Abstract
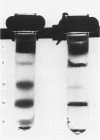
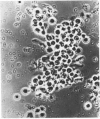
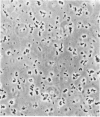
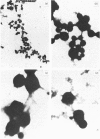
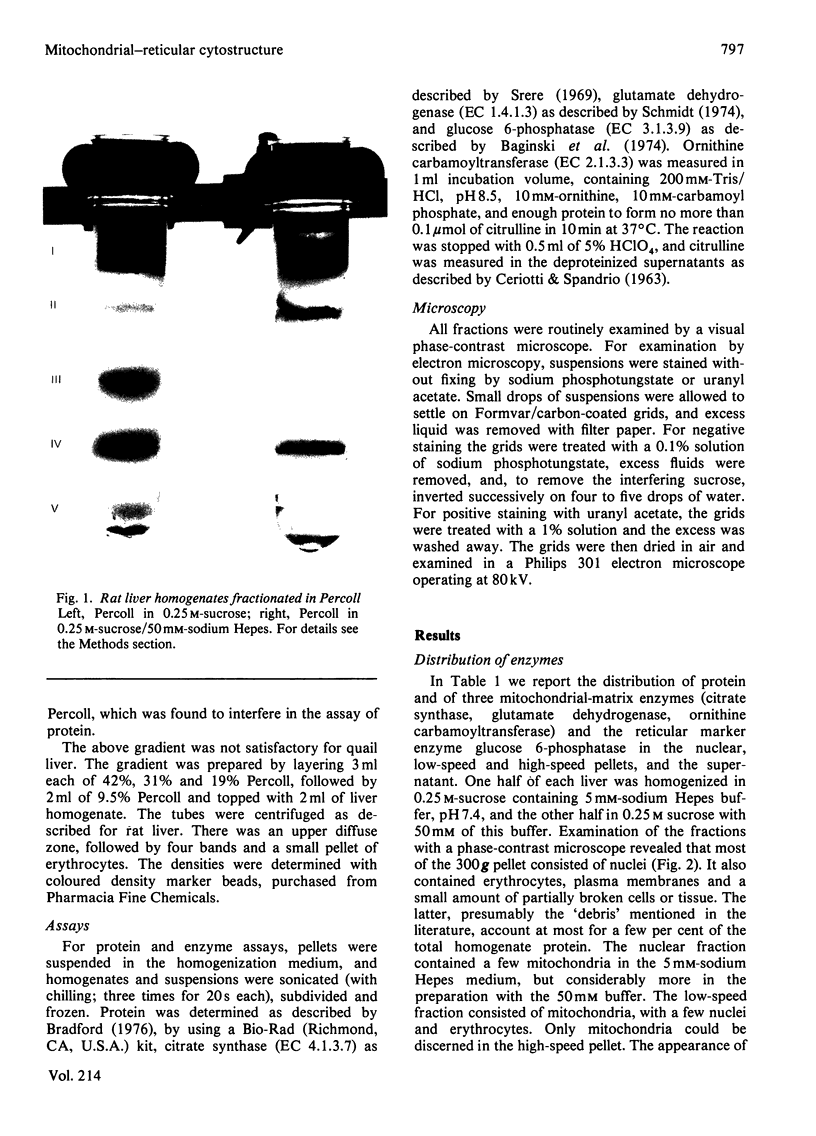
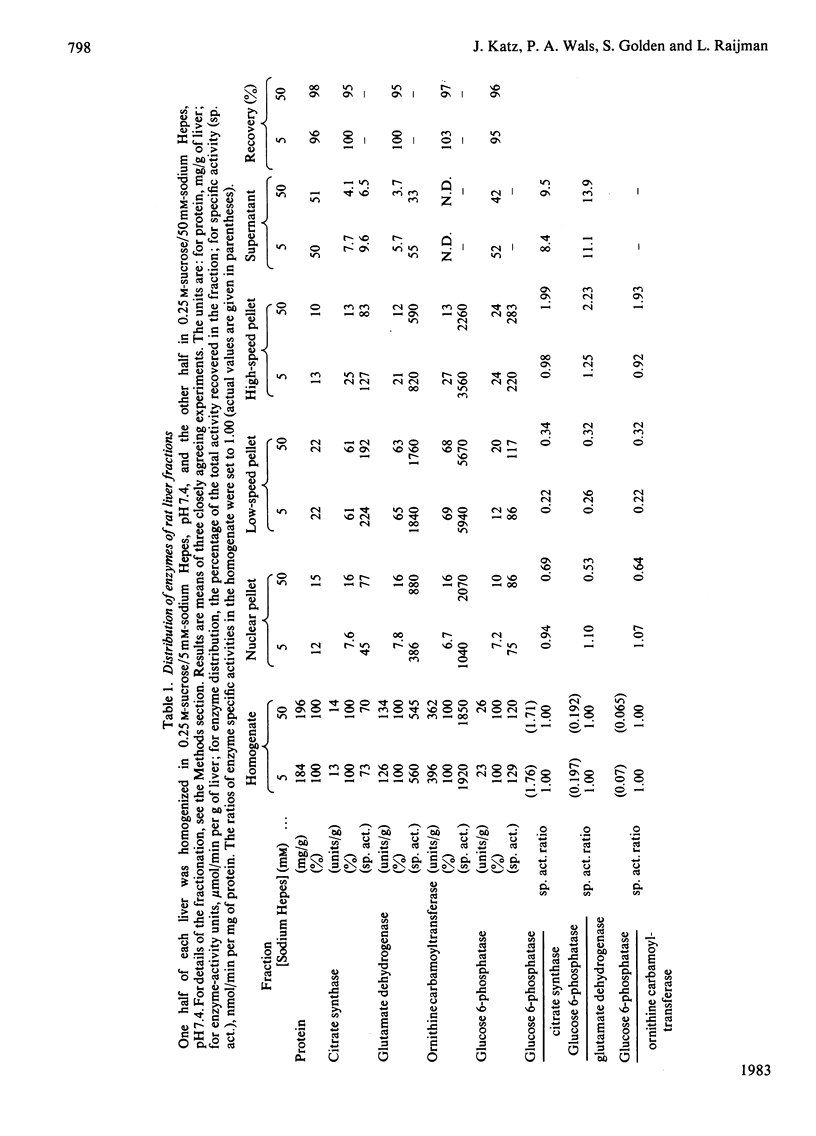
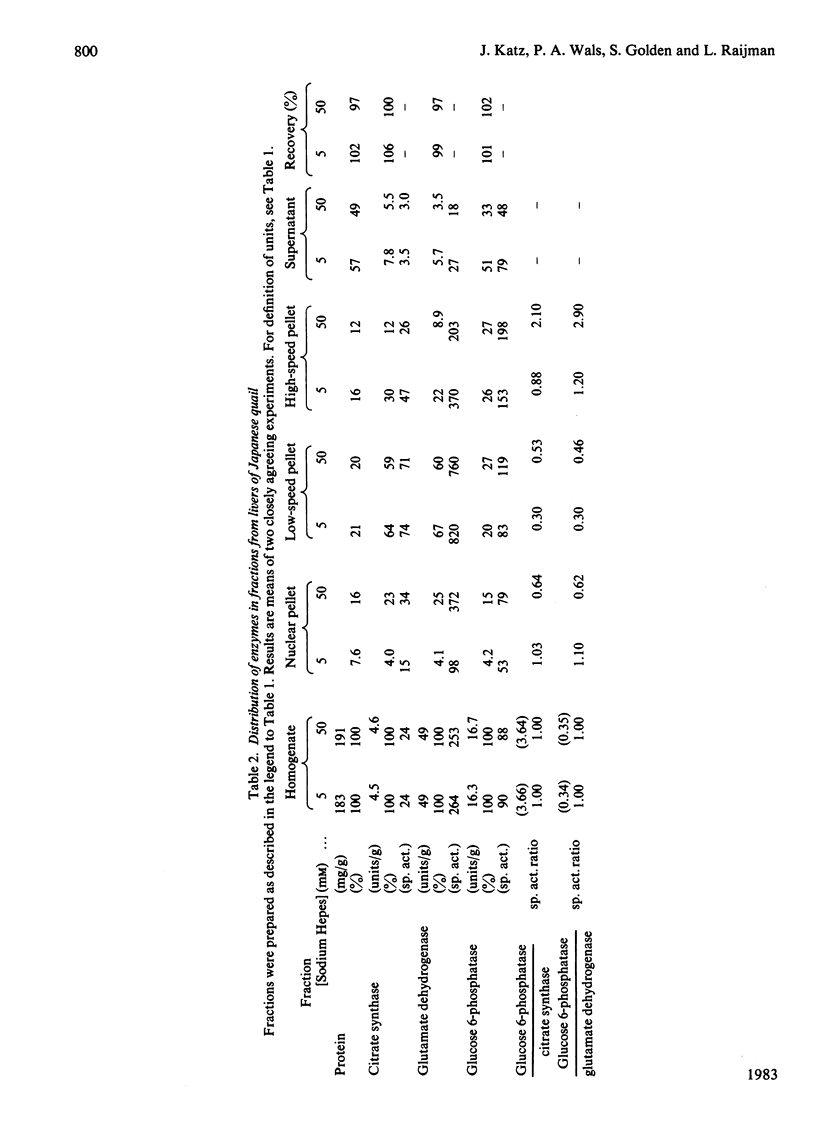
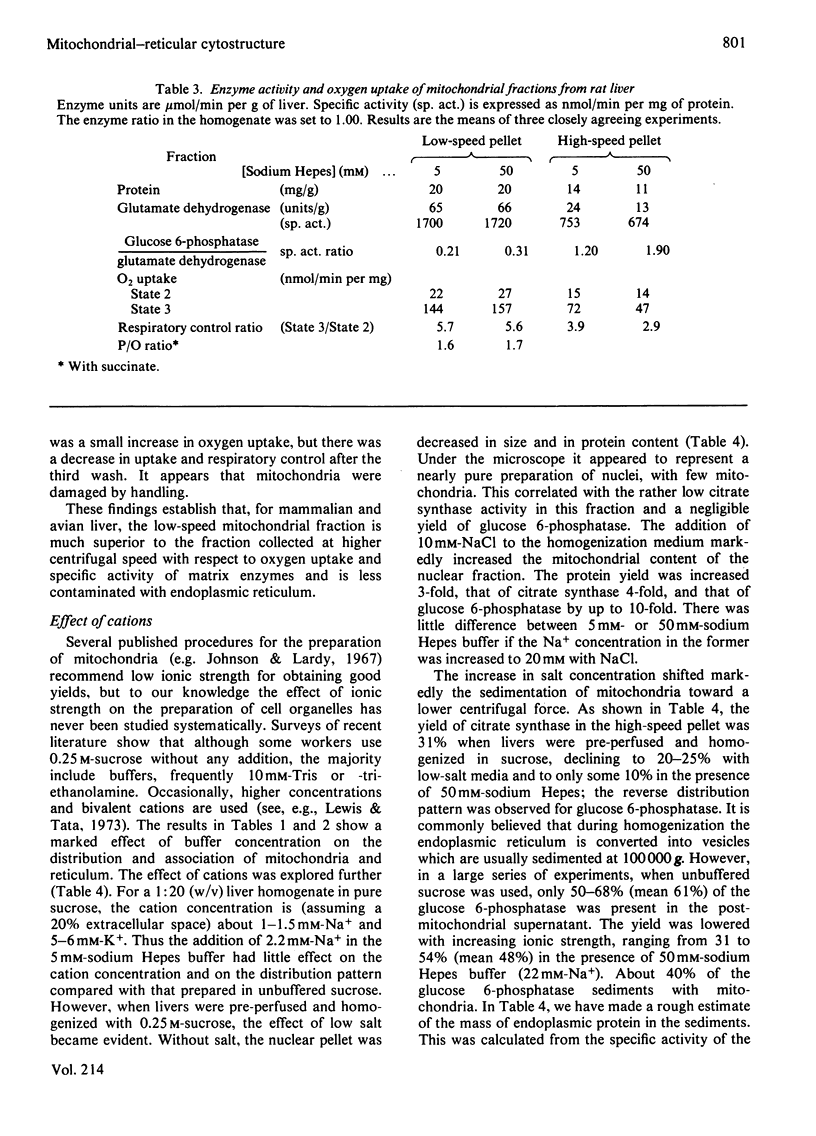
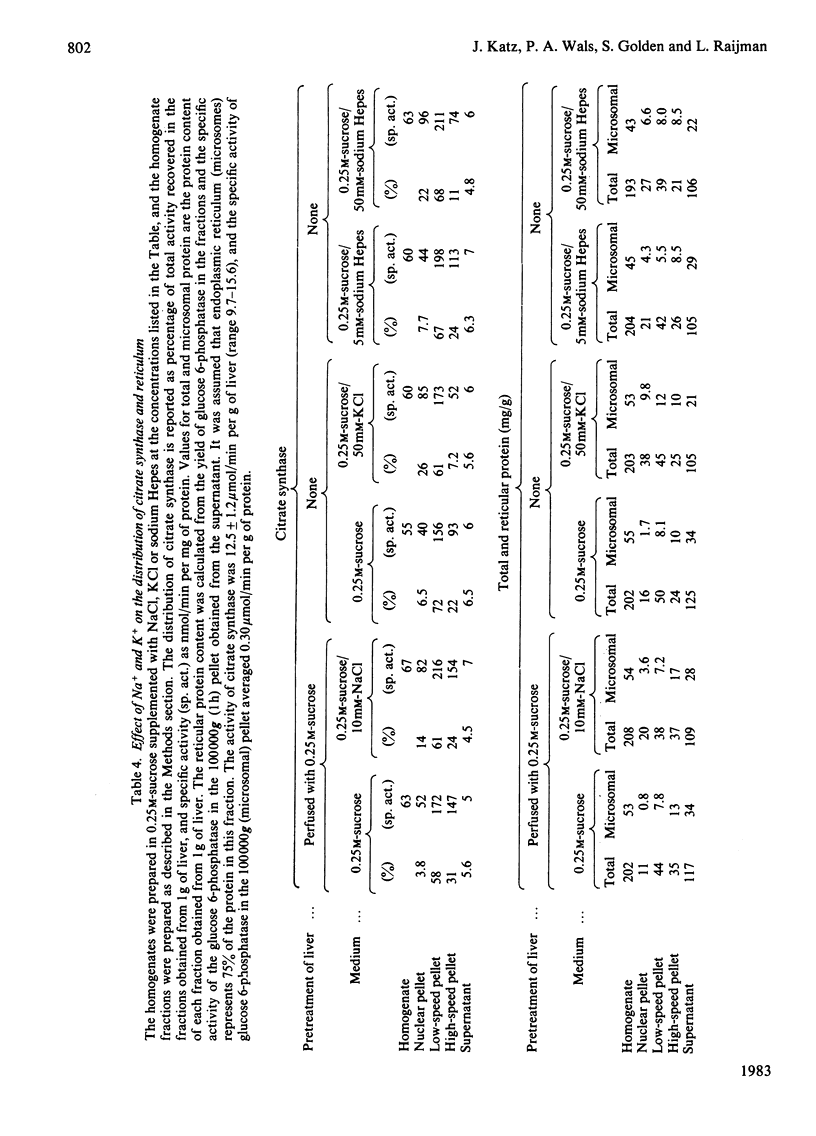
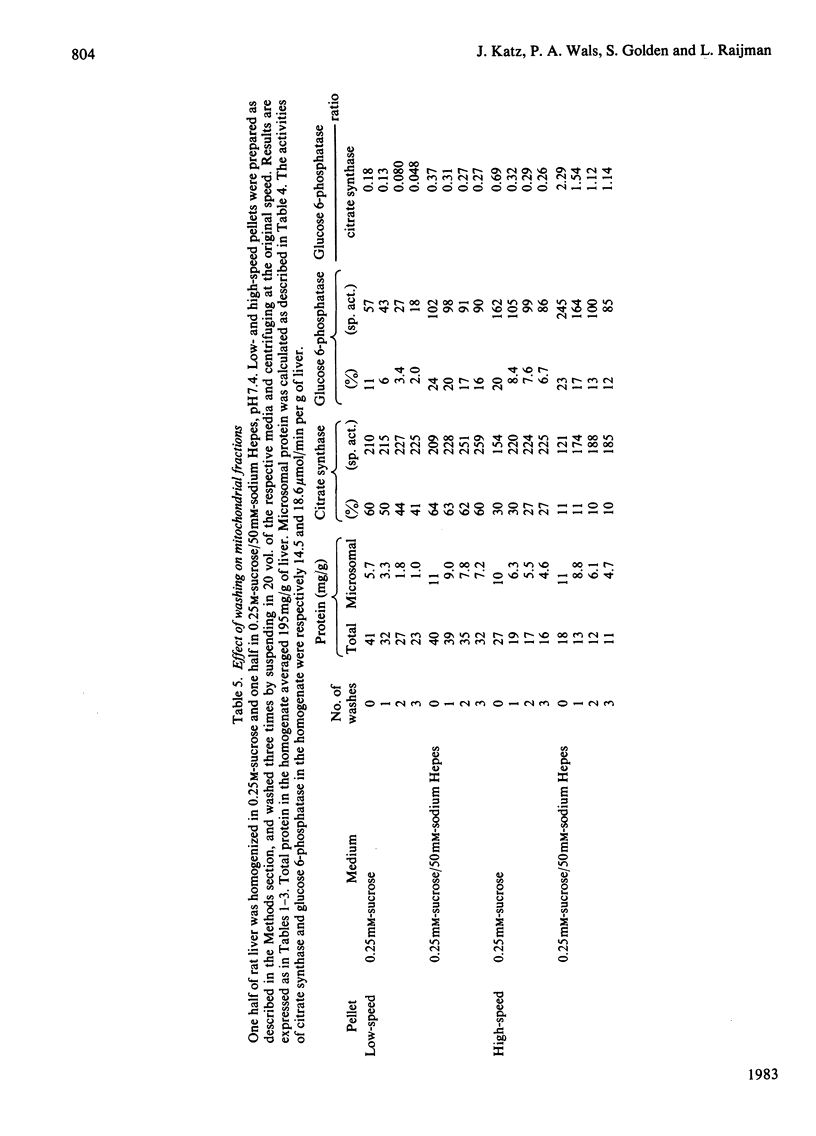
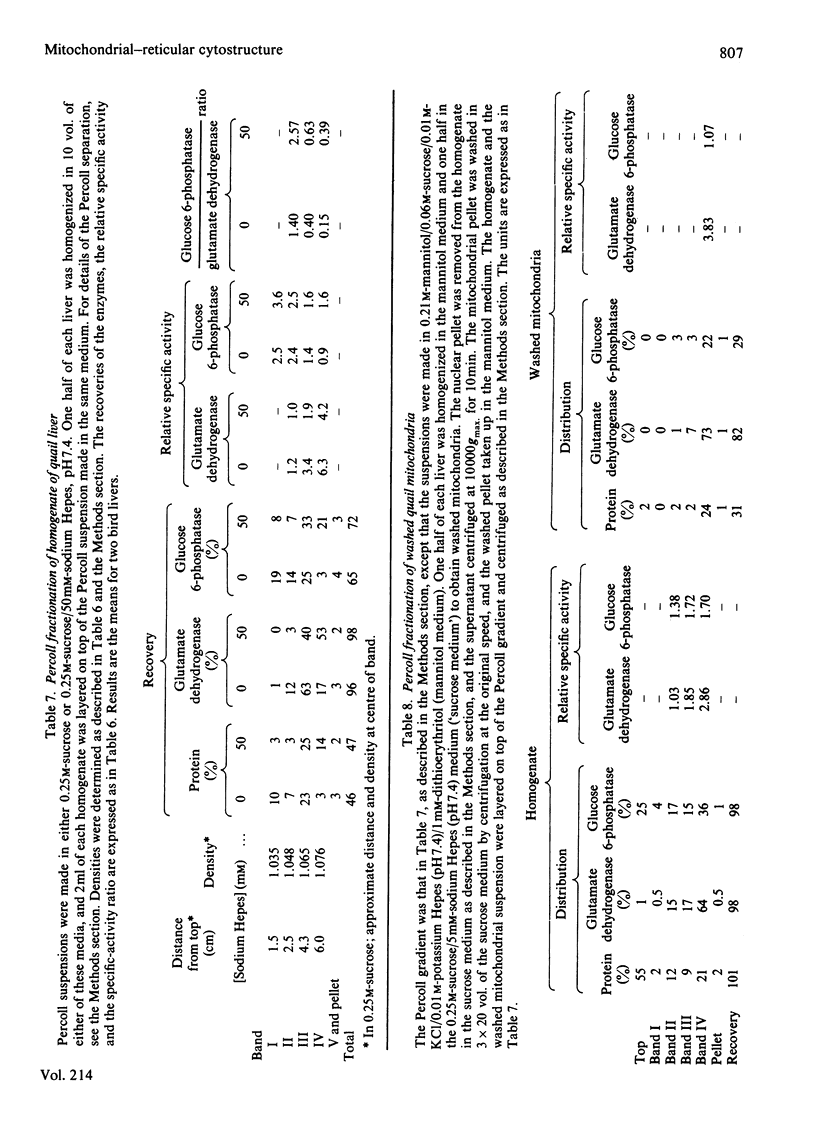
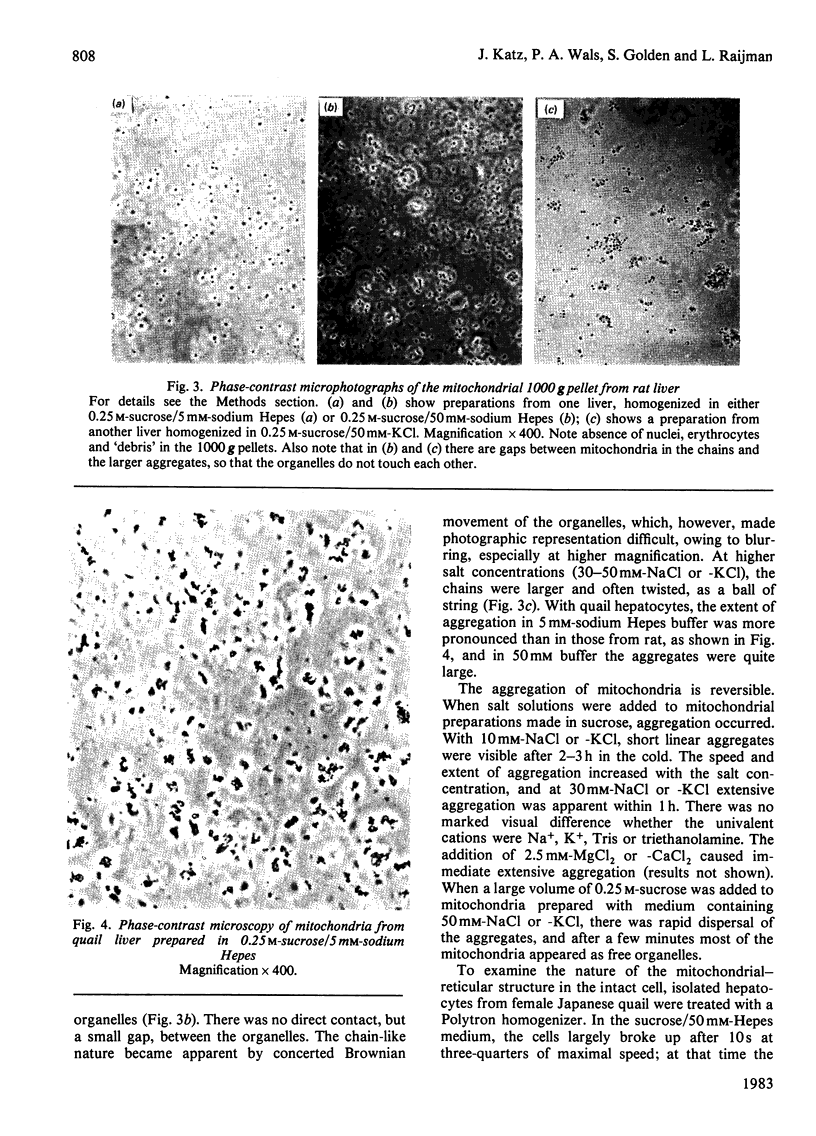
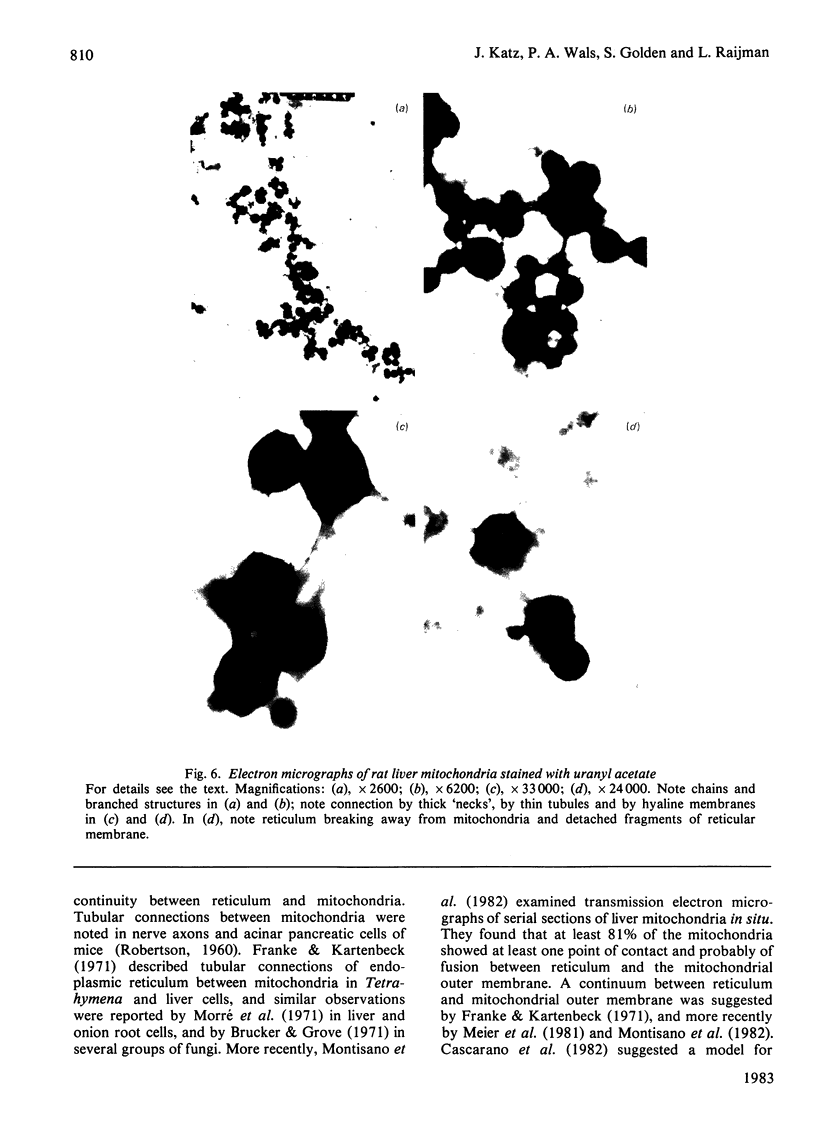
This study examines the structural relationship of mitochondria and the endoplasmic reticulum in liver. Livers of rat and Japanese quail were homogenized and fractionated in media of 0.25 M-sucrose, either 5mM or 50 mM in sodium Hepes [4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid], pH 7.4 (2.2 mM or 22 mM in Na respectively), designated here as low- and high-salt media. Three particulate fractions were prepared by sequential centrifugation. A nuclear pellet sedimenting at 300 g was obtained as described by Shore & Tata [(1977) J. Cell Biol. 72, 714-725], and from the resulting supernatant thereof a low-speed pellet (1100-1500 g) and a high-speed pellet (8000-10 000 g) were prepared. In the low-salt medium the yields of mitochondrial matrix enzymes (citrate synthase, glutamate dehydrogenase, ornithine carbamoyltransferase) and their specific activities in the low-speed pellet were over twice those in the high-speed pellet. In the high-salt medium the yield of matrix enzymes was 4-5 times, and the specific activities were up to 3 times, higher in the low-speed pellet than in the high-speed pellet. Oxygen uptake and respiratory control ratio were also much higher in the low-speed pellets in both media. Some 50-65% of the microsomal marker enzyme glucose 6-phosphatase was in the supernatant from the high-speed pellet, and the rest sedimented with the mitochondria. Repeated washing with the high-salt medium removes only a limited amount of reticulum. Washing with salt-free sucrose removes most of the reticulum, but a fraction remains strongly bound to mitochondria. Homogenates from quail and rat liver were fractioned isopycnically on Percoll gradients in either 0.25 M-sucrose or 0.25 M-sucrose/50 mM-sodium Hepes. Up to five particulate bands were separated and assayed. Mitochondria were present in two to three bands and were associated with endoplasmic reticulum. As seen in the phase-contrast microscope the mitochondria prepared in the low-salt medium consist of separate organelles. In the high-salt medium the mitochondria appear as chains of from three to ten organelles not touching each other. On addition of univalent ions at concentrations above 20 mM, the mitochondria aggregate into chains, and at higher ionic strength larger multidimensional aggregates are formed. The dispersion and aggregation of mitochondria are reversible. Negatively stained electron micrographs reveal a branched mitochondrial structure, with mitochondria held together by strands of reticulum.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracker C. E., Grove S. N. Continuity between cytoplasmic endomembranes and outer mitochondrial membranes in fungi. Protoplasma. 1971;73(1):15–34. doi: 10.1007/BF01286408. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner G., Bygrave F. L. Microsomal marker enzymes and their limitations in distinguishing the outer membrane of rat liver mitochondria from the microsomes. Eur J Biochem. 1969 Apr;8(4):530–534. doi: 10.1111/j.1432-1033.1969.tb00558.x. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G., SPANDRIO L. A spectrophotometric method for determination of urea. Clin Chim Acta. 1963 Mar;8:295–299. doi: 10.1016/0009-8981(63)90171-2. [DOI] [PubMed] [Google Scholar]
- Cascarano J., Montisano D. F., Pickett C. B., James T. W. Rough endoplasmic reticulum-mitochondrial complexes from rat liver. An extramitochondrial succinic dehydrogenase associated with this rough endoplasmic reticulum. Exp Cell Res. 1982 May;139(1):39–50. doi: 10.1016/0014-4827(82)90316-0. [DOI] [PubMed] [Google Scholar]
- Dallner G., Bergstrand A., Nilsson R. Heterogeneity of rough-surfaced liver microsomal membranes of adult, phenobarbital-treated, and newborn rats. J Cell Biol. 1968 Aug;38(2):257–276. doi: 10.1083/jcb.38.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. M., Phillips M. J. Cytoskeleton of the hepatocyte. Prog Liver Dis. 1979;6:105–121. [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73(1):35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Spycher M. A., Meyer U. A. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim Biophys Acta. 1981 Aug 20;646(2):283–297. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Spycher M. A., Meyer U. A. Isolation of a subfraction of rough endoplasmic reticulum closely associated with mitochondria. Evidence for its role in cytochrome P450 synthesis. Exp Cell Res. 1978 Feb;111(2):479–483. doi: 10.1016/0014-4827(78)90197-0. [DOI] [PubMed] [Google Scholar]
- Montisano D. F., Cascarano J., Pickett C. B., James T. W. Association between mitochondria and rough endoplasmic reticulum in rat liver. Anat Rec. 1982 Aug;203(4):441–450. doi: 10.1002/ar.1092030403. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Merritt W. D., Lembi C. A. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73(1):43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Montisano D., Eisner D., Cascarano J. The physical association between rat liver mitochondria and rough endoplasmic reticulum. I. Isolation, electron microscopic examination and sedimentation equilibrium centrifugation analyses of rough endoplasmic reticulum-mitochondrial complexes. Exp Cell Res. 1980 Aug;128(2):343–352. doi: 10.1016/0014-4827(80)90070-1. [DOI] [PubMed] [Google Scholar]
- Raijman L., Bartulis T. Effect of ATP translocation on citrulline and oxaloacetate synthesis by isolated rat liver mitochondria. Arch Biochem Biophys. 1979 Jun;195(1):188–197. doi: 10.1016/0003-9861(79)90340-0. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Two fractions of rough endoplasmic reticulum from rat liver. II. Cytoplasmic messenger RNA's which code for albumin and mitochondrial proteins are distributed differently between the two fractions. J Cell Biol. 1977 Mar;72(3):726–743. doi: 10.1083/jcb.72.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]