosing and comparable efficacy to the existing IFN-β forms, sc. PEG-IFN-β-1a (Plegridy®, Biogen, Cambridge, MA, USA) was approved by the EMA in 2014.
Figure 1.
Biochemical structure and study design. (a) The biochemical structure of (i) Rebif® and (ii) Plegridy®; sc. IFN-β-1a has the same amino acid sequence as human IFN-β and is glycosylated. The difference between the two formulations results from the addition of a non-toxic polymer to the α-amino group of the N-terminus of IFN-β-1, the so-called methoxy-PEG-O2-methylpropionaldehyde. (b) Study design: Out of a total of 128 patients with CIS or RRMS, 65 were treated with sc. PEG-IFN-β-1a and 63 were treated with sc. IFN-β-1a. (c) The absolute leukocyte count was significantly lower under treatment with sc. PEG-IFN-β-1a (red) compared to sc. IFN-β-1a (blue) (p = 0.046). The incidence of (d) leukopenia (p = 0.006) and (e) neutropenia (p = 0.03) was significantly higher in patients treated with sc. PEG-IFN-β-1a compared to patients treated with sc. IFN-β-1a.
*p < 0.05.**p < 0.01.
CIS, clinically isolated syndrome; IFN-β, interferon-beta; RRMS, relapsing-remitting multiple sclerosis.
Previous studies reported that the most common adverse events in patients treated with IFN-β were flu-like symptoms, injection-site reactions, fever, depression, and headache. 2 Furthermore, in the ADVANCE study, a majority of patients in the intervention group had a change in laboratory parameters such as increased liver enzymes or decreased white blood cell counts. 12 Similar results were obtained by the PRISM study. Here, patients under sc. IFN-β-1a had significantly higher rates of lymphopenia, leukopenia, and granulocytopenia compared to a placebo group after 3 months of treatment. 8
In this work, we retrospectively compare both formulations in terms of leukopenia and its clinical effects in MS patients. Furthermore, since neutrophils are the first line of defense in the innate arm of the immune system, we investigate the effect of sc. IFN-β-1a and sc. PEG-IFN-β-1a on neutrophil count and function.
Patients and methods
Study design
Patients with CIS or RRMS fulfilling the revised McDonald criteria from 2017 13 visiting the neurology department of the University Medical Center of the Johannes Gutenberg University Mainz from 2010 to 2019 and treated with sc. PEG-IFN-β-1a or sc. IFN-β-1a (n = 202) were screened for eligibility. Patients who underwent regular laboratory controls in-house were included in our analysis (n = 128). Of these, 65 were under sc. PEG-IFNβ-1a therapy and 63 were under sc. IFNβ-1a therapy (Figure 1(b)). Patients treated with im. PEG-IFNβ-1a were not included, since it was only licensed after the observation period. Patients with incomplete follow-up were excluded from analysis. Observed parameters were sex (self-reported), age at treatment start, relapses, Expanded Disability Status Scale (EDSS), and previously received DMT. Patients receiving prior DMT fulfilled national guidelines on washing out or time lapse between cessation of previous therapy and initiation of interferon therapy.
Laboratory analysis
Observed laboratory values included liver enzymes and blood cell counts of thrombocytes, leukocytes, lymphocytes, neutrophils, basophils, and eosinophils. Leukopenia was defined as leukocyte counts below 3.5/nl, and neutropenia as neutrophil count below 43% of lymphocytes or <1500/µl. Baseline was the last visit before treatment initiation.
Assessment of clinical data and MRI data
To investigate disability progression during IFN treatment, the standardized EDSS score was calculated at baseline and either upon stopping IFN treatment, if applicable, or at the end of the investigational period. Clinical relapses during treatment were noted and relapse rate was calculated as follows: New T2 lesions or gadolinium-enhancing lesions during the period of observation were considered inflammatory MRI activity. Subclinical disease activity was defined by MRI activity and absence of clinical disease activity, such as relapse or EDSS progression.
Adverse events questionnaire
After retrospective analysis, 87 out of 128 patients were interviewed regarding adverse events via a standardized telephone questionnaire. Patients who did not participate were not included in the analysis regarding adverse events. The questionnaire included adverse events and reasons for possible termination of treatment. More specifically, the occurrence of infections such as herpes infection, mycosis, infections of the airways, or intestinal infections were covered. Furthermore, common side effects of IFN-β treatment, such as flu-like symptoms, skin rash, injection-site reactions, and joint or muscle pain were documented. The questionnaire (Supplemental Table 1) utilized in this study was not subjected to formal validation or pilot testing.
Neutrophil isolation and fluorescence-activated cell sorting analysis
To investigate the effect of IFN-β on neutrophils in MS patients, we isolated and analyzed neutrophils from patient blood through flow cytometry. Samples for fluorescence-activated cell sorting (FACS) analysis were selected prospectively, including 10 patients with sc. IFNβ-1a or sc. PEG-IFN-β-1a each, as well as 20 healthy controls. Healthy controls were age- and sex-matched. Blood was collected in ammonium heparin tubes. Blood was resuspended in dextran solution and set for sedimentation at room temperature by gravitation. Histopaque was overlaid with sample supernatant and centrifuged at 600g for 30 min. Neutrophils were isolated from the cell pellet, platelets were lysed with ammonium-chloride-potassium buffer for 5 min, and washed with phosphate-buffered saline at 600g for 5 min. This step was repeated once. Cell count was determined using trypan blue.
Cells were stained for extracellular markers. Cells were incubated with either cluster of differentiation (CD) 64 Alexa Fluor® (AF) 700, CD16 Pe-Cy7, CD66b Horizon, CD11b PE, fluorescent viability dye (FVD) APC or CD64 AF700, CD16 Pe-Cy7, CD15 FITC, CD62L Pe, HLA-DR Horizon, CD45 PerCP, FVD APC for 10 min at 4°C. After washing with FACS buffer at 550g for 5 min, cells were resuspended in FACS buffer. Samples were screened using a FACS Canto II (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo version 10 (FlowJo, LLC, Ashland, OR, USA).
Statistical analysis
Statistical analyses were performed with SPSS version 23 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9.5.1 (GraphPad Software, La Jolla, CA, USA). The normal distribution of data was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. We applied a Mann–Whitney U test or Kruskal–Wallis test with adjusted p values by Bonferroni correction, as appropriate. Nonparametric correlation was determined by Spearman’s rank correlation coefficient and partial nonparametric correlation when considering age as a covariate. A log-rank test Kaplan–Meier analysis was applied if there were differences in the incidence for the different types of treatment. Additionally, we ran a receiver operating characteristic (ROC) curve analysis to assess the prognostic performance of different factors on leukopenic or neutropenic incidence. The optimal threshold level of leukocytes at treatment initiation for incidence of leukopenia or neutropenia was obtained through the Youden-Index (defined as the sum of sensitivity and specificity minus one). Based on the maximal Youden-Index, the sensitivity and specificity were defined. p values < 0.05 were considered statistically significant.
Manuscript preparation
Manuscript was prepared following the STROBE guidelines.
Results
PEG-IFN-β-1a treatment (sc.) is associated with a greater incidence of leukopenia and neutropenia
We aimed to investigate whether patients under sc. PEG-IFN-β-1a treatment have a higher risk for leukopenia compared to patients treated with sc. IFN-β-1a. The laboratory examinations were conducted during routine visits in our outpatient clinic, typically every 3–6 months. Our analysis showed a greater reduction and thus a lower absolute leukocyte count (mean ± standard deviation (SD) 5.10 ± 1.76 vs 5.73 ± 1.7 cells/nl, p = 0.046, Figure 1(c) and Table 1) 8.5 ± 10.1 months (mean ± SD) after treatment initiation in the PEG-IFN-β-1a group and 5.2 ± 7.5 months in the IFN-β-1a group (p = 0.071). In addition, a trend toward a lower neutrophil count (2879.16 ± 1245.82 vs 3373.65 ± 1289.06 cells/μl, p = 0.095), as well as a higher incidence of both leukopenia (30.8% vs 11.1%, p = 0.006) and neutropenia (21.5% vs 7.9%, p = 0.03) was observed under sc. PEG-IFN-β-1a (Figure 1(d) and (e), Table 1). These parameters did not differ at baseline (leukocytes: 7.06 ± 1.69 vs 7.24 ± 2.2 cells/nl, p = 0.709; lymphocytes: 1830.12 ± 500.75 vs 2273.40 ± 3024.62 cells/μl, p = 0.388 and neutrophils: 4295.28 ± 1573.40 vs 4077.79 ± 1662.08 cells/μl, p = 0.305). Baseline time was mostly 2 months (mean) prior to treatment initiation. Three patients under sc. PEG-IFN-β-1a were previously treated with dimethyl fumarate and two patients with mitoxantrone, whereas none of those under sc. IFN-β-1a had previously been administered dimethyl fumarate or mitoxantrone. Since both of the aforementioned DMTs are also capable of causing leukopenia and might therefore interfere with our results, these five patients were excluded from our comparison of sc. PEG-IFN-β-1a and sc. IFN-β-1a. After the exclusion of patients who were previously treated with mitoxantrone and dimethyl fumarate, patients treated with sc. PEG-IFN-β-1a still showed a significantly lower absolute leukocyte count as well as a higher incidence of leukopenia and neutropenia compared to the patients treated with sc. IFN-β-1a (Supplemental Figure 1, Supplemental Table 2).
Table 1.
Comparison of patient characteristics in PEG-IFN-β-1a versus sc. IFN-β-1a treatment groups.
| Parameter | IFN-β-cohort | PEG-IFN-β-1a | sc. IFN-β-1a | p Value |
|---|---|---|---|---|
| N | 128 | 65 (50.78) | 63 (49.22) | |
| Female (sex) | 106 (82.8) | 55 (84.61) | 51 (80.95) | 0.583 |
| BMI | 25.08 (±4.90) | 26.08 (±5.72) | 24.28 (±4.03) | 0.186 |
| Age at treatment start | 33.71 (±11.16) | 33.55 (±10.80) | 33.87 (±11.59) | 0.849 |
| Age at first diagnosis | 31.25 (±10.41) | 31.33 (±10) | 31.17 (±10.89) | 0.862 |
| Diagnosis by treatment start | ||||
| CIS | 27 (21.1) | 14 (21.54) | 13 (20.63) | 0.900 |
| RRMS | 101 (78.9) | 51 (78.46) | 50 (79.36) | 0.760 |
| IFN treatment duration (months) | 23.12 (±18.74) | 18.88 (±13.41) | 26.30 (±21.68) | 0.080 |
| Pretreatment | ||||
| sc. IFN-β-1a | 11 (8.6) | 11 (16.92) | 0 | 0.001 |
| im. IFN-β-1a | 1 (0.8) | 1 (1.53) | 0 | 0.323 |
| sc. IFN-β-1b | 2 (1.6) | 1 (1.53) | 1 (1.59) | 0.982 |
| Fingolimod | 2 (1.6) | 1 (1.53) | 1 (1.59) | 0.982 |
| Dimethyl fumarate | 3 (2.3) | 3 (4.61) | 0 | 0.084 |
| Glatiramer acetate | 8 (6.3) | 8 (12.31) | 0 | 0.004 |
| Mitoxantrone | 2 (1.6) | 2 (3.1) | 0 | 0.161 |
| EDSS at treatment start | 1.082 (±1.2) | 1.15 (±1.25) | 1.02 (±1.14) | 0.574 |
| EDSS changea | 0.077 (±0.87) | 0.15 (±1.09) | 0.342 | |
| Disease activity | ||||
| Relapse | 49 (38.3) | 22 (33.85) | 27 (42.86) | 0.294 |
| Relapse rate | 0.59 (±1.58) | 0.70 (±1.93) | 0.477 (±1.12) | 0.578 |
| MRI availability b | 107 (83.6) | 51 (78.5) | 56 (88.9) | |
| MRI activity | 57 (53.27) | 26 (50.98) | 31 (55.36) | 0.650 |
| Subclinical activity c | 44 (41.12) | 23 (45.1) | 21 (37.5) | 0.375 |
| New T2-lesion | 57 (53.27) | 26 (50.98) | 31 (55.36) | 0.650 |
| Gd-enhancement | 23 (21.49) | 9 (17.65) | 14 (25) | 0.356 |
| Laboratory results | ||||
| Leukocytes baseline (cells/nl) | 7.14 (±1.94) | 7.06 (±1.69) | 7.24 (±2.20) | 0.709 |
| Leukocytes under treatment (cells/nl) | 5.27 (±1.59) | 5.10 (±1.76) | 5.73 (±1.7) | 0.046 |
| White blood cell differential at baseline d | 61 (47.65) | 38 (58.46) | 23 (36.50) | |
| Lymphocytes baseline (cells/µl) | 1997.26 (±1885.72) | 1830.12 (±500.75) | 2273.40 (±3024.62) | 0.388 |
| Neutrophils baseline (%) | 61.46 (±12.32) | 60.69 (±12.38) | 62.73 (±12.38) | 0.935 |
| Neutrophils baseline (cells/µl) | 4213.28 (±1597.13) | 4295.28 (±1573.40) | 4077.79 (±1662.08) | 0.305 |
| Lymphocytes under treatment (cells/µl) | 1505.90 (±478.85) | 1577.29 (±474.49) | 1421 (±475.04) | 0.054 |
| Neutrophils under treatment (cells/µl) | 3115.27 (±1285.04) | 2879.16 (±1245.82) | 3373.65 (±1289.06) | 0.095 |
| Neutrophils under treatment (%) | 57.26 (±9.52) | 54.79 (±8.49) | 60.12 (±9.88) | 0.003 |
| Leukopenia | 27 (21.1) | 20 (30.77) | 7 (11.1) | 0.006 |
| Neutropenia based on % | 19 (14.8) | 14 (21.53) | 5 (7.93) | 0.030 |
| Neutropenia based on cells count | 24 (18.75) | 16 (24.61) | 8 (12.7) | 0.084 |
| Adverse events e | ||||
| N | 87 (67.9) | 44 (67.69) | 43 (68.25) | 0.950 |
| Mycosis genitalis | 3 (3.4) | 2 (4.54) | 1 (2.32) | 0.570 |
| Herpes simplex labialis | 4 (4.6) | 4 (9.09) | 0 | 0.043 |
| Infection of respiratory tract | 10 (11.5) | 5 (11.36) | 5 (11.63) | 0.969 |
| Hair loss | 17 (19.5) | 6 (13.63) | 5 (11.63) | 0.778 |
| Skin rash f | 11 (12.6) | 6 (13.63) | 5 (11.63) | 0.778 |
| Injection-site reaction | 58 (66.7) | 36 (81.81) | 22 (51.16) | 0.002 |
| Diarrhea | 4 (4.6) | 2 (4.54) | 2(4.65) | 0.981 |
| Flu-like symptoms | 60 (69) | 38 (86.36) | 22 (51.16) | <0.001 |
| Joint/muscle pain | 45 (51.7) | 31 (70.45) | 14 (32.56) | <0.001 |
EDSS change is defined as the difference between EDSS at study end and EDSS at therapy start.
MRI data available in 107 of 128 patients.
Subclinical disease activity is characterized by MRI activity (new T2 lesions or Gd+ enhancing lesions) without clinical activity.
White blood cell differential at baseline refers to the number and percentage of patients who had a white blood cell differential test performed at the baseline.
Adverse events were reported via follow-up telephone questionnaire completed by 87 of 128 patients.
Skin rash was defined as a change of the skin especially regarding color after injection. Data shown as number (percentage) or mean (±standard error of the mean) as appropriate, unless stated otherwise. Significant p values are highlighted in bold.
BMI, body mass index; CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFN-β, interferon-beta; RRMS, relapsing-remitting multiple sclerosis; sc., subcutaneous.
Predictors for leukopenia and neutropenia under treatment with interferons
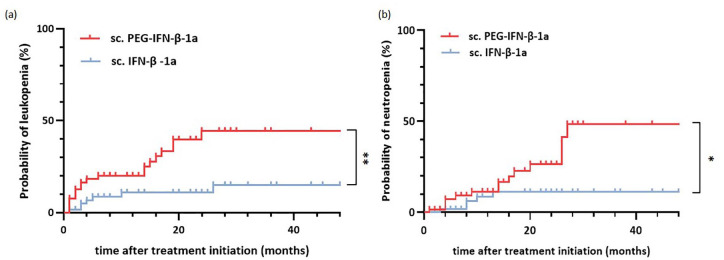
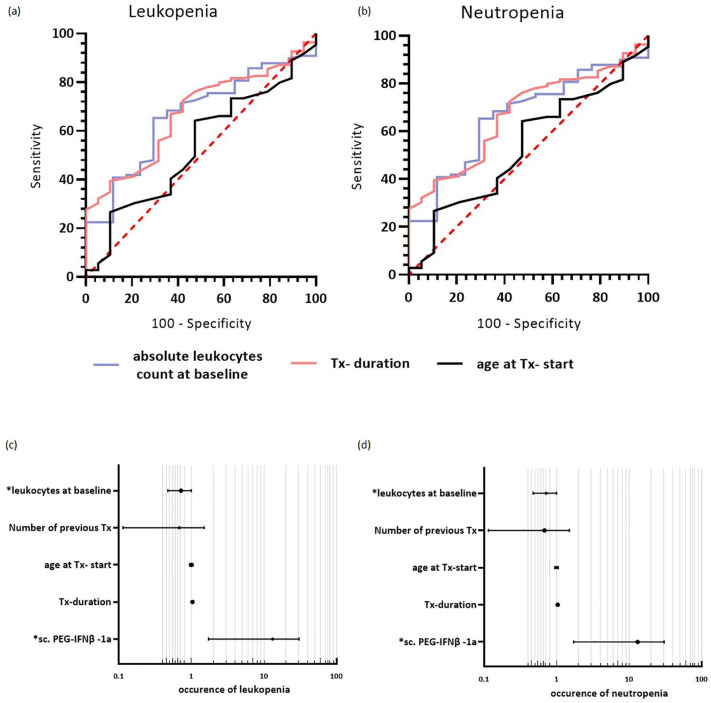
To unravel whether treatment with sc. PEG-IFN-β-1a could be a risk factor for developing leukopenia or neutropenia, a Kaplan–Meier risk analysis was performed. The two groups were compared using a log-rank test. This showed a significantly higher risk of developing leukopenia (p = 0.003, Figure 2(a)) and neutropenia (p = 0.01, Figure 2(b)) under sc. PEG-IFN-β-1a treatment compared to sc. IFN-β-1a. To identify risk factors for the development of leukopenia or neutropenia within the whole cohort, a ROC curve analysis was performed. Leukocytes at baseline (area under the curve (AUC) = 0.751, 95% confidence interval (CI): 0.648–0.854, p < 0.001) were predictive for the development of leukopenia with a cut-off value of ⩽6.99 leukocytes/nl (Youden-Index) (Figure 3(a)). Furthermore, leukocytes at baseline (AUC = 0.664, 95% CI: 0.540–0.787, p = 0.032) and treatment duration (AUC = 0.312, 95% CI: 0.199–0.425, p = 0.013) could predict neutropenia under treatment (Figure 3(b)). The cut-off value for leukocytes at baseline was ⩽6.55 leukocytes/nl (Youden-Index) and for treatment duration was >27 months (Youden-Index).
Figure 2.
Treatment with sc. PEG-IFN-β-1a predicts incidence of leukopenia and neutropenia. (a and b) Kaplan–Meier risk analysis for the occurrence of (a) leukopenia and (b) neutropenia under treatment with sc. PEG-IFN-β-1a (red) and sc. IFN-β-1a (blue). Log-rank test showed p = 0.003 for the occurrence of leukopenia and p = 0.01 for the occurrence of neutropenia. *p < 0.05. **p < 0.01.
IFN-β, interferon-beta.
Figure 3.
Predictive factors for the incidence of leukopenia and neutropenia. (a and b) ROC curve—predicting the occurrence of (a) leukopenia and (b) neutropenia in the whole cohort. (a) Leukocytes at baseline (AUC: 0.751, 95% CI: 0.648–0.854, p < 0.001, cut-off value of ⩽6.99 leukocytes/nl (Youden-Index)) was identified as a significant risk for leukopenia; treatment duration and age at treatment were not significant (treatment duration: AUC = 0.588, 95% CI: 0.497–0.674, p = 0.149; age at treatment start: AUC = 0.500, 95% CI: 0.388–0.612, p = 0.997 ). (b) Regarding the occurrence of neutropenia, leukocytes at baseline (AUC = 0.664, 95% CI: 0.540–0.787, p = 0.032, cut-off value for leukocytes at baseline ⩽6.55 leukocytes/nl (Youden-Index)) and treatment duration with interferons (AUC = 0.312, 95% CI: 0.199–0.425, p = 0.013, treatment duration >27 months (Youden-Index)), significantly identify patients developing neutropenia; age at treatment initiation was not significant. (c and d) Forest plots showing the determined OR for the occurrence of (c) leukopenia and (d) neutropenia. The binary logistic regression model revealed “use of sc. PEG-IFN-β-1a” and “leukocyte count at baseline” as significant predictors for the dependent variable “occurrence of leukopenia” under IFN therapy; “use of sc. PEG-IFN-β-1a” and “leukocyte count at baseline” were significant predictors for the dependent variable “occurrence of neutropenia” under IFN therapy.
AUC, area under the curve; CI, 95% confidence interval; IFN-β, interferon-beta; OR, odds ratio; ROC, receiver operating characteristic; sc., subcutaneous; Tx, drug treatment.
The aforementioned risk factors to develop leukopenia and neutropenia were confirmed by multivariate analysis. After considering covariates unbalanced at the univariate level, two factors were revealed as independent predictors for the development of leukopenia and neutropenia under treatment with interferons. Leukopenia and neutropenia were associated with treatment with sc. PEG-IFN-β-1a (leukopenia: odds ratio (OR) 7.736, 95% CI: 2.165–27.637, p = 0.002; neutropenia: sc. PEG-IFN-β-1a: OR 7.231, 95% CI: 1.721–30.391, p = 0.007) and leukocytes at baseline (leukopenia: OR 0.506, 95% CI: 0.346–0.739, p < 0.001; neutropenia: OR 0.686, 95% CI: 0.473–0.997, p = 0.048; Tables 2 and 3, Figure 3(c) and (d)).
Table 2.
Multivariable analysis with the occurrence of leukopenia as dependent variable.
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| sc. PEG-IFN-β-1a | 7.736 (2.165–27.637) | 0.002 |
| Treatment duration | 1.020 (0.990–1.052) | 0.190 |
| Age at Tx-start | 0.996 (0.948–1.047) | 0.882 |
| Number of previous therapies | 0.499 (0.179–1.393) | 0.185 |
| Leukocytes at baseline | 0.506 (0.346–0.739) | <0.001 |
Significant p values are highlighted in bold.
CI, confidence interval; IFN-β, interferon-beta; OR, odds ratio; sc., subcutaneous; Tx, drug treatment.
Table 3.
Multivariable analysis with the occurrence of neutropenia as dependent variable.
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| sc. PEG-IFN-β-1a | 7.231 (1.721–30.391) | 0.007 |
| Treatment duration | 1.032 (0.999–1.066) | 0.060 |
| Age at Tx-start | 0.996 (0.943–1.052) | 0.879 |
| Number of previous therapies | 0.423 (0.114–1.499) | 0.179 |
| Leukocytes at baseline | 0.686 (0.473–0.997) | 0.048 |
Significant p values are highlighted in bold.
CI, confidence interval; IFN-β, interferon-beta; OR, odds ratio; sc., subcutaneous; Tx, drug treatment.
In order to model the time-dependent occurrence of leukopenic incidences, we additionally performed a Cox regression analysis, revealing sc. PEG-IFN-β-1a (hazard ratio (HR) 0.256, 95% CI: 0.096–0.681, p = 0.006) and leukocytes at baseline (HR 0.580, 95% CI: 0.428–0.784, p ⩽ 0.001) as independent predictors for the occurrence of leukopenia (Tables 4 and 5).
Table 4.
Cox regression analysis with leukopenia as dependent variable.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| PEG-IFN-β-1a (vs sc. IFN-β-1a) | 0.256 (0.096–0.681) | 0.006 |
| Treatment duration (months) | 0.993 (0.964–1.022) | 0.619 |
| Age at Tx-start (years) | 0.998 (0.961–1.037) | 0.934 |
| Number of previous therapies | 0.649 (0.300–1.403) | 0.272 |
| Leukocytes baseline (leukocytes/nl) | 0.580 (0.428–0.784) | <0.001 |
Significant p values are highlighted in bold.
CI, confidence interval; HR, hazard ratio; IFN-β, interferon-beta; Tx, drug treatment.
Table 5.
Cox regression analysis with neutropenia as dependent variable.
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| PEG-IFN-β-1a (vs sc. IFN-β-1a) | 0.264 (0.078–0.889) | 0.032 |
| Treatment duration (months) | 0.989 (0.955–1.025) | 0.539 |
| Age at Tx-start (years) | 1 (0.955–1.025) | 0.992 |
| Number of previous therapies | 0.522 (0.171–1.591) | 0.253 |
| Leukocytes baseline (leukocytes/nl) | 0.712 (0.506–1.002) | 0.051 |
Significant p values are highlighted in bold.
CI, confidence interval; HR, hazard ratio; IFN-β, interferon-beta; Tx, drug treatment.
Differences in clinical effectiveness and side effects under sc. PEG-IFN-β-1a versus sc. IFN-β-1a treatment
Analysis of the clinical characteristics such as relapse rate and EDSS during treatment revealed no significant differences between the two groups. There were also no differences between the effects of both therapies on radiological activity (new T2 lesions or gadolinium-enhancing lesions). Treatment groups were similar in terms of age, sex, diagnosis, and treatment duration. However, patients treated with sc. PEG-IFN-β-1a reported a higher occurrence of herpes simplex labialis infection (9% vs 0%, p = 0.043), flu-like symptoms (86.4% vs 51.2%, p < 0.001), and joint/muscle pain (70.5% vs 32.6%, p < 0.001, Table 1) compared to patients treated with sc. IFN-β-1a. After repeating the analysis excluding the five patients pre-treated with dimethyl fumarate or mitoxantrone, we still observed a higher occurrence of flu-like symptoms (84.2% vs 50%, p = 0.001) and joint/muscle pain (68.4% vs 31.0%, p = 0.002) in the PEG-IFN-β-1a compared to the sc. IFN-β-1a group (Supplemental Table 2). However, we now additionally observed an increased rate of injection-site reaction (81.6% vs 52.4%, p = 0.009), but no longer increased occurrence of herpes simplex labial infection in the PEG-IFN-β-1a compared to the sc. IFN-β-1a group (Supplemental Table 2).
Clinical relevance of leukopenia in IFN-treated patients
In order to assess the clinical relevance of leukopenia and neutropenia, we divided the whole cohort by the incidence of leukopenia and neutropenia, independent of immunomodulatory treatment regimen. Patients with leukopenia had a lower count of leukocytes (5.9 ± 1.4 vs 7.5 ± 1.9 leukocytes/nl, p < 0.001) and neutrophils (3417.9 ± 1530.1 vs 4428.7 ± 1560.85 neutrophils/μl, p = 0.038) before treatment initiation. Furthermore, a reduction in leukocytes, neutrophils, and lymphocytes occurred during IFN treatment (leukocytes: 3.27 ± 0.5 vs 5.95 ± 1.4 leukocytes/nl, p < 0.001; neutrophils: 1569.6 ± 425.4 vs 3564.6 ± 1085.8 neutrophils/μl, p < 0.001; lymphocytes: 1188.16 ± 256.30 vs 1586.93 ± 492.01, p < 0.001). Per the questionnaire, leukopenic patients experienced genital mycosis (14.3% vs 0%, p = 0.002) and joint/muscle pain (71.4% vs 44.6%, p = 0.033) significantly more often than non-leukopenic patients (Supplemental Table 3). Neutropenic patients (14.84%) had been on the treatment significantly longer (29.8 ± 16.65 vs 21.9 ± 18.9 months, p = 0.017). Regarding further side effects, neutropenic patients also more commonly presented joint/muscle pain (83.3% vs 46.6%, p = 0.018, Supplemental Table 4). However, in our cohort, leukopenia or neutropenia did not significantly affect disease activity or progression.
PEG-IFN-β-1a (sc.) may decrease migration of neutrophils
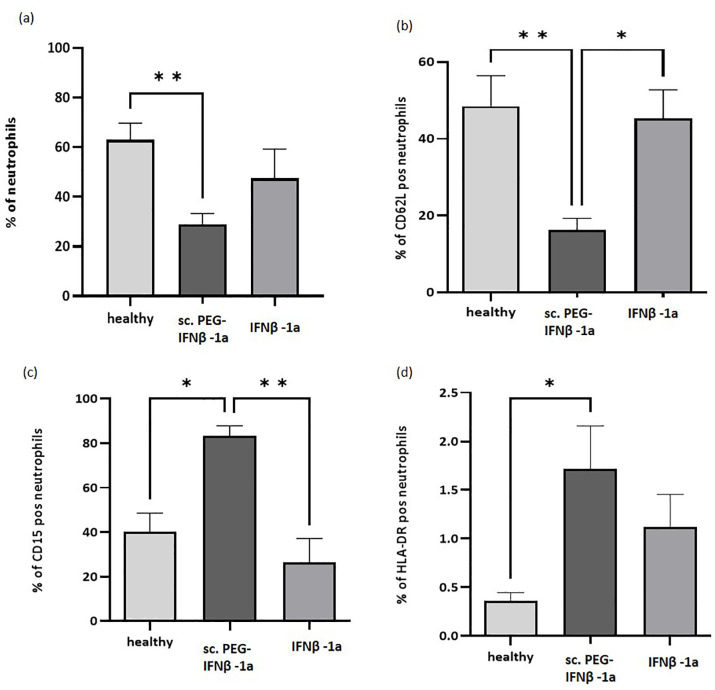
When isolating granulocytes from human blood, a decreased number of neutrophils could be observed within the sc. PEG-IFN-β-1a treatment cohort by FACS staining (Figure 4). Analyzing the single-cell living population, neutrophil numbers dropped in comparison to healthy controls, whereas sc. IFN-β-1a-treated patients retained stable neutrophil numbers (not significantly different from controls, Figure 4(a)). Neutrophils from patients treated with sc. PEG-IFN-β-1a displayed differential properties in comparison to both sc. IFN-β-1a-treated and healthy control neutrophils (Figure 4(b)–(d)). These neutrophils expressed significantly lower levels of the lymphocyte homing receptor CD62L (L-selectin) in comparison to neutrophils retrieved from both healthy controls and sc. IFN-β-1a-treated patients with MS (Figure 4(b)). Additionally, neutrophils of patients treated with sc. PEG-IFN-β-1a displayed a significantly higher proportion of the granulocyte-associated CD15, as well as the immune-presenting HLA-DR (Figure 4(c) and (d)).
Figure 4.
FACS analysis of neutrophils. (a) The number of neutrophils was significantly reduced in patients treated with sc. PEG-IFN-β-1a compared to healthy controls. (b) The neutrophils from patients under sc. PEG-IFN-β-1a shows significantly less CD62L in comparison to neutrophils from both healthy controls and sc. IFN-β-1a-treated patients. Neutrophils from patients treated with sc. PEG-IFN-β-1a displayed significantly more (c) CD15 and (d) HLA-DR than those from sc. IFN-β-1a-treated patients or healthy controls.
*p < 0.05. **p < 0.01.
FACS, fluorescence-activated cell sorting; IFN-β, interferon-beta.
Discussion
In this study, we demonstrated that the incidence of leukopenia, neutropenia, and adverse events including infections was higher in patients under treatment with IFN-β and especially sc. PEG-IFN-β-1a. However, there was no significant difference regarding lymphocyte counts in patients treated with sc. PEG-IFN-β-1a compared to patients treated with sc. IFN-β-1a. These findings most likely indicate a shift in blood cell lines and underline the importance of measuring differential blood counts under interferon treatment. Moreover, the drop in neutrophil counts was greater with longer exposure to treatment. Leukopenic patients, independent of IFN-formulation, also displayed a higher incidence of fungal disease. Whereas the results regarding leukopenia and side effects are still significant after exclusion of patients pretreated with mitoxantrone or dimethyl fumarate, the previously reported effects of PEG-IFN-β on increased rates of infections could no longer be demonstrated. Our observation identifies a lower baseline level of leukocytes as a significant risk factor for the occurrence of both leukopenia (6.99 leukocytes/nl) and neutropenia (6.55 leukocytes/nl). Furthermore, patients with a treatment duration over 27 months were at a higher risk for the occurrence of neutropenia. Patients receiving sc. PEG-IFN-β-1a reported more side effects than patients under sc. IFN-β-1a. The Plegridy Observational Program is an ongoing phase IV real-world study for the safety and efficacy of sc. PEG-IFN-β-1a in RRMS patients. Preliminary results have shown an adverse event profile like that reported in the pivotal ADVANCE/ATTAIN trials.11,12 Presence of these was shown to result in higher rates of early discontinuation of therapy when compared to patients without these symptoms. 14
In the safety profile analysis of IFN-β preparations with more than 16 years of follow-up, no new unknown complications or adverse events were discovered. The known complications are mostly reversible and manageable.3,15,16 Laboratory tests could show a reduction in neutrophils, platelets, and less frequently lymphocytes, as well as an increase in transaminases. 10 The ADVANCE study showed that there was a reduction in hematologic parameters (leukocyte, lymphocyte, and neutrophil counts) in more patients taking sc. PEG-IFN-β-1a compared to the placebo group. However, according to the study, these were not clinically relevant. A reduction in leukocyte counts below 3 leukocytes/nl was seen in 7% of patients with application of sc. PEG-IFN-β-1a every 2 weeks and in only 4% of patients with application every 4 weeks. 14 Although the guidelines suggest a white blood cell differential before therapy,17,18 it is not always applied in clinical routine. Our results emphasize regular laboratory examinations to detect changes in leukocyte and neutrophil counts under treatment with interferon preparations, as suggested. 17 We suggest a monitoring schedule entailing a follow-up examination after 4 weeks in case of leukocyte count below a certain threshold (in this study, below 6.55 leukocytes/nl), followed by quarterly checks thereafter; in case of leukocyte counts above 6.55 leukocytes/nl at treatment initiation, quarterly follow-up checks are deemed sufficient.
It remains unclear why the application of sc. PEG-IFN-β-1a leads to leukopenia, particularly neutrophil decrease. Pegylation enlarges the molecule, reducing the glomerular filtration rate and extending the half-life. This also allows the biweekly application regimen of sc. PEG-IFN-β-1a.10,12 We hypothesize that a longer interaction of the immune system or leukocytes with the drug might result in more leukocytes or neutrophils perishing. CD62L is an adhesion molecule involved in the attachment of neutrophils to the endothelial cell layer upon inflammation. 19 The observed reduction in CD62L expression on neutrophils from patients treated with sc. PEG-IFN-β-1a may hint at potential shedding of the molecule. It has been described in other inflammatory diseases that CD62L is shed upon challenge of the expressing neutrophils via an immunoglobulin E-dependent pathway.20,21 Whether that is also the case upon PEG-IFN-β-1a treatment remains to be elucidated. It is possible that the reduced expression of CD62L explains the increased occurrence of infection, as neutrophils might not be able to enter inflammatory tissue.
Additionally, we observed that neutrophils from sc. PEG-IFN-β-1a-treated people with MS displayed higher levels of CD15. Increased CD15 levels, especially on neutrophils, have been associated with the modulation of the adaptive and innate immune system, potentially contributing to the effects of sc. PEG-IFN-β-1a-treatment.22,23 Finally, a small but significant increase in MHC class II expression (HLA-DR) could be visualized on sc. PEG-IFN-β-1a neutrophils. It has been shown before that IFN is able to induce HLA-DR expression on neutrophils, which in turn drives super-antigen-mediated activation of T-cells. 24 Moreover, HLA-DR expression on neutrophils has been associated with increased neutrophilic activity and phagocytic capacity. 25 These FACS findings support a decrease in migratory capacities and activation of neutrophils. However, the small number of patients included in this analysis is not sufficient to demonstrate a causal relation between the occurrence of neutropenia in people with MS and sc. PEG-IFN-β-1a treatment.
While our study provides important information about the monitoring of patients treated with sc. PEG-IFN-β-1a, is not without limitations. We did not conduct a power analysis prior to study initiation, which might result in an underpowered sample size thereby limiting the generalizability of our findings. Especially the small number of patients included in our FACS analysis cohort prohibits drawing causal conclusions. An additional limitation of the study is that the questionnaire used to collect reported adverse events was not pilot tested and was applied retrospectively so the occurrence of side effects with regard to injection time was based on participants’ recollection. Further prospective studies are needed to investigate reasons for treatment discontinuations due to adverse events or leukopenia, as well as examinations on the duration until the blood cell counts return to a normal range. Finally, prospective longitudinal studies with blood sampling directly after application of sc. PEG-IFN-β-1a or sc. IFN-β-1a is necessary to explore this relationship.
Based on the above results, laboratory monitoring including differential blood count prior to initiation of therapy can be recommended. However, cut-off values have to be established and confirmed in a prospective study. In this way, patients at risk of developing relevant leukopenia or neutropenia could be identified. For those patients, closer monitoring or an alternative DMT could be discussed.
Conclusion
This study shows a higher incidence of leukopenia, neutropenia, and infections in patients treated with sc. PEG-IFN-β-1a compared to sc. IFN-β-1a. Moreover, sc. PEG-IFN-β-1a also resulted in more side effects. Lower baseline leukocyte counts were a significant risk factor for leukopenia and neutropenia. Establishing cut-off values for pretreatment leukocyte counts could help identify patients at risk and thereby enable closer monitoring or initiation of alternative treatment in these patients.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241286497 for Lower leukocytes pretreatment as a possible risk factor for therapy-induced leukopenia in interferon-beta-treated patients with multiple sclerosis by Maria Protopapa, Samantha Schmaul, Muriel Schraad, Katrin Pape, Frauke Zipp, Stefan Bittner and Timo Uphaus in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors thank Dr Cheryl Ernest for proofreading and editing the manuscript and Birgit Hohmann for technical assistance.
Footnotes
ORCID iDs: Maria Protopapa  https://orcid.org/0009-0000-7707-9050
https://orcid.org/0009-0000-7707-9050
Katrin Pape  https://orcid.org/0000-0001-9211-5873
https://orcid.org/0000-0001-9211-5873
Frauke Zipp  https://orcid.org/0000-0002-1231-1928
https://orcid.org/0000-0002-1231-1928
Stefan Bittner  https://orcid.org/0000-0003-2179-3655
https://orcid.org/0000-0003-2179-3655
Timo Uphaus  https://orcid.org/0000-0001-5526-0510
https://orcid.org/0000-0001-5526-0510
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria Protopapa, Department of Neurology, Focus Program Translational Neuroscience, (FTN), and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Samantha Schmaul, Department of Neurology, Focus Program Translational Neuroscience, (FTN), and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Muriel Schraad, Department of Neurology, Focus Program Translational Neuroscience, (FTN), and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Katrin Pape, Department of Neurology, Focus Program Translational Neuroscience, (FTN), and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Frauke Zipp, Department of Neurology, Focus Program Translational Neuroscience, (FTN), and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Stefan Bittner, Department of Neurology, Focus Program Translational Neuroscience, Rhine-Main Neuroscience Network, University Medical Center of the Johannes Gutenberg University Mainz, Langenbeckstrasse 1, Mainz 55131, Germany.
Timo Uphaus, Department of Neurology, Focus Program Translational Neuroscience, Rhine-Main Neuroscience Network, University Medical Center of the Johannes Gutenberg University Mainz, Langenbeckstrasse 1, Mainz 55131, Germany.
Declarations
Ethics approval and consent to participate: The study was performed in compliance with the Declaration of Helsinki and was approved by the ethics committee of the Aertzekammer Rheinland Pfalz (2019-14758_1); participants gave written informed consent.
Consent for publication: Not applicable.
Author contributions: Maria Protopapa: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Samantha Schmaul: Methodology; Writing – original draft; Writing – review & editing.
Muriel Schraad: Writing – review & editing.
Katrin Pape: Methodology; Writing – review & editing.
Frauke Zipp: Funding acquisition; Supervision; Writing – review & editing.
Stefan Bittner: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Writing – review & editing.
Timo Uphaus: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Else Kröner-Fresenius-Foundation (EKFS) grant 2022_EKCS.10 (to T.U.), the German Research Foundation (DFG) projects SFB-TR-128 (to T.U., F.Z., and S.B.) and TRR 355 (to S.B.), and the Hermann and Lilly-Schilling Foundation (to S.B.).
Competing interests: This study is a part of the doctoral thesis (MD) of M.P. at the University Medical Center of the Johannes Gutenberg University Mainz, Germany. F.Z. has recently received research grants and/or consultation funds from Biogen, Ministry of Education and Research (BMBF), Bristol-Meyers-Squibb, Celgene, German Research Foundation (DFG), Janssen, Max-Planck-Society (MPG), Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sanofi Genzyme, and Sandoz. S.B. has received honoraria from Biogen Idec, Bristol Meyer Squibbs, Merck Healthcare, Novartis, Roche, Sanofi Genzyme, and TEVA. His research is funded by Deutsche Forschungsgemeinschaft (DFG), Hertie Foundation, and the Hermann and Lilly-Schilling Foundation. T.U. reports personal fees from Merck Serono and Pfizer, and grants from Else Kröner-Fresenius Stiftung. All other authors report no disclosures relevant to the manuscript.
Availability of data and materials: The raw data used in the preparation of the figures and tables will be shared in anonymized format on request of a qualified investigator to the corresponding author for purposes of replicating procedures and results.
References
- 1. Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine 2020; 56: 102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tolley K, Hutchinson M, You X, et al. A network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing-remitting multiple sclerosis. PLoS One 2015; 10: e0127960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zettl UK, Hecker M, Aktas O, et al. Interferon β-1a and β-1b for patients with multiple sclerosis: updates to current knowledge. Expert Rev Clin Immunol 2018; 14: 137–153. [DOI] [PubMed] [Google Scholar]
- 4. Gilli F, Valentino P, Caldano M, et al. Expression and regulation of IFNalpha/beta receptor in IFNbeta-treated patients with multiple sclerosis. Neurology 2008; 71: 1940–1947. [DOI] [PubMed] [Google Scholar]
- 5. Sanford M, Lyseng-Williamson KA. Subcutaneous recombinant interferon-β-1a (Rebif®): a review of its use in the treatment of relapsing multiple sclerosis. Drugs 2011; 71: 1865–1891. [DOI] [PubMed] [Google Scholar]
- 6. Runkel L, Meier W, Pepinsky RB, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res 1998; 15: 641–649. [DOI] [PubMed] [Google Scholar]
- 7. Neuhaus O, Kieseier BC, Hartung HP. Pharmacokinetics and pharmacodynamics of the interferon-betas, glatiramer acetate, and mitoxantrone in multiple sclerosis. J Neurol Sci 2007; 259: 27–37. [DOI] [PubMed] [Google Scholar]
- 8. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998; 352: 1498–1504. [PubMed] [Google Scholar]
- 9. Goodin DS. Disease-modifying therapy in multiple sclerosis: update and clinical implications. Neurology 2008; 71: S8–S13. [DOI] [PubMed] [Google Scholar]
- 10. Hu X, Miller L, Richman S, et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol 2012; 52: 798–808. [DOI] [PubMed] [Google Scholar]
- 11. Newsome SD, Scott TF, Arnold DL, et al. Long-term outcomes of peginterferon beta-1a in multiple sclerosis: results from the ADVANCE extension study, ATTAIN. Ther Adv Neurol Disord 2018; 11: 1756286418791143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon β-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 2014; 13: 657–665. [DOI] [PubMed] [Google Scholar]
- 13. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 14. Salvetti M, Wray S, Nelles G, et al. Safety and clinical effectiveness of peginterferon beta-1a for relapsing multiple sclerosis in the real-world setting: interim results from the Plegridy Observational Program. Mult Scler Relat Disord 2022; 57: 103350. [DOI] [PubMed] [Google Scholar]
- 15. Jakimovski D, Vaughn CB, Eckert S, et al. Long-term drug treatment in multiple sclerosis: safety success and concerns. Expert Opin Drug Saf 2020; 19: 1121–1142. [DOI] [PubMed] [Google Scholar]
- 16. Alba Palé L, León Caballero J, Samsó Buxareu B, et al. Systematic review of depression in patients with multiple sclerosis and its relationship to interferonβ treatment. Mult Scler Relat Disord 2017; 17: 138–143. [DOI] [PubMed] [Google Scholar]
- 17. Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 2021; 14: 17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolb-Mäurer A, Sunderkötter C, Kukowski B, et al. An update on Peginterferon beta-1a Management in Multiple Sclerosis: results from an interdisciplinary Board of German and Austrian Neurologists and dermatologists. BMC Neurol 2019; 19: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci 2009; 56: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mastej K, Adamiec R. Neutrophil surface expression of CD11b and CD62L in diabetic microangiopathy. Acta Diabetol 2008; 45: 183–190. [DOI] [PubMed] [Google Scholar]
- 21. Monteseirín J, Chacón P, Vega A, et al. L-selectin expression on neutrophils from allergic patients. Clin Exp Allergy 2005; 35: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 22. Gadhoum SZ, Sackstein R. CD15 expression in human myeloid cell differentiation is regulated by sialidase activity. Nat Chem Biol 2008; 4: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, et al. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med 2005; 201: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reinisch W, Lichtenberger C, Steger G, et al. Donor dependent, interferon-gamma induced HLA-DR expression on human neutrophils in vivo. Clin Exp Immunol 2003; 133: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis RE, Sharma S, Conceição J, et al. Phenotypic and functional characteristics of HLA-DR(+) neutrophils in Brazilians with cutaneous leishmaniasis. J Leukoc Biol 2017; 101: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241286497 for Lower leukocytes pretreatment as a possible risk factor for therapy-induced leukopenia in interferon-beta-treated patients with multiple sclerosis by Maria Protopapa, Samantha Schmaul, Muriel Schraad, Katrin Pape, Frauke Zipp, Stefan Bittner and Timo Uphaus in Therapeutic Advances in Neurological Disorders