Abstract
Stem cell factors (SCFs) are pivotal factors existing in both soluble and membrane-bound forms, expressed by endothelial cells (ECs) and fibroblasts throughout the body. These factors enhance cell growth, viability, and migration in multipotent cell lineages. The preferential expression of SCF by arteriolar ECs indicates that arterioles create a unique microenvironment tailored to hematopoietic stem cells (HSCs). Insufficiency of SCF within bone marrow (BM)-derived adipose tissue results in decreased their overall cellularity, affecting HSCs and their immediate progenitors critical for generating diverse blood cells and maintaining the hematopoietic microenvironment. SCF deficiency disrupts BM function, impacting the production and differentiation of HSCs. Additionally, deleting SCF from adipocytes reduces lipogenesis, highlighting the crucial role of SCF/c-kit signaling in controlling lipid accumulation. This review elucidates the sources, roles, mechanisms, and molecular strategies of SCF in bone renewal, offering a comprehensive overview of recent advancements, challenges, and future directions for leveraging SCF as a key agent in regenerative medicine.
Keywords: Stem cell factors, regeneration, ligands, viability, medicine, bone renewal
Introduction
Stem cell factors (SCFs), also known as kit ligands, steel factor, or mast cell growth factor, 1 play a critical role in various biological processes, notably bone renewal. Serving as a hematopoietic cytokine within stem cell niches, 2 SCFs support and regulate hematopoietic stem cells (HSCs) in the bone marrow (BM) niche, enhancing HSC survival and facilitating their self-renewal and maintenance. 3 SCF engages its receptor, c-kit, on the cellular surface. This binding initiates the activation of various signaling pathways, including those crucial for cell proliferation, survival, and migration, and facilitates their homing to sites requiring tissue repair or regeneration. 4
Moreover, SCF has been shown to enhance adhesion, thereby ensuring the retention of HSCs within the niche and potentially facilitating their homing back to the BM environment. The SCF also serves with the related cytokines for the in vitro cultivation of HSCs and hematopoietic precursors, which are significant for the transplant of BM and blood formation. The HSCs and hematopoietic progenitor cells (HPCs) developmental facts are also assumed to be influenced by the original hematopoietic organs, such as in the adult BMs of vascular endothelial cells (ECs) that carry out distinct roles. This role extends to malignant conditions, where aberrant vascular niche cells are recruited to enhance the propagation of leukemic cells. 5 Arterial (A)ECs interact with HSCs in the BM, creating a specialized microenvironment tailored to HSC maintenance and regulation. It has been demonstrated that AECs selectively secrete SCF and support the concept of an arterial-associated HSC niche. 6 Research indicates that AECs contribute to the proliferation of human cord blood hematopoietic stem and progenitor cells (HSPCs), suggesting their involvement in establishing a supportive environment for HSCs. 7
Furthermore, AECs have been found to express specific markers that distinguish them from other subtypes of ECs, and they have been shown to regulate HSC maintenance and localization within the BM. 8 ECs, working in collaboration with stromal cells, represent substantial origins of the leading niche factors, SCF and C-X-C motif chemokine ligand 12 (CXCL12). The concurrent secretion of SCF and CXCL12 by stromal cells and ECs facilitates the maintenance and positioning of HSCs within the perivascular niche. While stromal cells typically exhibit higher expression levels of these factors, the synthesis of SCF and CXCL12 by ECs is essential for sustaining the proper function of the niche. Depleting SCF or CXCL12 from ECs using Tie2-Cre, receptor tyrosine kinase specific to the ECs, within the perivascular niche leads to the reduction of stem cells in the BM. This evidence highlights the significant interaction between ECs and HSCs, emphasizing the role of the former in regulating HSC maintenance and creating a specialized microenvironment tailored to HSC subsets.9–12
The scarcity of SCF in BM adipose tissue (AT) substantially affects both hematopoiesis and systemic metabolism. SCF, produced by various cells within the BM niche, including ECs, leptin receptor+ (LepR+) stromal cells, and adipocytes, maintains stable hematopoiesis. Furthermore, it supports the differentiation process of human pluripotent stem cell-derived brown adipocytes and is indispensable for regulating systemic metabolism through its cellular source. 13 Deleting SCFin adipocytes results in reduced platelets, neutrophils, monocytes, lymphocytes, eosinophils, and basophils in male and female mice, indicating that adipocytes create a niche responsible for producing SCF to promote uninterrupted hematopoiesis. BM adipocytes (A) stem from osterix-positive progenitor cells and release SCF, adiponectin, and other functional factors. SCF from BMA is essential for maintaining hematopoietic balance during aging, irradiation, and obesity, as well as in response to β3-adrenergic agonists.
Under homeostatic conditions, SCF deficiency in BMAT results in decreased HSPCs, as well as common myeloid progenitors (CMPs), megakaryocyte-erythrocyte progenitors (MEPs), and granulocyte-monocyte progenitors (GMPs). Mice that lack SCF from adipocytes are characterized by macrocytic anemia and depletion of circulating neutrophils, monocytes, and lymphocytes before transplants.13,14 Furthermore, the role of BMAT in bone health and regeneration is complex. While BMAT accumulation is often associated with detrimental effects on bone health, evidence implies that BMAT might play significant roles within the bone microenvironment. The biochemical characteristics of BMAT include its ability to store energy and release nutrients, such as free fatty acids, in response to suitable stimuli. 15
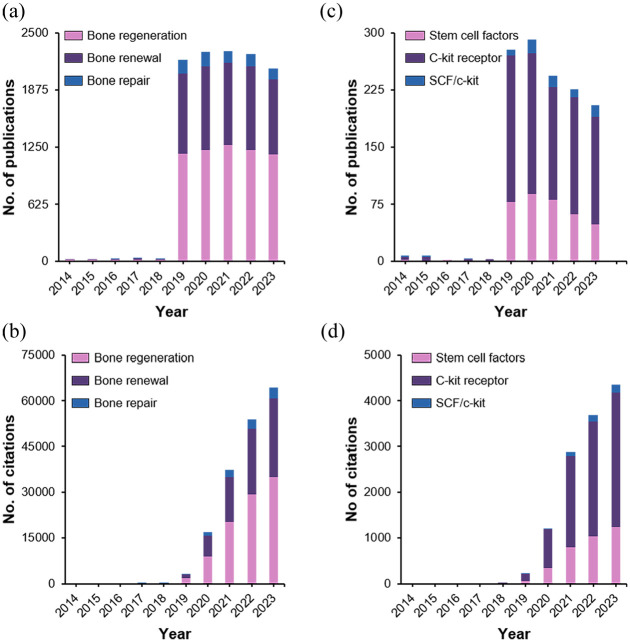
A comprehensive literature review of SCF for bone regeneration can become a critical basis for further development of our comprehension of the mechanisms of bone healing, the improvement of tissue engineering techniques, and an assessment of the potential of SCF-based therapies in clinical settings. Given the significance of bone regeneration in clinical applications and medical science, stem cell-driven bone regeneration is a meticulously researched field that has remained a crucial focus for decades. We conducted a citation analysis (Supplemental Information) to assess the importance and evolving interest in this field from 2014 to 2023. The findings of this analysis are presented in Figure 1. Figure 1(a) and (b) show the number of publications and citations, respectively, for the keywords “bone regeneration,” “bone repair,” and “bone renewal” using stem cells. Similarly, Figure 1(c) and (d) present the number of publications and citations, respectively, for the keywords “stem cell factors,” “C-kit receptors,” and “SCF/c-kit.” The growing number of citations, reflecting expanding interest and research activity, underscores the significance of this field. Despite the high demand in the field of bone regeneration, significant challenges remain to be addressed. Bone regeneration therapies face numerous challenges and limitations, such as the lower regenerative capacity of large bone defects than small ones; insufficient vascularization for regenerated bones to supply oxygen, nutrients, biological cues or cells in pre-clinical studies-and challenges translating results into clinical practice. 16 The difficulty to optimize mechanical properties is still a challenge of the engineered scaffolds, as natural polymers have poor strength and synthetic materials may lack bioactivity.17,18 Cell sourcing and controlling stem cell behavior in vivo is complex, and there is a lack of comprehensive long-term studies on the efficacy and safety of tissue engineering approaches. 19 The scalability and manufacture of tissue constructs for clinical application is a significant challenge, with some approaches also raising concerns about immunological morbidity.20,21 Regulatory hurdles and cost issues also impede clinical translation of many promising laboratory techniques.22,23 The major research gaps include the lack of vascularization strategies combined with bone regeneration, development of materials that can truly mimic native bone and innovative strategies to control the behavior or implanted stem cells.24–28 Furthermore, long-term clinical evaluations of tissue-engineering strategies are warranted to identify viable solutions for treating segmental defects using engineered bone constructs that can be manufactured on a large scale and in an open regulatory guideline-compatible environment when combined with the necessary advances needed in understanding bone healing biology.29–31 Addressing these challenges and research gaps is crucial for advancing bone regeneration therapies toward widespread clinical use, potentially through the integration of multiple strategies such as combining advanced biomaterials with cell and growth factor therapies. This review focuses on the main attributes and role of SCF as a fundamental trophic factor, highlighting the effects, sources, and pathways of this mediator in the context of bone regeneration. Additionally, we dig into recent research on the interactions and impacts of SCF on niche cells and HSCs within the BM microenvironment. Furthermore, we address the challenges associated with utilizing SCF as a regenerative tool and propose potential solutions based on our current understanding. To provide a comprehensive overview of the key points discussed, scheme 1 and S1 summarize the core aspects covered in this review. Following the introduction to the significance of SCF in regenerative medicine, it is vital to understand the several sources of these cell-derived growth factors fully. In the next section, we examine the genesis of SCF and many other diverse sources supporting its accessibility and functionality.
Figure 1.
Growing interest in stem cell-based therapy in bone regeneration: (a) number of publications and, (b) number of citations per year related to keywords like bone regeneration, bone renewal, and bone repair using stem cells, (c) number of publications, and (d) number of citations per year related to keywords like stem cell factors, c-kit receptor, and SCF/c-kit.
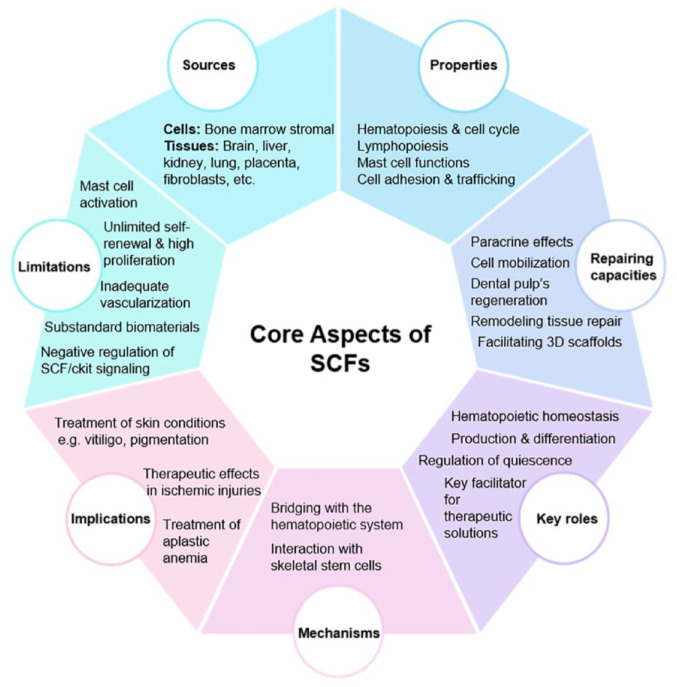
Scheme 1.
Summary of the core aspects discussed in this review. The aspects include critical sources, functional properties, repairing and regenerative abilities, key roles in maintaining hematopoietic homeostasis, mechanisms in bone regeneration, clinical implications on disease therapy and limitations of SCFs. The crucial sources of SCF encompass a diverse array of tissues (brain, liver, kidney, lung, placenta, fibroblasts, etc.) and cellular (BM stromal). In vitro, SCFs singlehandedly promote the viability and self-performance of HSCs and play a crucial role in mast cell biology. There exist various potential applications of SCFs, including but not limited to improving dental pulp’s regenerative abilities, remodeling tissue and repair, facilitating 3D scaffold stem, cell transplant via mobilization, improving and exerting paracrine effects in the field of regenerative medicine. SCFs are important to help BM function properly for the production and differentiation of HSCs. The scarcity of SCFs affects the ability of BM to facilitate the HSC function, leading to development of hematological diseases. Mechanistically, SCF plays a pivotal role in bone repair by bridging BMA with the hematopoietic system and through their interactions with skeletal stem cells. SCFs are used in clinically to treat diseases such as aplastic anemia and skin disorders. SCFs are potential tools for regeneration and recovery of ischemic injuries. SCFs, despite showing great potential in regenerative medicine, possess several limitations including mast cell-driven cytotoxicity, challenges associated with available cell sources, poor quality biomaterials insufficient supply of blood and oxygen to defect sites and negative regulation of SCFs/c-kit signaling. These limitations need to be addressed to maximize the efficacy of SCFs.
Sources of SCF
The crucial origins of SCF encompass a diverse array of tissues and cellular sources. Stem cells exhibit provenance from adult bodily tissues such as BM, blood, and AT. Furthermore, their derivation extends to embryonic origins and alternative reservoirs like umbilical cord blood. Notably, induced pluripotent stem cells (iPSCs) can be synthetically induced within laboratory settings, utilizing skin cells and other tissue-specific counterparts. Functionally akin to embryonic stem cells, iPSCs harbor considerable therapeutic promise across various applications. Moreover, the SCF reservoir includes BM stromal cells, brain tissue, liver, kidney, lung, placenta, fibroblasts, oocytes, and testicular tissue. This expansive diversity shows the richness of stem cell research and its potential clinical implications.
Numerous researchers (Kim et al. 2021) have explained the production of SCF in granulosa cells and its consequential impacts on cellular development and growth. Within follicular fluid (FF), SCF is predominantly secreted by granulosa cells (GCs). Through entangled ligand-receptor interactions, SCF fosters communication between oocytes and GCs, actively participating in the development and enlargement of primordial follicles, as well as the emergence of preovulatory dominant follicles. Moreover, this signaling pathway holds pivotal importance in early embryonic development and the subsequent formation of blastocysts. 32 Notably, SCF expression has been detected in granulosa cells across various mammalian species, including mice, 33 humans, 34 and porcine 35 (Figure S1a).
When the SCF/kit signaling pathways are disrupted, stem cell maintenance and function are compromised, which holds a significant relevance to the function of HSPCs in vivo. A functionally compromised kit receptor reduces the competitiveness of HSPCs and, consequently, enables HSPC transplantation without conditioning possible.36,37 Additionally, SCF-mediated signals have distinct effects on HSCs that express different amounts of the kit receptor indicating that the origin and quantity of the available SCF may impact the activity of HSPC. 38 LepR+ perivascular mesenchymal stromal cells (MSCs) and ECs contribute the majority of the SCFs in the BM, 39 with SCFs from both sources being essential to sustain normal HSC numbers and function in vivo. 40 A significant discovery was made (Zhou et al. 2017) regarding the fundamental sources of SCF in 2-month-old Leprcre; R26tdTomato; SCF-GFP (Green fluorescent protein) mice, particularly following irradiation. They identified LepR+ stromal cells and adipocytes as the main contributors of SCF within the BM microenvironment. Notably, SCF-GFP expression was substantially elevated in Tomato+CD45Ter119 stromal cells while relatively lower in VE-cadherin+CD45Ter119 ECs.
Interestingly, irradiation did not significantly alter the expression levels of SCF-GFP within these cell populations, with the majority of high SCF-GFP expressing cells remaining Tomato+ both pre- and post-irradiation in the BM. Furthermore, Tomato+ stromal cells surrounding sinusoids and small-diameter arterioles consistently exhibited positive SCF-GFP expression, contrasting with those around large-diameter arterioles, which remained negative. Following a 2-week post-irradiation period, SCF-GFP+ Tomato+ stromal cells were predominantly localized around sinusoids and small-diameter arterioles within the BM. In the BM of these mice, SCF-GFP generation was absent in hematopoietic cells or osteoblasts/osteocytes regardless of the irradiation status. 41
In the complex microenvironment of hematopoietic niches, the contribution of different cell types in supplying essential cues for hematopoiesis has been the focus of extensive research. (Stefano et al., 2019) shed light on the nuanced dynamics of SCF provision within this context. Contrary to previous assumptions, their findings suggest an operational specialization among niche cell types in delivering SCF to support hematopoietic progenitors. Through the targeted depletion of SCF from LepR+-expressing MSCs and Tie2-expressing ECs using mouse models (Stefano et al., 2019), it was demonstrated that while MSC-derived SCF is crucial for the maintenance and function of HSCs as well as specific lineage-restricted progenitors, such as common lymphoid progenitors (CLPs), CMPs, MEPs, and GMPs, EC-derived SCF appears to be exclusively vital for HSC function. Importantly, they observed comparable effects on progenitor cell populations irrespective of the dosage of SCF depletion, suggesting a robust dependence on MSC-derived SCF. Notably, despite the reduction in progenitor cell numbers, mature cell populations, particularly B and T lymphocytes, have remained largely unaffected, underscoring the resilience of lymphocyte pool size to fluctuations in progenitor numbers.
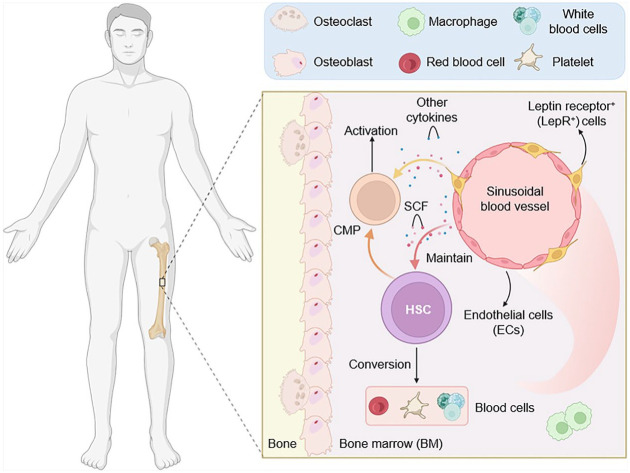
Further investigations are warranted to elucidate the fate of committed lymphoid progenitor cells upon loss of SCF production by MSCs. Microscopic examination revealed a close association between LepR-expressing MSCs and erythroid progenitors, providing visual validation of the niche role played by these cells in supporting erythroid lineage development. Questions persist regarding the homogeneity of MSC populations and their specific contributions to HSC versus progenitor cell maintenance. Although LepR-expressing MSCs outnumber HSCs significantly, their abundance alone may not be the sole determinant of HSC pool size. Researchers (Comazzetto et al. 2019 and Kara et al. 2023) introduced the concept of cellular specialization within the niche environment, where distinct niche cell subsets provide tailored support to different hematopoietic cell populations, thereby contributing to the precise regulation of HSPCs’ dynamics. As demonstrated in Figure 2, in the early postnatal BM, LepR+ and ECs express significant and high amounts of SCF, which is vital for preserving progenitors and HSCs. In early postnatal BM, LepR+/Prx1+ cell-derived SCF is required to activate myeloid and erythroid progenitors, while ECs-derived SCF is essential for HSC maintenance. Also, early postnatally, ECs, especially AECs and SECs, secrete high SCF levels, promoting HSC maintenance. LepR+ cells are significant sources of SCF in adult BM; however, their scarcity in the early postnatal phases is not vital for maintaining HSCs.42,43
Figure 2.
Early postnatal BM shows HSCs adjacent to sinusoidal blood vessels. Both LepR+ and ECs display elevated SCF levels within the BM. Myeloerythroid progenitors such as CMP depend on LepR+ cell-derived SCF for activation, while ECs-derived SCF is essential for HSC maintenance during this early postnatal period.
Schwann cells (SCs), along with LepR-expressing cells and ECs, represent another source of SCF, kit Ligand. Researchers (Feng-Chun et al., 2003) discovered that homozygous Nf1 mutant (Nf1–/–) SC secrete SCF, stimulating mast cell migration, particularly in Nf1+/– mast cells displaying heightened motility in response to SCF. This finding indicates the potent chemotactic stimulus provided by Nf1–/– SC through the secretion of soluble SCF and subsequent activation of specific rat sarcoma (Ras) effector-signaling pathways in mast cells.
Current studies specify the importance of hematopoietic cell attachment to integrins alpha 4 beta 1 (α4β1) and α5β1 for mast cell migration in response to SCF. Notably, the mast cells expressed both integrins. For instance, they (Kara et al. 2023 and Yang et al. 2003) found that conditioned media (CM)-derived from both wild type (WT) and Nf1–/– SCs proved to have elevated levels of several chemokines and growth factors, including vascular endothelial growth factor (VEGF), monocyte chemotactic factor 1 (MCP-1), chemokine; chemokine (C-C motif) ligand 5 (CCL5); and SCF. However, higher concentrations of the discussed factors were observed in Nf1–/– CM. At the same time, recombinant VEGF, MCP-1, or CCL5, separately or in combination, did not cause robust migration of the mast cells either WT or Nf1+/–, except for slight enhancement detected in the presence of recombinant SCF. Further experiments involved preincubating mast cells with an anti-c-kit antibody, which significantly inhibited their migration toward both WT and Nf1–/– CM, indicating the significance of the secreted SCF/c-kit signaling pathway in mast cell migration.43,44 Having elucidated the genesis of SCF, it is vital to comprehend how SCF regulates the stem cell microenvironment. Stem cell behavior and their functional properties are directly or indirectly connected to sources of the SCF, as the stem cell niches and extracellular matrix in which these factors are secreted can significantly influence their biological activity. 45 Table 1. summarizes the physical forms of SCF and highlights the critical cellular sources that produce and secrete SCF, including fibroblasts, ECs, BM-derived stromal cells, granulosa, adipocytes and thymic epithelial cells.
Table 1.
Summary of existing forms and critical sources of SCFs discussed in the indicated references.
| Forms | Critical sources | Reference |
|---|---|---|
| Liquid form (Protein supplementation) | Granulosa cells and pig ovaries | Kim et al. 32 |
| Membrane-associated KL proteins | Human ovaries, cultured granulosa-luteal cells, gonadotropins | Laitinen et al. 34 |
| Soluble forms of SCF (full-length and internal isoforms) | Granulosa cell layer, endothelial tissue, theca layer, oocytes, corpus luteum | Brankin et al. 35 |
| Membrane-bound and soluble forms | BM-derived stromal cells | Cosgun et al. 37 |
| Cytokine form | BM-derived stromal cells | Grinenko et al. 38 |
| Soluble and membrane-bound forms | BM-derived endothelial and perivascular cells | Ding et al. 40 |
| Human SCF and SCF-GFP forms | Adipocytes, Lepr+ cells, and ECs | Zhou et al. 41 |
| Human SCF | Lepr+ cells, and ECs | Comazzetto et al. 42 |
| Membrane-bound and secreted forms | Lepr+ cells, and ECs | Kara et al. 43 |
| Soluble SCF | Schwann cells (nf1 mutant) | Yang et al. 44 |
In the following section, we discussed the functional properties of the SCF and how their sources perform specific roles in stem cell maintenance, differentiation, and regeneration.
Functional properties of SCF
SCF is essential for maintaining a proportion of HSCs and c-kit+-limited progenitors and viral in promoting erythropoiesis, mast cell development, and lymphopoiesis (Figure S1b).46,47 In vitro experiments suggested that SCF may sustain HSCs and hemopoietic progenitors by facilitating their growth and differentiation in specific cellular lineages, including the erythroid cells.48,49 SCF is highly required in vivo to sustain the replication and survival of the youngest hematopoietic precursors. SCF alone promotes the survival and self-renewal of HSCs in in vitro research. As hematopoiesis proceeds, SCF, in synergy with other cytokines, promotes the cloning efficacy of HPCs from all lineages.
In addition, SCF is also pertinent for the survival of primordial germ cells and melanocyte precursors during development. Moreover, in vitro experiments show that SCF increases the number of mast cells and activates various other functions, including chemotaxis, adherence, adhesion, and secretory and degranulation responsiveness.
Hematopoiesis and cell cycle
SCF is necessary in vivo to preserve the integrity of the most primitive hematopoietic precursors. In vitro, SCF alone is enough to promote the viability and self-performance of HSCs. Similarly, with the advancement of hematopoiesis, SCF boosts proliferation in lymphoid and myeloid cells as long as extra growth factors are stimulated. In vitro experiments also reveal that SCF fosters the growth of mast cells and stimulates various functions, including chemotaxis, adhesion, and secretion. Mice deficient in SCF, such as the natural mutant Sl (steel), typically succumb before or shortly after birth due to severe anemia. It is proposed that SCF primarily primes stem cells to respond to the influence of other cytokines. It is essential to know that SCF has a synergistic impact with cytokines such as erythropoietin (EPO), interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and G-CSF for the direct colony growth of multiple progenitor cells including burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte, macrophage (CFU-GM), and colony-forming unit-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) in semisolid media. In addition, it promotes the survival of progenitor cells and enhances their stem cell’s progression in the cell cycle. It also acts as a chemokine and chemotactic factor for these cells. Its potent chemoattractant properties draw mucosal and connective tissue-type mast cells to specific locations within tissues.50–52
Lymphopoiesis and supporting the immune system
Regarding lymphopoiesis, SCF is vital for increasing the proliferative reaction of pre-B cells to IL-7, and it also has a role in the early stages of T-cell development. 53 Moreover, it supports differentiating CD34+ human hematopoietic cells into natural killer (NK) cells in the presence of IL-2 to help the immune response to viral infections and tumors. 54 Besides, SCF increases the expansion of dendritic cell (DC) progenitors, which is important for effective antigen presentation and T-cell immunity. DCs, efficient antigen-presenting cells involved in T-cell immunity, can be amplified in number by SCF. When certain types of cells, such as CD34+ human BM, are exposed to substances like GM-CSF and tumor necrosis factor (TNF-α), SCF notably boosts the growth of dendritic precursor cells for cells in a lab setting. This highlights capacity of SCFs to enhance the production of cell precursors in vitro. Besides, SCF works together with GM-CSF and TNF-α to directly improve the development of cells from these BM cells. These resulting DCs can activate T-cells during a response involving different genetic backgrounds.54,55 According to research studies, SCF is essential for B lymphopoiesis since it efficiently maintains the B-lymphoid potential of primitive hematopoietic cells conditioned in combinations of hematopoietic growth factors, especially when combined with IL-7. In the case of in vitro B cell lymphopoiesis, it appears that despite the synergistic effect of SCF and IL-7 on pre-B cell replication, the synergistic effect of SCF being recruited to this pre-B cell replication and caused pre-B cells to differentiate into surface Ig+B lymphocytes. This is because SCF was not the only requirement for the reaction. In addition, the SCF/c-kit receptor interaction does not rely on the proliferative response of B lymphocytes, which was not required for mitogens and vice versa. This shows that in the case of in vitro B cell lymphopoiesis, SCF affects B lymphopoiesis by enhancing the pre-B cells’ proliferative response to IL-7.56–59
In living organisms, SCF contributes to the initial phases of T-cell formation.60,61 Research conducted on W/W mice has shown that the developed thymocyte group is notably smaller than their counterparts. When W/W fetal liver cells are transplanted into recombination activating gene 2 (RAG 2)−/− mice, they do not contribute to thymocyte production. Additionally, when SCF and IL-7 are combined, they can stimulate the growth of typical CD4− CD8− CD3− thymocytes in a laboratory setting, indicating how important SCF is in developing T-cells. Moreover, SCF presentation by the thymic microenvironment is crucial for T-cell differentiation, as indicated by experiments using thymic tissue implants. The dysregulation of intestinal intraepithelial lymphocyte populations in W/Wv and Sl/Sld mice further determines the significance of SCF/c-kit receptor interaction in the normal function of the intestinal immune system. The function of T-cells could be influenced by SCF, which might boost the mixed lymphocyte reaction.62,63
NK cells, crucial for combating infections and cancers, can be generated from CD34+ hematopoietic cells in a laboratory setting with the help of SCF and IL-2. Additionally, SCF increases the capacity of NK cells to proliferate in response to IL-2. However, SCF induction does not increase the cell toxicity of CD56 bright NK cells against human erythroleukemic K562 cells (NK activity) or augment IL-2–induced lymphokine-activated killer activity.64–66
In another context, eosinophils from blood contribute to the development and maturation of mast cells by producing SCF, which controls these processes and ensures mast cell survival. This highlights the significance of SCF in fostering the growth and functionality of mast cells within the immune system.67,68
Mast cell functions
Mast cells originate from BM progenitors, migrating into the bloodstream and peripheral tissues. There, they undergo expansion and maturation influenced by cytokines in the local environment. 69 SCF plays a pivotal role in mast cell biology. It is essential for their proliferation, differentiation, activation, secretory function, and chemotaxis, uncovering their significance in allergic responses and immune regulation. As a result of the binding of its ligand SCF secreted in tissues by stromal cells, Kit activation is crucial for the progression of mast cells from BM progenitor cells and their eventful buildup, maturation, and survival in tissues. 70 In mice, SCF regulates mast cell development. Studies (Galli et al. 1993) demonstrated that treatment with recombinant human SCF in baboons and cynomolgus monkeys led to significant expansions of mast cell populations.
The mast cell expansion depends on mice-driven SCF secretion, which mirrors cytokine-induced proliferation and maturation—however, the influence of SCF on human mast cell development changes across experiments. Discontinuation of SCF treatment resulted in a decline in mast cell numbers. Additionally, local variations in endogenous SCF levels may influence tissue mast cell densities. Notably, animals treated with SCF did not exhibit clinically apparent issues related to mast cell activation. 71 SCF treatment enhances pro-survival B cell lymphoma (Bcl)-2 family proteins Bcl-2 and Bcl-XL (extra-large) levels in mast cells. However, it does not impact the expression of Bcl-2-related protein A1 (A1/Bfl-1), crucial for mast cell survival upon high-affinity receptor for the Fc region of immunoglobulin E (FcεR1) activation. SCF exerts its effects via the kit receptor, activating downstream pathways like phosphoinositide 3-kinase (PI3-K) and the Ak strain transforming gene (Akt). It is unclear how important the BH3(Bcl-2 homology 3)-only protein Bad (Bcl-2-associated death promoter) is for the survival of hematopoietic cells. However, Akt phosphorylation may render it inactive. Additionally, forkhead transcription factors (FKHR/FOXO1a, FKHRL1/FOXO3a, AFX/FOXO4) are phosphorylated by Akt, which inhibits their transcriptional activity. For instance, FOXO3a controls the expression of the proapoptotic Bim (Bcl-2 interacting mediator of cell death) gene. 72 Taylor et al. 73 investigated the function of SCF in developing various cell lineages. They observed increased responsiveness of rat peritoneal mast cells to SCF and anti-IgE during culture, possibly due to alterations in receptor expression and shared signaling pathways. SCF, produced by different cell types, elicits diverse effects. Mast cells cultured for varying durations exhibit heightened responsiveness to SCF and anti-IgE, independent of exogenous factors or purification procedures. In vivo, peritoneal mast cells degranulate in response to SCF and anti-IgE. Cultured tissue mast cells may better reflect in vivo secretory function and are thus suitable for investigating SCF- or IgE-dependent responses. Antigen-induced mast cell activation via FcεR1 triggers the release of inflammatory mediators, contributing to allergic reactions in atopic disease. These findings suggest that cultured tissue mast cells offer a more representative model for studying SCF- or IgE-mediated secretory responses in vivo. Unlike human mast cells, mouse mast cell development and survival are promoted by IL-3 in SCF-free culture.74,75 However, adding SCF as a supplementary markedly improves the cell proliferative rate. It is explored to alter the development of the cells to that of a serosal/connective tissue phenotype. In contrast, cells cultured solely in IL-3 are assumed to more closely mimic the mucosal phenotype, as indicated by the kind of proteases they express.76,77
Limited understanding exists regarding how SCF and other external factors influence mast cell phenotype and responsiveness to antigens. Under acute experimental conditions, SCF accelerates antigen-mediated mast cell degranulation and cytokine secretion, among other endogenous agents. However, repetitive subcutaneous SCF injection over 21 days in mice may protect against fatal anaphylactic reactions, with mast cells at injection sites showing minimal degranulation after IgE-induced anaphylaxis, suggesting a differential impact of chronic SCF exposure on mast cell activation. It was hypothesized (Ito et al. 2012 and Grabarek et al. 1994) that prolonged SCF exposure induces transcriptional modifications in mast cells, potentially altering their activation properties.78,79
Cell adhesion and trafficking
SCF functions in cell adhesion and trafficking processes, functioning as both an adhesion structure for megakaryocytes and a growth factor for these cells. Additionally, SCF generates progenitor cell adhesion to fibronectin, possibly by altering integrin avidity or binding directly to the c-kit receptor on HSCs. This interaction supports anchor HSCs within the BM microenvironment, facilitating cellular trafficking. Bendall et al. 80 investigated this phenomenon and found that exposure of Acute myeloid leukemia (AML) cells to SCF improves their adhesion to fibronectin, a protein abundant in the BM. The increased adhesion occurs through the b-1 integrin VLA-5, with a 5-min swift onset and persistent effects lasting beyond 2 h, unlike the transient response observed in mast cells. Using the c-kit receptor, hemopoietic progenitors may initially attach to stromal fibroblasts that express transmembrane (tm) SCFs. This is followed by acceleration in adhesion to fibronectin-containing environments via VLA-5. Loss of c-kit during normal myeloid maturation may reduce independent adhesion ligands and increase binding through VLA-5.80,81
Similarly, Pesce et al. 81 provided the initial evidence suggesting that the SCF/c-kit interaction mediates primordial germ cells’ (PGCs) adhesion to somatic cells, possibly in conjunction with other molecules. Their study demonstrated that in vitro, PGC adhesion to cell monolayers expressing membrane-bound SCF (such as TM4, gonadal somatic cells, STO (Sandoz inbred mouse strain Thymic Organism) fibroblasts, and BM stromal cells) was significantly decreased by antibodies against the c-kit receptor or SCF, or by soluble SCF. However, these therapies did not completely prevent PGC adhesion, but they suggest the involvement of additional mechanisms in PGC adhesion in these systems. The presence of both SCF/c-kit-dependent and -independent adhesion mechanisms in embryonic germ cells is also reinforced by the observation that PGCs demonstrated a constant but limited ability (15%–30%) to bind to cells with no tmSCF (such as Sl/Sld -1 BM stromal cells). Other epitopes will likely contribute to adhesive connections between PGCs and somatic cells. Notably, oocytes from 14.5 dpc (days post coitum) expressing low levels of the c-kit receptor demonstrated efficient SCF/c-kit-independent mechanisms for adhesion to TM4 cells, indicating the presence of distinct and developmentally regulated mechanisms modulating germ cell adhesiveness to somatic cells. 82 For easy understanding, we have summarized the functional properties of SCF in Table 2. Understanding the functional properties of SCF offers a fundamental insight into their respective roles in many biological processes. The key role of SCFs in fostering regeneration makes them stand out among other growth factors. Based on the functional attributes we discussed, we now probed into SCF as a regenerative key, focusing on its unique roles and signaling pathways in tissue repair and regeneration.
Table 2.
Summary of functional properties of SCFs.
| Functional properties | Potential applications | Target cells | Reference |
|---|---|---|---|
| Regulating cell survival, proliferation, hematopoiesis, and development Activating and binding to c-kit to turn on tyrosine kinase pathways for proliferation |
Regulates cell survival, proliferation, and stem cell maintenance plays a role in melanogenesis and tumor progression |
Mast | Annese et al. 46 |
| Regulating of hematopoiesis and cell survival Affecting fetal and adult compartments |
Shows therapeutic potential in stem cell mobilization and transplantation crucial for hematopoiesis and lymphopoiesis in adult life plays a pivotal role in the maintenance of lymphocyte development |
HSCs | Waskow et al. 47 |
| Regulating quiescence and long-term maintenance in BM | Stimulates HSC proliferation and survival induces rapid proliferation in HSCs provides a strong proliferative stimulus to HSCs |
HSCs | Domen and Weissman 48 |
| Providing strong proliferative stimulus Facilitating differentiation and preventing apoptosis |
Essential for hematopoietic stem cell maintenance and differentiation Plays a role in controlling the number of HSCs and MPPs Regulates hematopoietic cell lineage-restricted progenitors |
HSCs | Cordeiro Gomes et al. 49 |
| Preserving HSCs’ proliferation and differentiation Enhancing cloning efficacy and stimulating growth of Reducing chemotherapy-induced apoptosis in CD34+ |
Plays a role in thymocyte development and expansion. SCF-c-kit interactions are involved in erythropoiesis and myelopoiesis. SCF-driven expansion affects very early thymic immigrants |
Leukemic progenitor | Rodewald et al. 50 |
| Maintaining self-renewal and differentiation Playing avital role for long-term hematopoiesis post-transplantation Enhancing self-renewability of isolated HSCs |
Stimulates growth of CD34+ leukemic progenitor cells in AML Reduces chemotherapy-induced apoptosis in CD34+ human leukemia |
HSCs | Hassan and Zander 51 |
| Activating progenitor cells to protect against irradiation, and eliciting hematopoietic responses Enhancing BM cellularity, boosts mobilization in clinical studies |
Increases PBPC mobilization clinically SCF combined with filgrastim shows enhanced PBPC mobilization clinically |
Peripheral blood progenitor | Glaspy et al. 52 |
| Driving expansion in vivo
Promoting intrathymic proliferation of recent thymic immigrants |
Supports T-cell survival, differentiation, and proliferation. Used in optimizing cytokine concentrations for T-cell maturation. |
Thymocyte | John and Peter 53 |
| Facilitating cell proliferation and differentiation Exerting positive effects on DP stages |
Possess clinical applications in HSC transplantation. Used in the treatment of certain types of anemia |
Pro T | Lee et al. 54 |
| Supporting differentiation of multiple hematopoietic lineages from progenitors and facilitating cell differentiation in vivo | Enhances DC production by expanding lineage-restricted precursors | Human DC | Curti et al. 55 |
| Maintaining CFU-DC in long-term cultures Collaborating with FLT3-L in DC progenitor expansion Increasing CFU-DC by 10-fold |
Enhances pre-B cell expansion and inhibits maturation into B cells Used in the study of early human B cell development |
CD34DR | Kraus et al. 56 |
| Improving cell expansion and inhibiting maturation into B cells Suppressing differentiation into immature B cells Enhancing proliferation rate of CLP |
Plays a role in hematopoiesis and immune system regulation Essential for HSC maintenance and differentiation in the BM |
Pre-B, B, pro-B & CLPs | Ichii et al. 57 |
| Supporting maintenance and differentiation Regulating HSC stemness and differentiation via ligand-receptor interactions |
Supports the production of mature granulocytes in vitro | BM stromal cells (OP9) | Cho et al. 58 |
| Enhancing cell lineage commitment and differentiation Synergizing with IL-7 to induce B cell progenitor proliferation Contributing to the generation of antigen-specific surface Ig |
Serves as a growth factor for B-cell precursors | B cell progenitor | LeBien 59 |
| Improving growth | Promotes T-cell proliferation and differentiation in clinical applications Enhances proT-cell proliferation and differentiation in T-cell development |
HSC, fibroblast & ECs | Edgar et al. 60 |
| Promoting cell proliferation and differentiation Inhibiting differentiation to the double-positive stage in T-cell development |
Served in clinical settings for HSC transplantation | Pro T | Famili et al. 61 |
| Regulating cell survival Promoting cell proliferation and differentiation |
Impacts mucosal immune responses and lymphoid tissue | HSCs | Yoshino et al. 62 |
| Playing a vital role in lymphocyte and Peyer’s patches’ development Contributing to the organogenesis |
Regulates thymocyte growth in T cell development Initiates early developing thymocytes |
Mesenteric lymph node | Han and Zúñiga-Pflücker 63 |
| Regulating thymocyte survival and proliferation via Notch signaling Contributing to early thymocyte development by providing survival signals |
boosts innate immune surveillance against malignancy and infection | Double-negative | Fehniger et al. 64 |
| Enhancing cell proliferation, differentiation and expansion | Influences cell growth, useful in cancer therapy, and can enhance immunotherapy. studying SCF helps understand lymphopoiesis and leukemia, guiding targeted treatments | IL-2-induced NK | Benson et al. 65 |
| Inducing cell differentiation in cord blood cells Promoting cell proliferation and maturation Enhancing cell cytotoxic activities against tumor |
Crucial for blood cell development, gene therapy efficiency, and enhancing NK cell activity in cancer treatment | NK | Cavazzana-Calvo et al. 66 |
| Influencing cell activation and degranulation Enhancing cell adhesion to ECM proteins Playing a central part to hematopoiesis and encoded by the Steel locus |
Vital for blood cell formation, mast cell activity, suggesting its use in treating blood disorders and allergic diseases | Mast | Hartman et al. 67 |
| Promoting proliferation and proteoglycan accumulation in tissues Contributing to extracellular matrix protein synthesis in autoimmune diseases |
Influences immune cell activity, aiding in the regulation of immune responses and potentially offering therapeutic strategies | Fibroblast | Diny et al. 68 |
| Regulating cell activation and degranulation Producing a multimolecular signaling complex for cell activation Collaborating with LAT-scaffolded signaling complex for cellular responses |
Could enhance immune responses and improve vaccine efficacy | Mast | Rivera and Gilfillan 69 |
| Acting as a major chemotactic factor Facilitating cell proliferation, survival, and differentiation |
Manages allergic diseases by modulating mast cell activity | Mast | Okayama and Kawakami 70 |
| Regulating cell development in primates | rhSCF-treated monkeys can model systemic mastocytosis, improving treatment development | Mouse & human mast | Galli et al. 71 |
| Promoting cell survival through inactivation of FOXO3a Inducing phosphorylation of Akt and FOXO3a Regulating cell survival via PI3-K-dependent pathway Increasing levels of prosurvival Bcl-2 family members |
Supports mast cell survival, aiding in allergy and asthma treatment Highlights the potential in cancer therapy, regenerative medicine, autoimmune disease treatment improving transplant success by enhancing cell survival and integration |
Mast | Möller et al. 72 |
| Inducing cell degranulation in vitro and in vivo Acting as a potent activator |
Shows clinical applications in stem cell development and mast cells Potent activator of mast cell degranulation Induces direct degranulation of rat peritoneal mast cells |
Rat peritoneal mast | Taylor et al. 73 |
| Playing a key role for cell development and survival Serving CD34 cells gives rise to mast cells Helping define mast cell lineage in CD34 cells |
Shows potential applications in mast cell lineage commitment studies Crucial for the development of human mast cells |
Mast & CD34 | Kirshenbaum et al. 74 |
| Promoting mast cell growth and differentiation Enhancing lipid mediator production in mast cells |
Stimulates hematopoiesis and enhances stem cell survival Serves in research for cell culture and regenerative medicine studies |
Mast | Razin et al. 75 |
| Exerting synergistic effects with IL-3 and GM-CSF in human megakaryocytopoiesis Increasing colony growth and number of M, GMM, GM, and MAST colonies |
Enhances proliferation of murine megakaryocytic progenitors in serum-free culture Interacts with IL-3 to increase colony formation in mice shows synergism with IL-3 and GM-CSF in human megakaryocytopoiesis |
BM-derived mast | Tanaka et al. 76 |
| Enhancing proliferation | Used in pulmonary mast cell activation for allergic responses Critical for histamine release in mast cells |
progenitors | Lukacs et al. 77 |
| Programming cell activation phenotype Inducing graded hypo-responsiveness Downregulating mediator release based on a concentration Affecting the responses of cells mediated by tyrosine kinases Protecting against fatal anaphylactic reactions under chronic exposure |
May offer novel treatments for allergic disorders like asthma Can program mast cell activation phenotype for potential therapeutic strategies Can lead to hypo-responsive mast cells in allergic responses Downregulates mediator release in a concentration-dependent manner |
Mast | Ito et al. 78 |
| Inducing proliferation Modulating platelet activation and serotonin secretion in vitro Interacting with c-Kit receptor to mediate pleiotropic effects |
May modulate hemostasis through platelet interaction could potentiate platelet activation and aggregation May play a role in wound healing and atherosclerosis |
Progenitor megakaryocyte& mast | Grabarek et al. 79 |
| Enhancing AML cell adhesion to fibronectin Inhibiting AML cell apoptosis and enhancing proliferation Augmenting fibronectin-mediated anti-apoptotic and proliferative signals |
Enhances AML cell adhesion to fibronectin Augments anti-apoptotic and proliferative signals in AML cells |
Fibronectin | Bendall et al. 80 |
| Promoting survival, proliferation, and migration Functioning as an adhesion molecule |
Informs therapies for cancer and genetic disorders Enhances stem cell growth in lab settings, and targeting the SCF/c-kit pathway may improve outcomes in stem cell-related diseases |
Mouse primordial germ | Pesce et al. 81 |
SCF as a regenerative key
Several methodologies and factors could be considered to uncover the regenerative characteristics of SCF in bone repair. Studies indicate that MSCs are essential for the regeneration of bone tissue. These versatile cells can be obtained from sources like BM and fat tissue and have been utilized in bone regeneration studies for many years. 83 Researchers have also found results by combining human-derived biomaterials, such as scaffolds, with stem cells to improve bone regeneration. The impact of stem cells’ paracrine effects on bone healing is currently a research subject focusing on the factors released by MSCs during their transformation into bone-forming cells that could attract and develop progenitor cells.
Additionally, the use of growth factors like transforming growth factor-beta (TGF-β), VEGF, and SCF has been shown to promote bone regeneration by stimulating blood vessel growth and enhancing bone formation. For instance, VEGF, a crucial angiogenic growth factor involved in bone healing, plays a vital role in bone repair by enhancing angiogenesis and stimulating important skeletal cell populations, including osteoblasts, chondrocytes, and osteoclasts. Animal studies have demonstrated that combining scaffolds with stem cells and growth factors improves bone healing outcomes.84,85 To fully utilize the potential of SCFs in renewing bones, a comprehensive approach involving MSCs, biomaterials, and growth factors like SCF, TGF-β, and VEGF is recommended. These methods hold promise for advancing bone regeneration efforts and warrant exploration for potential clinical applications. The versatility of SCF in addressing tissues and conditions highlights its applicability in regenerative medicine. SCF acting as a growth stimulant is essential for encouraging stem cells’ growth, specialization, and survival, which play a role in regenerative treatments. Herein, we elucidate various potential applications of SCF, summarized in Figure S1c, including improving dental pulp’s regenerative abilities, remodeling tissue and repair, facilitating 3D scaffold stem cell transplant via mobilization, and improving and exerting paracrine effects in the field of regenerative medicine.
Dental pulp regeneration
Another crucial factor in bone regeneration is the surrounding environment. In cell therapy for bone regeneration, the injected cells interact with and influence the surrounding cells. 86 SCF has shown promise in dental pulp regeneration, especially in enhancing the angiogenic and neurogenic properties of dental pulp stem cells (DPSCs). Treatments for pulpitis and other dental disorders may benefit from this function, which is significant for the regeneration of tooth pulp tissue.87,88 Using SCF in dental pulp regeneration stimulates stem cell proliferation and differentiation, which promotes the repair and regeneration of damaged pulp tissue, as a detailed illustration is presented in Figure 3(a). SCF plays a significant role in tissue regeneration, extending beyond its applications in dental and liver tissues. Its ability to promote cell migration, angiogenesis, and tissue remodeling makes it a versatile agent in regenerative medicine. For example, SCF has been shown to promote the migration and proliferation of dental pulp progenitor cells, thereby speeding up tissue repair. In studies using collagen sponges, SCF application led to a significant increase in cell numbers and capillary formation, demonstrating its potential in regenerating various mesenchymal tissues.88–90 Beyond dental applications, the properties of SCF can be used to treat issues including cardiac ischemia and neurodegenerative diseases-in cases when stem cells are needed for tissue repair. In tissue engineering, SCF has been successfully employed to promote cardiac repair, liver regeneration, neurogenesis, corneal wound healing and to maintain germ cells.91–95 Given the multipotency and accessibility of dental stem cells, SCF could form a part of diverse tissue engineering strategies to help generate bone and nerve tissues. Despite the considerable promise that SCF offers in a number of regenerative contexts, however, the complexity of tissue interactions and different needs for individual tissues remain problems which need further investigation.
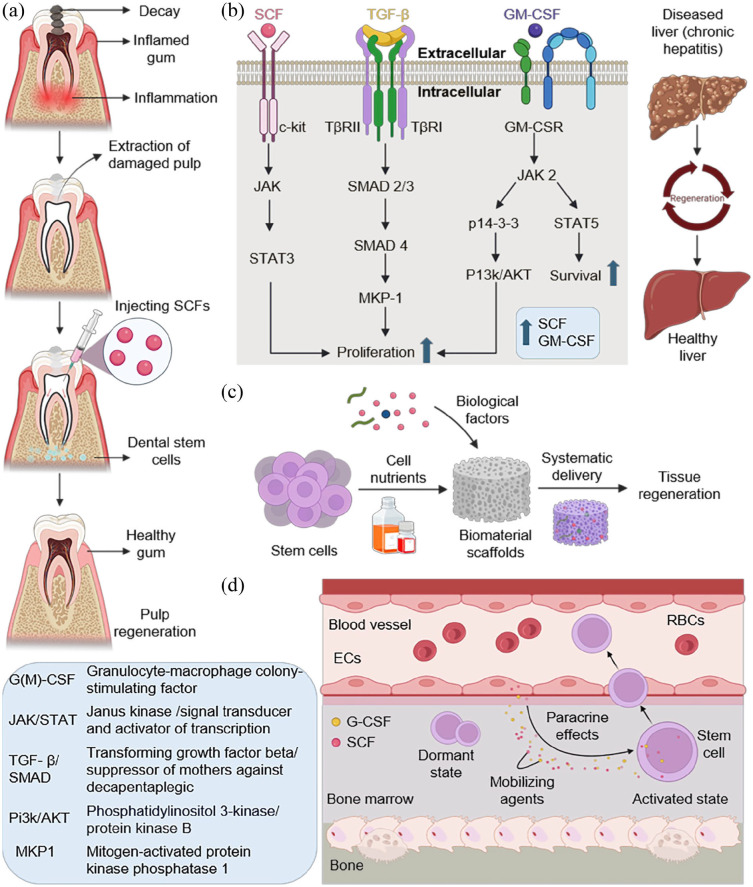
Figure 3.
The role of SCF in regeneration. (a) SCFs are administered into the pulp using a syringe after cleaning damaged tooth areas. The interaction between dental stem cells and SCF helps in the regeneration of dental pulp. (b) Mechanism for the regeneration of liver tissue. The combined effect of SCF, TGF-β and GM-CSF with their respective signaling pathways facilitates the liver cell proliferation and survival, which aid in inducing liver regeneration. (c) SCF facilitates the construction of scaffolds as a basis for tissue regeneration. The cocktail of stem cells and biological growth factors are introduced separately into the biomaterials of choice. Cocktail laden biomaterials are implanted symmetrically in vivo, enhancing tissue regeneration. (d) The paracrine effects of SCFs exerted on stem cells for activation and mobilization into blood circulation from BM. SCFs and G-CSF secreted by ECs act as mobilizing agents that activate dormant stem cells. The activated stem cells trespass into blood vessel form bone microenvironment.
Liver tissue remodeling and regeneration
SCF plays a critical role in liver tissue remodeling and regeneration in the liver illness and injury paradigm, especially when combined with other growth factors such as GM-CSF. 96 Liver tissue regeneration is aided by the synergistic interaction of GM-CSF and SCF, which is made possible via pathways such as TGF-β signaling. The key components of TGF-β signaling pathway are the TGF-β receptor complex containing Type I (TβRI) and Type II (TβRII) receptors, which initialize the process upon binding to TGF-β ligands. 97 Other components include suppressor of mothers against decapentaplegic (SMAD) proteins such as SMAD2, SMAD 3, and SMAD 4 which act as downstream mediators of the signaling. 98 Disruption of TGF-β signaling pathways, as observed in the β2SP knockout mouse model, results in a notable two to fourfold increase in the density of Oct3/4-positive cells with activated wingless/integrated (Wnt) signaling, occupying a progenitor cell niche after a partial hepatectomy. This indicates that TGF-β signaling is crucial in regulating hepatocyte proliferation, transitional phenotypes, and activation of hepatic progenitor cells during liver regeneration. 98 The TGF-β signaling pathway is vital for the synergistic effects of SCF and GM-CSF on stem/progenitor cell properties such as self-renewal, differentiation, and their involvement in liver regeneration following injury (Figure 3(b)).96,98–100 This application gains significance due to the remarkable regenerative capacity of liver, rendering SCF a valuable asset in therapies targeting liver repair and recovery from conditions like chronic hepatitis C (CHC). 101 Mahla et al. 102 investigated the role of SCFs in the liver using a murine model of acetaminophen-induced liver toxicity. Their findings reveal a substantial presence of SCF in the liver of normal mice, which significantly diminishes during injury concomitant with hepatocyte damage. As the liver initiates the regenerative process, SCF levels rebound, correlating with hepatocyte proliferation. Neutralizing SCF during the acetaminophen response markedly attenuates regeneration, exacerbating liver damage. Conversely, administering exogenous SCFs to acetaminophen-treated animals mitigates lethality. These studies depict significant role of SCFs in liver function and protection from injury.
Tissue engineering and organoid development
In tissue engineering and organoid development, the influence of SCF is significant. By promoting the proliferation and differentiation of diverse stem cells, SCF facilitates the construction of 3D scaffolds and organoids that replicate the structure and functionality of natural tissues. 103 An important role of SCF in organoid development and culture has been observed, specifically for some types of organoids such as neural and hematopoietic organoid development. SCF is one of the growth factors that are employed to induce formation of brain organoids from iPSCs and embryonic stem cells in vitro. 104 Sequential treatment of SCF with other factors can mimic the early stages of brain development (e.g., neurepithelium formation, neural progenitor generation, and neuronal birth) in brain organoids. SCF might be a hematopoietic cytokine during development of hematopoietic organoids arising from pluripotent stem cells or hematopoietic stem/progenitor cells. 96 Hematopoiesis critically depends on self-renewal coupled with differentiation capacities exhibited by HSCs; hence, defects thereof result in non-functional blood organs for developmental research or therapy. Commonly used to expand various types of stem cells including MSCs and HSCs before starting the organoid generation process, SCF serves as a reliable tool in such endeavors. Successful creation of functional human liver organs primarily relies on adequate multiplication rates which facilitate cellular organization into tissue like structures termed liver buds having hepatocytes at their core. Similar to other tissue engineering applications, extracellular matrix components (e.g., Matrigel) used for preparing an ideal environment for growing organoids may be potentially supplemented with immobilized molecules like SCFs so as to stimulate the formation and full maturation processes. 105 However, the specific role of SCFs in organoids formed from adult stem cells (of intestine, liver, pancreas and others) is yet to be investigated. However, the need for SCF may differ depending on the type of organoid and the source of stem cells. Generally, SCF appears as a crucial determinant in organoid systems that are either based on pluripotent stem cells or include hematopoietic lineages. SCF is an essential cytokine for expanding HSCs ex vivo for BM transplantation and other applications when used in combination with other biological factors. It also enhances stem cell proliferation and mobilization singlehandedly, which makes mobilized stem cells useful in regenerating ischemic tissues and for therapeutic use, thus making SCF a good candidate for MSC expansion for tissue engineering applications. 106 The immobilization of SCF on biomaterial surfaces results in increased cell adhesion, proliferation, and differentiation for tissue engineering purposes. It is more effective than soluble form of the protein to expand HSCs. Besides this, it promotes angiogenesis by enlisting endothelial progenitor cells as well as aiding the formation of new blood vessels which are indispensable in vascularizing engineered tissues. Furthermore, SCF enhances survival, migration, and differentiation of neural stem/progenitor cells during neural tissue engineering implying its potential application in repair of spinal cord injuries.96,106 This utilization holds immense importance for restoring damaged tissues and organs, opening up innovative approaches for addressing severe injuries and chronic diseases (Figure 3(c)).
Stem cell mobilization and transplantation
Beyond its direct regeneration capabilities, SCF is essential for recruiting stem cells for transplantation. The ability of this substance to specifically target HSCs and facilitate their growth and survival is a prerequisite for the effective treatment of BM transplants and other stem cell-based treatments. This feature is crucial for treating hematological ailments and enhancing the efficacy of stem cell transplants. Studies have shown that SCF induces heightened mobilization of long-term repopulating stem cells across various species, including mice, dogs, baboons, and humans. 107 When combined with G-CSF, SCF is an effective mobilizing agent for stem cells. The mechanism underlying SCF-driven mobilization of stem cells involves factors such as the upregulation of surface (C-X-C chemokine receptor type 4) CXCR4 expression on human CD34 cells and the release of membrane-bound SCF, which stimulates the proliferation of dormant stem cells in the BM before their release into circulation.107,108 Moreover, SCF directly promotes the development of specific cell populations, such as granulocyte-macrophage colony-forming cells. 109 This features critical role of SCFs in enhancing the efficiency and success of stem cell transplantation procedures through its mobilization effects (Figure 3(d)).
Paracrine actions and tissue regeneration
SCF, alongside other growth factors released by stem cells, can exert paracrine effects that enhance tissue repair and regeneration.110,111 These paracrine actions involve modulating the local tissue environment to facilitate the recruitment and function of progenitor cells, thereby aiding in the healing process. Leveraging paracrine effects of SCFs introduces novel strategies for regenerative medicine, emphasizing the indirect mechanisms by which stem cells promote tissue repair. The potential applications of SCF in regenerative medicine are vast, encompassing direct tissue regeneration and engineering, supporting stem cell transplantation, and utilizing paracrine effects for tissue repair. 112 These applications highlight the crucial role of SCF in advancing regenerative therapies and improving outcomes for patients with diverse diseases and injuries, including bone defects. After establishing a pivotal role of SCF as a regenerative key, exploring its specific role in bone regeneration is necessary. SCF’s regenerative qualities extend and apply to the skeletal stem cell system, promoting bone remodeling and renewal. 113 In the following section, we investigated how SCF deficiency interrupts the function of the BM, affecting the production and differentiation of HSC.
Role of SCFs in BM homeostasis
The deficiency of SCFs is detrimental to function BM as it is essential for the production and differentiation of HSCs. This tight regulation is required to ensure the balance between HSC self-renewal and differentiation, which maintains hematopoietic homeostasis. The BM microenvironment, or niche, provides essential signals for HSC maintenance and function. Growth factors like CXCL12 and SCF, secreted by niche cells, are critical for HSC proliferation and quiescence. A deficiency in these factors can lead to the depletion of HSCs, as they are unable to receive the necessary signals for survival and proliferation, ultimately disrupting the BM microenvironment.114,115 SCFs also influence the differentiation of HSCs into specific blood cell lineages. SCF deficiency significantly disrupts the BM niche, leading to impaired HSC self-renewal and differentiation. This disruption manifests through various mechanisms, such as c-kit signaling pathway, PI3K/Akt pathway and MAPK pathway (Figure 4). Deficiencies in SCFs can skew this process, potentially leading to an imbalance in blood cell types, such as an increase in myeloid cells over lymphoid cells. This imbalance can contribute to diseases like myeloproliferative disorders and leukemia, which are associated with altered hematopoietic lineage commitment. 116 SCFs play a pivotal role in controlling the proliferation and differentiation of HSCs, helping to maintain a balance between self-renewal and differentiation across various blood cell lineages. Without adequate levels of these factors, this balance is disrupted, leading to insufficient production of blood cells and potential hematological disorders.116,117 Quiescence, a state of dormancy, protects HSCs from exhaustion and maintains their long-term functionality. SCFs are involved in regulating this quiescent state. A deficiency can cause HSCs to exit quiescence inappropriately, leading to premature exhaustion, a reduction in the HSC pool, and loss of HSC quiescence.3,117 SCF deficiency affects the ability of BM to provide support for HSC function with significant effects on hematopoiesis and is a potential cause of hematological diseases. This knowledge is key for the development of therapeutic solutions against these deficiencies. In the following section, we investigated how SCF induces regenerative properties, emphasizing the therapeutic potential in curing bone-related ailments.
Figure 4.
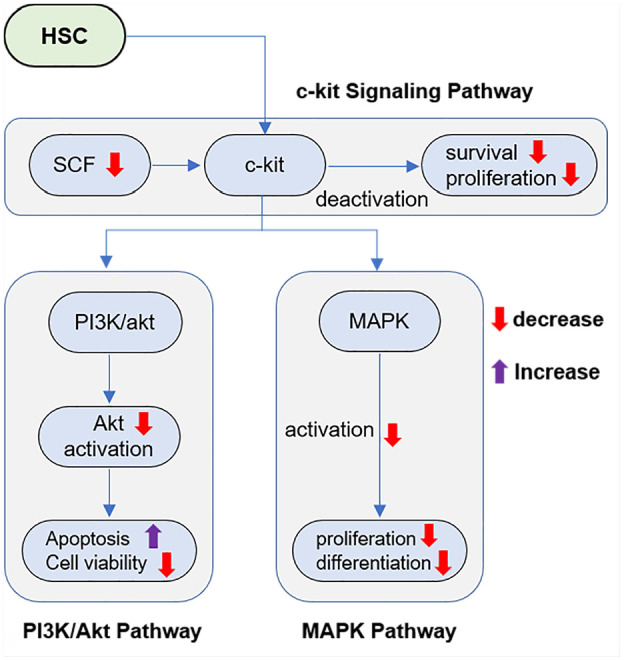
SCF binds with its receptor c-kit present on the surface of HSCs, which then activates downstream signaling pathways vital for the survival and self-renewal. Downregulation of SCFs decreases activation inherent to this pathway, leading to impaired HSC maintenance and function loss. The c-kit signaling activates PI3K/Akt pathway, an important regulator of cell survival. Consider that failure to induce AKT by SCF could result in increased apoptosis and reduced HSC viability. SCF also upregulates the MAPK pathway, a key cellular signaling network that controls cell proliferation and differentiation. SCF-deficiency disrupts this pathway and the differentiation of HSC into multiple blood cell lineages is impaired.
Mechanisms of SCF in bone renewal
Bone possesses strong regenerative potential, yet its ability to repair critical-sized defects is limited. 118 Bone cells, including osteoblasts, osteoclasts, bone lining cells, and osteocytes, act as mechanosensors (Figure 5). 85 Bone regeneration involves a complex and well-coordinated physiological process of bone formation. A healthy bone exhibits an intrinsic capacity to regenerate and repair itself at optimal conditions. However, when the ossification becomes extensive, as we see after fractures or in significant bone defects, the lifespan of bone repair cannot be overcome. Therefore, effective interventions are of utmost desire.119,120 SCF plays a pivotal role in bone regeneration by bridging BMA with the hematopoietic system. It supports the self-renewal and maintenance of HSCs and influences the BM microenvironment, crucial for stem cell regeneration and hematopoiesis. These processes are vital for maintaining the balance and functionality of blood cells and contribute significantly to skeletal system health. SCF, primarily secreted by BMA, substantially aids in HSC regeneration and hematopoiesis, especially following irradiation-induced stress, thus ensuring blood cells’ continued balance and function.121,122
Figure 5.
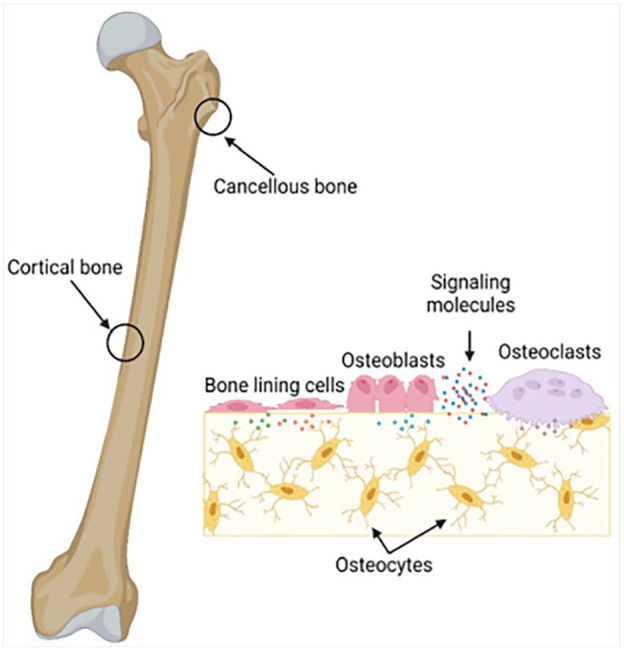
The structure of bone and the interactions between cortical and trabecular (cancellous) bone, along with key cells in bone remodeling. Cortical bone is the compact outer layer and cancellous or spongy bone inside. The process shows how remodeling occurs in response to loading and unloading. Bone forming osteoblasts are activated, whereas old bone resorbing cells called osteoclasts get deactivated. Osteocytes, descendants of osteoblasts, support bone tissue and lining cells cover inactive areas on bones. These cells are regulated by signaling molecules to maintain the balance between bone formation and resorption. 85 This figure is taken from article which can be distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Furthermore, the role of SCFs in bone regeneration is skillfully linked to its interaction with skeletal stem cells (SSCs), essential for bone development, homeostasis, and repair. SSCs exhibit an impressive ability to differentiate into crucial cell types necessary for bone regeneration, such as chondrocytes, osteoblasts, and marrow stromal cells. The responsiveness and versatility of SSCs in regenerative environments depict the complex and compelling nature of bone repair mechanisms. SCF, among other factors, contributes to attracting and retaining HPCs within the marrow space, thereby supporting the regenerative functions of SSCs. In addition, modifications to the stem cells’ milieu caused by biomolecules may affect the trophic factors secreted by stem cells, affecting their functioning. Research has indicated that brief exposure of human osteoblasts to TNF-α might enhance osteogenic differentiation and trigger the release of soluble molecules that support osteogenic differentiation in adipose-derived stem cells (ADSC). Likewise, a brief stimulation period with IL-1β, G-CSF, stromal cell-derived factor 1 (SDF1), and SCF can significantly impact the expression of many cytokine genes and proteins in MSCs. While SCF controls the synthesis of proteins involved in proliferation, chondrogenesis, and extracellular matrix modulation, IL-1β stimulates the release of proteins with chemotactic, proinflammatory, and angiogenic qualities.123–125
Likewise, adipocytes exhibit a direct impact on hematopoiesis in an assortment of ways. However, the precise functions adipocytes play in this process are still debatable because of disparities in how they affect stem or progenitor cells and AT locations. For instance, depleting adipocytes in fat-enriched tail vertebrae increases the expansion of progenitor cells post-irradiation. In contrast, adipocytes in long bones promote HSC regeneration via increased SCF secretion after radiation-induced stress. Moreover, adiponectin has been shown to maintain HSC self-renewal and protect HSCs from inflammatory cytokine insults, particularly in aging. These effects are specific to HSCs and less prominent in differentiated cells. 126 Expansion of HSCs ex vivo poses a significant challenge in clinical practice. Along with SCF, enhancing the identification of optimal growth factors like thrombopoietin, fms-like tyrosine kinase 3 (Flt3) ligand, IL-6, and G-CSF can support sustained growth of HSC colonies outside the body. Various strategies have been devised to utilize umbilical cord blood-derived HSCs (UCB-CD34+) for ex vivo expansion, capitalizing on the self-renewal capabilities of HSCs as a therapeutic strategy to renew hematopoietic progenitors hierarchically. While tissue-specific HSC aspiration is considered invasive, ex vivo expansion strategies aim to enhance HSC self-renewal and proliferation. For instance, a synergistic effect of SCF and Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 1 (SHP-1) inhibitor, NSC87877, has been demonstrated to activate c-kit and inhibit negative regulators (SHP-1/SHP-2), promoting cellular proliferation of erythroid and megakaryoblast cells.127–129 In the BM environment, SCF and GM-CSF interact synergistically to control hematopoiesis and bone formation. Here we clarified some of main key points regarding their interaction. SCF and GM-CSF work in tandem to facilitate the proliferation and differentiation of HSCs and HPCs in the BM niche. This mutual enhancement leads to improved production of various blood cell types, including those involved in bone remodeling. Both in the absence and presence of GM-CSF, SCF seems to be necessary for the development of mature, committed and primitive HPCs in the BM.130–132 SCF supports the survival and expansion of these progenitors. GM-CSF, SCF and in conjunction with other cytokines such as IL-3, promote the growth and differentiation of megakaryocyte progenitors from BM-derived primitive bone cells. 133 Megakaryocytes play a vital role in controlling bone remodeling mechanism. However, it has been demonstrated that GM-CSF inhibits the production of SCF and its receptor c-kit in BMDCs. This implies an effective regulatory mechanism where GM-CSF modulates the effects of SCF in certain BM microenvironments or cell types. The complementary effect between SCF and GM-CSF involves distinct signaling pathways such as activating transcription factors like signal transducer and activator of transcription 3 (STAT3), which regulate cell proliferation and differentiation. 134 While SCF and GM-CSF synergize to promote expansion of HPCs and differentiation in the BM, GM-CSF can also negatively regulate the SCF/c-Kit axis in certain BM cell types like DCs. This complicated interaction between these cytokines helps supervise the sophisticated mechanisms of hematopoiesis and bone remodeling in the BM niches. We have summarized the role of SCFs in regulating hematopoiesis, elucidating the respective fucntions, target cells and microenvironments in Table 3.
Table 3.
Role of SCFs in regulating hematopoiesis process.
| Function | Target | Condition | Reference |
|---|---|---|---|
| • Promote proliferation • Maintain the long-term repopulating ability |
Densely populated HSCs | In vitro secretion | Broudy
2
Hasan et al. 51 |
| • Support the survival, proliferation, and differentiation • Enhance production of various blood cell lineages. |
HSCs and progenitor cell | Cytokine interaction (EPO, IL-3, GM-CSF, and G-CSF) | Broudy
2
Hasan et al. 51 Molineux et al. 135 |
| • Facilitate Mobilization and migration | HSCs | BM | Shah et al.
119
Kurzrock et al. 136 |
| • Play a anchoring role | HSCs | BM | Kurzrock et al. 136 |
| • Create the specialized microenvironment necessary for HSC self-renewal and differentiation | HSCs | Stromal cells | Broudy
2
Hasan et al. 51 Kurzrock et al. 136 |
| • Enhance the proliferative response • Play a role in early lymphopoiesis and T-cell development |
HSCs | Cytokine interaction (IL-7) | Kurzrock et al. 136 |
Conclusively, SCF plays a dual role in hematopoietic regeneration and bone repair by supporting stem cell regeneration and influencing the behavior of skeletal stem cells. This multifactorial approach highlights the importance of SCF in maintaining bone health and systemic blood cell production. Despite the limitations associated with the clinical utilization of SCF, we believe that advances in the mechanistic understanding of bone repair and regeneration will continue to provide exciting new treatments for patients suffering from delayed bone healing and large-volume defects. After demonstrating the specific role of SCF in tissue and bone regeneration, it is necessary to explore the clinical implications of its therapeutic use. The following section highlight the clinical implications, examining the challenges from biological, technical, and practical perspectives.
Clinical implications of SCF-based therapies
SCFs have several clinical applications due to their role in promoting the proliferation, survival, and differentiation of HSCs and other progenitor cells. Here, we elucidate some of the key clinical applications that exploit SCFs as regenerative keys. SCFs are used in clinical settings to mobilize peripheral blood progenitor cells. When combined with G-CSF, SCFs significantly increase the number of progenitor cells that can be harvested from peripheral blood. This combination is more effective than G-CSF alone in mobilizing progenitor cells, which is crucial for stem cell transplantation procedures.137,138 SCFs have also been explored for their potential in treating various marrow failure states and aplastic anemia. They can stimulate the production of multiple blood cell lineages, providing therapeutic benefits in conditions where bone marrow function is compromised. 138 Additionally, SCFs are used in ex vivo culture systems to expand hematopoietic progenitor cells. This application enhances the yield of stem cells for transplantation purposes, allowing for better engraftment and recovery in patients undergoing bone marrow transplants.138,139 Recent studies have explored the use of tmSCFs in regenerative therapies. Unlike their soluble form, tmSCFs do not activate mast cells, reducing the risk of anaphylaxis. tmSCF-based therapies have shown promise in promoting angiogenesis and stem cell mobilization without off-target effects, making them a potential tool for tissue regeneration and recovery from ischemic injuries. 106 SCFs have also been investigated for their role in skin pigmentation disorders due to their ability to influence melanocyte biology. This could open avenues for treating conditions like vitiligo and other pigmentation disorders.138,140,141 Overall, SCFs hold significant promise in regenerative medicine and hematology, although their clinical use is limited by some adverse effects, such as mast cell activation. Advances in delivery methods, such as tmSCFs, may help mitigate these issues and expand their therapeutic applications.
SCF therapies that can revolutionize the clinical scopes have been found in regenerative as well as hematopoietic treatments. Their translation into clinical practice requires some consideration like cost, patient outcomes and how they can be practiced efficiently. SCF-based therapeutics are costly, given the large expenses of protein therapeutic development and administration. Costs associated with producing and purifying SCF may be high, compared to many other biologics (presently in clinic), such that scalable production of the secreted growth factor could seem unlikely unless economical strategies are established. 106 While the up-front price tag might be steep, SCF therapies could save money in the long run by preventing costlier interventions and hospitalizations. Measures that enable successful mobilization of HSCs could shorten hospital stays and diminish the requirement for transfusions in patients who have received chemotherapy or bone marrow transplants. 142 For patients with diseases such as aplastic anemia to those undergoing chemotherapy, improving the survival and expansion of HSCs through SCF has promise for significantly better tissue regeneration. On the other hand, SCF therapies have been hindered by adverse events like mastocytosis and anaphylaxis caused due to generation of high serum levels of free mediators. 143 The more recent approaches, like tmSCF besides the supposed increased efficiency and quality of ex vivo expanded stem cells are reported to have an additional advantage for such a purpose as they avoid activation of mast cells that induce these side effects. Such therapies, with improved safety profiles that are also more effective in animal models than current treatments (therefore potentially leading to better patient outcomes), would have an added benefit: no risk of anaphylaxis. Adoption of SCF-based therapies into clinical practice is associated with regulatory challenges, in addition to the need for robust data from appropriate clinical trials. The use of standardized protocols for administration and monitoring is critical to allow safe and efficacious use. Similar to other biologics (e.g., biosimilars), adoption of SCF-based therapies in clinical practice may require overcoming challenges that are largely unique to hematopoietic growth factors. But to enter the mainstream, they will have to conquer skepticism, and show that they are as good—or better—than what already exists.106,142,144 In conclusion, although SCF-based therapies have great potential for enhancing patient outcomes in a range of hematological and regenerative contexts, it is clear from these studies that overcoming many challenges all the way downstream to regulatory issues needs to also be addressed before this may ever occur routinely in clinical practice. Improvements in delivery and adverse effect profiles could facilitate their inclusion into mainstream medical practice. While SCF possesses a promising therapeutic effect in regenerative medicine, to fully capitalize on these effects, several obstacles exist that need to be eliminated. The following section thoroughly examines these obstacles in detail.
Limitations
While SCF has shown great potential in promoting bone repair and renewal, several limitations, summarized in Figure S1d, need addressing to maximize their efficacy. These limitations include mast cell toxicity, uncontrollable self-renewal cellular behaviors, insufficient vascularization, availability of subpar biomaterials, and negative regulators causing antagonism in SCF/c-kit signaling. One of the primary challenges is their linkage to cell toxicity via mast cell activation. Additionally, identifying the most effective cell type and analyzing risk factors such as tumor formation in stem cell therapy pose significant obstacles. Furthermore, improving the cost-effectiveness and scalability of producing and delivering these factors for widespread clinical use is a prerequisite. 145
Mast cell activation
A major limitation of using exogenous soluble SCF clinically is its tendency to activate mast cells, leading to the risk of inducing anaphylaxis in large animal models and clinical trials, significantly restricting its therapeutic potencies. Thus, it is crucial to acknowledge that clinical utility of SCFs has been constrained by the toxicity linked to mast cell activation. Researchers have explored the use of tmSCF supplied in lipid nanodiscs or proteoliposomes, which do not trigger mast cell activation, as an alternative to overcome drawbacks associated with soluble SCF. However, there are still challenges in developing and improving these alternative delivery modalities.73,106,146 Further research is needed to address these limitations and fully harness the therapeutic potential of SCF in clinical settings. 147
Cell source challenges
Due to the properties of unlimited self-renewal and high proliferation rate, cells such as induced pluripotent and embryonal stem cells are considered to carry very high risk if not perceived to be acceptable. In contrast, most small-scale clinical trials indulging MSCs in applications related to regenerative medicine have not raised significant health concerns, indicating that MSC-based treatments may be relatively safe. However, some clinical trials have reported severe adverse effects, focusing on the need for more research, especially in long-term safety and biological processes. As a component of stem cell therapy, SCF is subject to risk factors such as the type of stem cells used, their procurement and culturing history, and the level of manipulation, all of which can impact the safety and efficacy of stem cell-based treatments.148–151
Vascularization
Adipose tissue-derived microvessels have been utilized as natural vascularization units in angiogenesis research and tissue regeneration due to their exceptional capacity to quickly reassemble into microvascular networks. 152 Ensuring adequate vascularization at the defect location is still challenging because insufficient blood supply can hamper the success of regenerative modalities. 153 Vascularization plays a vital role in the survival and integration of engineered tissue grafts. The core area of the large tissue grafts is susceptible to ischemia and cell death due to elusive diffusion from the surrounding blood vessels. Rapid vascularization within days after implantation requires tissue grafts to function properly. Significant morbidity and fatalities also occur due to these ischemic diseases, such as atherosclerotic artery blockage, that are not adequately addressed by the currently available treatments. Therapeutic angiogenesis uses angiogenic factors to stimulate the formation of nascent microvascular networks.154,155 One possible solution to circumvent the lack of blood supply is to utilize the implants of pre-vascularized tissue constructs that are microsurgically linked to the local vasculature site.
Techniques that have shown promising results in improving vascularization include creating an arteriovenous (AV) loop in the construct before transplantation.25,156 However, no optimized methods for vascularizing tissue constructs in vitro exist. Therefore, it is recommended that both in vitro and in vivo assays be conducted to determine optimal culturing conditions for stable capillary development. In regenerative medicine, achieving sufficient vascularization is still a significant challenge that requires more in-depth studies and the generation of cutting-edge vascularization strategies.
Biomaterials and scaffolds
Although growth factors, including SCF, biomaterials, and scaffolds, play a vital role in promoting stem cell-based bone regeneration, replacing native and fully functional bone tissue remains a major challenge. Both stem cell and BM niches are highly complicated and homeostatically maintained with the complex interplay of structural, biochemical, and biophysical stimuli in the BM niches. In order to resemble the entangled native bone microenvironment, ongoing research is focused on refining the composition, structure, and integration of biomaterial scaffolds with stem cells and other regeneration agents (e.g. growth factors, hormones, and cytokines). Some essential scaffold attributes that must be refined include biocompatibility, biodegradability, mechanical qualities, porosity, and the capacity to deliver specific biochemical cues to control stem cell behavior. Indeed, beyond the physicochemical properties of the material, cell-material interactions have been shown to significantly impact cell differentiation. 157 Both SCFs and scaffolds/biomaterials have distinct advantages and challenges. SCFs serve as local stimulators for specific cell proliferation and they regulate stem cells directly by orchestrating their activity in terms of proliferation, differentiation and migration that combined increase the regenerative potential. They can often be delivered through minimally invasive methods, such as injections, bypassing the requirement for surgical implantation into body tissues. SCFs can also be delivered along with scaffolds/ biomaterials to develop more effective combination therapies.158,159 Nevertheless, SCFs face obstacles regarding controlled release and in vivo bioactivity. The dosage requirement and the need for safety, especially in cases of growth factors (BMPs), are critical to prevent side effects. 160 Moreover, certain SCFs are very costly for manufacturing and utilizing in a clinical context, especially recombinant growth factors. 161 In contrast, scaffolds and biomaterials provide structure support through a 3D framework that mimics the natural ECM, directing tissue growth. They are extensively designable with specific architecture, porosity and mechanical properties frequently by 3D printing. There are some scaffold properties and strategies that promote cell adhesion, proliferation, differentiation. 162 Nevertheless, scaffolds encounter troubles when it comes to achieving enough vascularization even in large constructs, implying that sufficient nutrient supply and waste removal are still hampered. 163 It can be tricky, especially if you want an optimal balance of strength and stiffness with degradation rate for load-bearing applications. It can also be challenging to ensure that subsequent tissue integrates into the host and is not encased in collagen. Both approaches have shown promise in combination therapies. For example, combining stem cells with BMP-2 resulted in new bone generation, quick healing and great-regenerative outcomes. 164 Similarly, scaffolds can be engineered to include both growth factors and cells rendering the scaffold capable of marked regenerative potential. 165 Overall, while both SCFs and scaffolds/biomaterials have their own strengths and limitations, the future of bone regeneration likely lies in combining these approaches to leverage their respective advantages while mitigating individual challenges. Despite tremendous advancements, recapitulating all the complex properties of the native bone microenvironment remains an unmet bottleneck in bone tissue engineering. More scientific developments are still required to create biomaterials that comprehensively mimic the natural bone microenvironment.123,124,166,167
Regulation of SCF/c-kit signaling
The main obstacle in employing SCF for bone renewal is the suitable regulation of the SCF/c-kit signaling axis. SCF/c-kit signaling participates in the regulation of stem cell activity and bone regeneration. However, lymphocyte adapter protein (Lnk) negatively regulates the duo signaling. The phosphorylated tyrosine 567 within the juxtamembrane domain of the KIT protein binds directly and preferentially to the Lnk SH2 domain, inhibiting SCF-induced signaling. Loss in Lnk enhances both SCF/c-kit signaling and SCF-driven activities, including the accelerated proliferation of BM mast cells, with increased mitogen-activated protein kinase (MAPK) and Jun N-terminal kinase signaling and enhanced Rac and p38 MAPK activation, together with improved migration of BM mast cells (as a result of the Ras-related C3 botulinum toxin substrate). Enhanced recruitment and mobility of endothelial progenitor cells and HSC/HPCs to bone defect sites. Moreover, the other benefits of accelerated SCF/c-kit signaling in Lnk-deficient cells include increased osteoblast development and the creation of a mineralized matrix that supports bone regeneration.168–170 Strategies to block the Lnk-dependent negative regulation of SCF/c-kit signaling may be required to maximize the regenerative potential of this pathway for bone regeneration. The SCF/c-kit-Lnk signaling axis must be carefully modulated to have the desired effects on bone regeneration and stem cell activity.
While the limitations of SCF in bone regeneration draw attention to the obstacles still present, they also indicate the areas that need more investigation and scientific creativity. In the final section, we summarized the main ideas and presented our conclusions on current state and prospects of SCFs in regenerative medicine.
Conclusions and prospects
In summary, the authors critically scrutinized the role of SCF in hematopoiesis and bone regeneration. Hematopoiesis and bone regeneration are two interconnected and fundamental processes in human health. SCF is intriguing due to its potential therapeutic applications in regenerative medicine and its role in maintaining HSCs microenvironment. It is well known for its regulatory functions in various cell lineages. This review addressed the mechanisms through which SCF affects myriad biological processes, including cell migration, cell viability, bone repair, and cell survival, to clarify the broader aspects and implications for clinical practices in treating BM and systemic metabolic disorders. Using a thorough literature review, our work investigated the details of SCFs’ cellular sources, molecular signaling routes, and effects on hematopoietic and bone niches. It highlights how SCF insufficiency affects BMAT, leading to decreased cellularity and impaired hematopoiesis.
Additionally, the interaction between SCF and other niche factors, such as CXCL12, is explored, emphasizing the role of microenvironment in sustaining HSCs. The main findings suggest that SCF is vital for maintaining HSC populations and regulating BM function, including lipid accumulation in adipocytes. The review also emphasized the prospective of SCF as a therapeutic target in bone regeneration strategies and hematopoietic treatments. These insights contribute to a deeper understanding of sophisticated roles of SCFs and pave the way for its application in regenerative medicine, particularly in addressing aging, irradiation, and obesity-related BM dysfunctions.
In conclusion, SCFs play a pivotal role in bone regeneration through their effects on stem cell maintenance and differentiation. Furthermore, SCFs trigger activation of angiogenesis and ensure adequate blood flow to the regenerating tissue as well as have immunomodulatory effects which set in motion a favorable environment for tissue repair. Their combination with biomaterials and scaffolds has potential to improve bone repair outcome, paving the way for novel SCF-assisted therapeutic strategies to further accelerate MSC therapy on fracture healing or large bone defects. Significant advancements in tissue engineering and regenerative medicine have been achieved over recent years, as SCF with different functionalities have been studied. Though many approaches have been attempted and investigated to improve bone regeneration, only a limited number of in vivo applications have been successfully demonstrated. These approaches use various stem cells, biomaterials, scaffolds, and their mixed forms with bioengineered functions. Of them, using SCF is a potentially effective tactic for establishing a favorable BM niche to enhance the regeneration of damaged bones. Nevertheless, there are legitimate concerns regarding the safety of SCF-mediated tissue regeneration.
Further study on the roadmap of regenerative medicine is a prerequisite for transforming experimental research into clinical therapy. Additionally, both in vitro and in vivo investigations must be conducted, and the discrepancies between these studies must be recognized to better assess the outcomes of their clinical application. Although SCF holds a propitious depiction, the real focus should be to alleviate concerns regarding SCF-driven activated mast cell toxification by identifying its causes and developing strategies for reversing them or nullifying the toxic effect to a minimum. In addition, research should target the specific cargo profiles of stem cells and their interactions with growth factors to enhance regenerative potential. While SCFs are crucial, understanding their interactions with cellular components and optimizing delivery methods is key to advancing bone regeneration therapies. Clinical research also depends on understating the rejuvenation process since the advantages and disadvantages of bone repair must be considered. Additional examination is needed to determine the safety measures and tissue-specific responses of SCF-based bone regeneration.
Supplemental Material
Supplemental material, sj-tif-1-tej-10.1177_20417314241287491 for Unlocking the regenerative key: Targeting stem cell factors for bone renewal by Gul Karima and Hwan D. Kim in Journal of Tissue Engineering
Footnotes
Author contributions: G.K. outlined and investigated the study, created the figures and tables, and wrote the original manuscript draft. H.D.K. provided guidance, designed the study, edited the entire manuscript, and supervised the study from the beginning.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and ICT of Korea (NRF-2021R1C1C2004576 and RS-2023-00222737). This research was also supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Health & Welfare: Code: KFRM 22A0105L1-11).
ORCID iD: Hwan D. Kim  https://orcid.org/0000-0001-7656-9920
https://orcid.org/0000-0001-7656-9920
Supplemental material: Supplemental material for this article is available online.
References
- 1. Tsai M, Valent P, Galli SJ. KIT as a master regulator of the mast cell lineage. JACI 2022; 149(6): 1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broudy VC. Stem Cell Factor and hematopoiesis. Blood 1997; 90(4): 1345–1364. [PubMed] [Google Scholar]
- 3. Mann Z, Sengar M, Verma YK, et al. Hematopoietic stem cell factors: their functional role in self-renewal and clinical aspects. Front Cell Dev Biol 2022; 10: 664261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilayov T, Hingaly T, Greenshpan Y, et al. Engineering stem cell factor ligands with different c-Kit agonistic potencies. Molecules 2020; 25(20): 4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Itkin T, Duarte D, Passaro D. Editorial: the dynamic interface between vascular blood vessels to blood forming hematopoietic stem cells in health and disease. Front Cell Dev Biol 2022; 10: 870129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu C, Gao X, Wei Q, et al. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat Commun 2018; 9(1): 2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Pei H, Wang S, et al. Arterial endothelium creates a permissive niche for expansion of human cord blood hematopoietic stem and progenitor cells. Stem Cell Res Ther 2020; 11(1): 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramalingam P, Poulos MG, Butler JM. Regulation of the hematopoietic stem cell lifecycle by the endothelial niche. Curr Opin Hematol 2017; 24(4): 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505(7483): 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell 2021; 56(13): 1848–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perlin JR, Sporrij A, Zon LI. Blood on the tracks: hematopoietic stem cell-endothelial cell interactions in homing and engraftment. J Mol Med 2017; 95(8): 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495(7440): 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Z, Huang Z, Ong B, et al. Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica 2019; 104(9): 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Z, MacDougald OA. Stem cell factor: the bridge between bone marrow adipocytes and hematopoietic cells. Haematologica 2019; 104(9): 1689–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burkhardt LM, Bucher CH, Löffler J, et al. The benefits of adipocyte metabolism in bone health and regeneration. Front Cell Dev Biol 2023; 11: 1104709–20230221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riester O, Borgolte M, Csuk R, et al. Challenges in bone tissue regeneration: stem cell therapy, biofunctionality and antimicrobial properties of novel materials and its evolution. Int J Mol Sci 2020; 22(1): 20201227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li JJ, Ebied M, Xu J, et al. Current approaches to bone tissue engineering: the interface between Biology and Engineering. Adv Healthc Mater 2018; 7(6): 1701061. [DOI] [PubMed] [Google Scholar]
- 18. Huang YZ, Xie HQ, Li X. Scaffolds in bone tissue engineering: research progress and current applications. In: Zaidi M. (ed.) Encyclopedia of Bone Biology. Oxford: Academic Press, 2020, pp. 204–215. [Google Scholar]
- 19. Tsiklin IL, Shabunin AV, Kolsanov AV, et al. In vivo bone tissue engineering strategies: advances and Prospects. Polymers 2022; 14(15): 20220808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar R, Srivastava P, Chandra P. Bioengineering of bone tissues using bioreactors for modulation of mechano-sensitivity in bone. BGER 2023; 39(2): 688–728. [DOI] [PubMed] [Google Scholar]
- 21. Qin L, Yang S, Zhao C, et al. Prospects and challenges for the application of tissue engineering technologies in the treatment of bone infections. Bone Res 2024; 12(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roohani I, Newsom E, Zreiqat H. High-resolution vat-photopolymerization of personalized bioceramic implants: new advances, regulatory hurdles, and key recommendations. Int Mater Rev 2023; 68(8): 1075–1097. [Google Scholar]
- 23. Alageel S, Hildebrandt M, de Andrade A. Correction to: the regulatory landscape of cell- and tissue-based regenerative medicine: current challenges and emerging issues. In: Duscher D, Shiffman M. (eds) Regenerative medicine and plastic surgery: elements, research concepts and emerging technologies. Cham: Springer International Publishing, 2019, pp.C1–C2. [Google Scholar]
- 24. Xu J, Shen J, Sun Y, et al. In vivo prevascularization strategy enhances neovascularization of β-tricalcium phosphate scaffolds in bone regeneration. J Orthop Translat 2022; 37: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lv N, Zhou Z, Hou M, et al. Research progress of vascularization strategies of tissue-engineered bone. Front Bioeng Biotechnol 2023; 11: 1291969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellermann E, Meyer N, Cameron RE, et al. In vitro angiogenesis in response to biomaterial properties for bone tissue engineering: a review of the state of the art. Regen Biomater 2023; 10: rbad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bi M, Yang K, Yu T, et al. Cell-based mechanisms and strategies of co-culture system both in vivo and vitro for bone tissue engineering. Biomed Pharmacother 2023; 169: 115907. [DOI] [PubMed] [Google Scholar]
- 28. Li A, Sasaki J-I, Abe GL, et al. Vascularization of a bone organoid using dental pulp stem cells. Stem Cells Int 2023; 2023: 5367887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Re F, Borsani E, Rezzani R, et al. Bone regeneration using mesenchymal stromal cells and biocompatible scaffolds: a concise review of the current clinical trials. Gels 2023; 9(5): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furka D, Dueñas Santana JA, Furka S. Advances in new materials for bone tissue engineering simplifying postoperative rehabilitation: recent scientific breakthroughs. J REHABILITÁCIA 2023; 60(3): 16–18. [Google Scholar]
- 31. Grassie K, Khan Y. Chapter 1—bone tissue engineering. In: Chen Y. (ed.) Musculoskeletal tissue engineering. Farmington, CT: Elsevier, 2022, pp. 1–40. [Google Scholar]
- 32. Kim E, Cai L, Hyun SH. Effects of stem cell factor/c-Kit signaling on in vitro maturation of porcine oocytes and subsequent developmental competence after fertilization. Front Vet Sci 2021; 8: 745488–20211008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manova K, Huang EJ, Angeles M, et al. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of Spermatogonia. Dev Biol 1993; 157(1): 85–99. [DOI] [PubMed] [Google Scholar]
- 34. Laitinen M, Rutanen EM, Ritvos O. Expression of c-kit ligand messenger ribonucleic acids in human ovaries and regulation of their steady state levels by gonadotropins in cultured granulosa-luteal cells. Endocrinology 1995; 136(10): 4407–4414. [DOI] [PubMed] [Google Scholar]
- 35. Brankin V, Hunter MG, Horan TL, et al. The expression patterns of mRNA-encoding stem cell factor, internal stem cell factor and c-kit in the prepubertal and adult porcine ovary. J Anat 2004; 205(5): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waskow C, Madan V, Bartels S, et al. Hematopoietic stem cell transplantation without irradiation. Nat Methods 2009; 6(4): 267–269. [DOI] [PubMed] [Google Scholar]
- 37. Cosgun KN, Rahmig S, Mende N, et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell 2014; 15(2): 227–238. [DOI] [PubMed] [Google Scholar]
- 38. Grinenko T, Arndt K, Portz M, et al. Clonal expansion capacity defines two consecutive developmental stages of long-term hematopoietic stem cells. J Exp Med 2014; 211(2): 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014; 15(2): 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481(7382): 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 2017; 19(8): 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Comazzetto S, Murphy MM, Berto S, et al. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin receptor+ niche cells in the bone marrow. Cell Stem Cell 2019; 24(3): 477–486.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kara N, Xue Y, Zhao Z, et al. Endothelial and leptin receptor+ cells promote the maintenance of stem cells and hematopoiesis in early postnatal murine bone marrow. Dev Cell 2023; 58(5): 348–360.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang FC, Ingram DA, Chen S, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for nf1+/- mast cells. J Clin Investig 2003; 112(12): 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pereira AR, Trivanović D, Stahlhut P, et al. Preservation of the naïve features of mesenchymal stromal cells in vitro: comparison of cell- and bone-derived decellularized extracellular matrix. J Tissue Eng 2022; 13: 20417314221074453–20417314320220207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Annese T, Tamma R, Bozza M, et al. Autocrine/paracrine loop between SCF+/c-kit+ mast cells promotes cutaneous melanoma progression. Front Immunol 2022; 13: 794974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waskow C, Paul S, Haller C, et al. Viable c-KitW/W mutants reveal pivotal role for c-Kit in the maintenance of lymphopoiesis. Immunity 2002; 17(3): 277–288. [DOI] [PubMed] [Google Scholar]
- 48. Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; Bcl-2 can provide one of these, Kitl/C-KIT signaling the other. J Exp Med 2000; 192(12): 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cordeiro Gomes A, Hara T, Lim VY, et al. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity 2016; 45(6): 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodewald H-R, Kretzschmar K, Swat W, et al. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity 1995; 3(3): 313–319. [DOI] [PubMed] [Google Scholar]
- 51. Hassan HT, Zander A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol 1996; 95(3-4): 257–262. [DOI] [PubMed] [Google Scholar]
- 52. Glaspy J, Davis MW, Parker WR, et al. Biology and clinical potential of stem-cell factor. Cancer Chemother Pharmacol 1996; 38 Suppl: S53–S57. [DOI] [PubMed] [Google Scholar]
- 53. John M, Peter W. Inflammatory cytokines regulate T-cell development from blood progenitor cells in a stage and dose-specific manner. bioRxiv 2021: 2021.2001.2018.427186. [Google Scholar]
- 54. Lee J, Breton G, Oliveira TY, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med 2015; 212(3): 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Curti A, Fogli M, Ratta M, et al. Stem cell factor and FLT3-ligand are strictly required to sustain the long-term expansion of primitive CD34+DR-dendritic cell precursors. J Immunol 2001; 166(2): 848–854. [DOI] [PubMed] [Google Scholar]
- 56. Kraus H, Kaiser S, Aumann K, et al. A feeder-free differentiation system identifies autonomously proliferating B cell precursors in human bone marrow. J Immunol 2014; 192(3): 1044–1054. [DOI] [PubMed] [Google Scholar]
- 57. Ichii M, Oritani K, Kanakura Y. Early B lymphocyte development: similarities and differences in human and mouse. World J Stem Cells 2014; 6(4): 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cho SK, Webber TD, Carlyle JR, et al. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. PNAS 1999; 96(17): 9797–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. LeBien TW. Fates of human B-cell precursors. Blood 2000; 96(1): 9–23. [PubMed] [Google Scholar]
- 60. Edgar JM, Michaels YS, Zandstra PW. Multi-objective optimization reveals time- and dose-dependent inflammatory cytokine-mediated regulation of human stem cell derived T-cell development. NPJ Regen Med 2022; 7(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Famili F, Wiekmeijer AS, Staal FJ. The development of T cells from stem cells in mice and humans. Future Sci OA 2017; 3(3): Fso186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoshino N, Kanno H, Takahashi K, et al. Mucosal immune responses in W/W(v) and Sl/Sl(d) mutant mice. Exp Anim 2012; 61(4): 407–416. [DOI] [PubMed] [Google Scholar]
- 63. Han J, Zúñiga-Pflücker JC. A 2020 view of thymus stromal cells in T cell development. J Immunol 2021; 206(2): 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fehniger TA, Carson WE, Mrózek E, et al. Stem cell factor enhances interleukin-2-mediated expansion of murine natural killer cells in vivo. Blood 1997; 90(9): 3647–3653. [PubMed] [Google Scholar]
- 65. Benson DM, Yu J, Becknell B, et al. Stem cell factor and interleukin-2/15 combine to enhance MAPK-mediated proliferation of human natural killer cells. Blood 2009; 113(12): 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from gamma c transduced severe combined immunodeficiency X1 bone marrow cells. Blood 1996; 88(10): 3901–3909. [PubMed] [Google Scholar]
- 67. Hartman M, Piliponsky AM, Temkin V, et al. Human peripheral blood eosinophils express stem cell factor. Blood 2001; 97(4): 1086–1091. [DOI] [PubMed] [Google Scholar]
- 68. Diny NL, Rose NR, Čiháková D. Eosinophils in autoimmune diseases. Front Immunol 2017; 8: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. JACI 2006; 117(6): 1214–1225. [DOI] [PubMed] [Google Scholar]
- 70. Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res 2006; 34(2): 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Galli SJ, Iemura A, Garlick DS, et al. Reversible expansion of primate mast cell populations in vivo by stem cell factor. J Clin Investig 1993; 91(1): 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Möller C, Alfredsson J, Engström M, et al. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein bim. Blood 2005; 106(4): 1330–1336. [DOI] [PubMed] [Google Scholar]
- 73. Taylor AM, Galli SJ, Coleman JW. Stem-cell factor, the kit ligand, induces direct degranulation of rat peritoneal mast cells in vitro and in vivo: dependence of the in vitro effect on period of culture and comparisons of stem-cell factor with other mast cell-activating agents. Immunology 1995; 86(3): 427–433. [PMC free article] [PubMed] [Google Scholar]
- 74. Kirshenbaum AS, Goff JP, Semere T, et al. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13). Blood 1999; 94(7): 2333–2342. [PubMed] [Google Scholar]
- 75. Razin E, Ihle JN, Seldin D, et al. Interleukin 3: a differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol 1984; 132(3): 1479–1486. [PubMed] [Google Scholar]
- 76. Tanaka R, Koike K, Imai T, et al. Stem cell factor enhances proliferation, but not maturation, of murine megakaryocytic progenitors in serum-free culture. Blood 1992; 80(7): 1743–1749. [PubMed] [Google Scholar]
- 77. Lukacs NW, Kunkel SL, Strieter RM, et al. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood 1996; 87(6): 2262–2268. [PubMed] [Google Scholar]
- 78. Ito T, Smrž D, Jung MY, et al. Stem cell factor programs the mast cell activation phenotype. J Immunol 2012; 188(11): 5428–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grabarek J, Groopman JE, Lyles YR, et al. Human kit ligand (stem cell factor) modulates platelet activation in vitro. JBC 1994; 269(34): 21718–21724. [PubMed] [Google Scholar]
- 80. Bendall LJ, Makrynikola V, Hutchinson A, et al. Stem cell factor enhances the adhesion of AML cells to fibronectin and augments fibronectin-mediated anti-apoptotic and proliferative signals. Leukemia 1998; 12(9): 1375–1382. [DOI] [PubMed] [Google Scholar]
- 81. Pesce M, Di Carlo A, De Felici M. The c-kit receptor is involved in the adhesion of mouse primordial germ cells to somatic cells in culture. Mech Dev 1997; 68(1-2): 37–44. [DOI] [PubMed] [Google Scholar]
- 82. Shang F, Yu Y, Liu S, et al. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater 2021; 6(3): 666–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tollemar V, Collier ZJ, Mohammed MK, et al. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis 2016; 3(1): 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kwack KH, Lee HW. Clinical potential of dental pulp stem cells in pulp regeneration: current endodontic progress and future perspectives. Front Cell Dev Biol 2022; 10: 857066–20220411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ma Q, Miri Z, Haugen HJ, et al. Significance of mechanical loading in bone fracture healing, bone regeneration, and vascularization. J Tissue Eng 2023; 14: 20417314231172573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baek I, Bello AB, Jeon J, et al. Therapeutic potential of epiphyseal growth plate cells for bone regeneration in an osteoporosis model. J Tissue Eng 2022; 13: 20417314221116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Meng F, Francis H, Glaser S, et al. Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology 2012; 55(1): 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ruangsawasdi N, Zehnder M, Patcas R, et al. Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Eng Part A 2017; 23(3-4): 115–123. [DOI] [PubMed] [Google Scholar]
- 89. Pan S, Dangaria S, Gopinathan G, et al. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling—potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev Rep 2013; 9(5): 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sevari SP, Ansari S, Moshaverinia A. A narrative overview of utilizing biomaterials to recapitulate the salient regenerative features of dental-derived mesenchymal stem cells. Int J Oral Sci 2021; 13(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yaniz-Galende E, Chen J, Chemaly E, et al. Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells. Circ Res 2012; 111(11): 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Swenson ES, Kuwahara R, Krause DS, et al. Physiological variations of stem cell factor and stromal-derived factor-1 in murine models of liver injury and regeneration. Liver Int 2008; 28(3): 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jin K, Mao XO, Sun Y, et al. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Investig 2002; 110(3): 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miyamoto K, Kobayashi T, Hayashi Y, et al. Involvement of stem cell factor and c-kit in corneal wound healing in mice. Mol Vis 2012; 18: 1505–1515. [PMC free article] [PubMed] [Google Scholar]
- 95. Sette C, Dolci S, Geremia R, et al. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int J Dev Biol 2000; 44(6): 599–608. [PubMed] [Google Scholar]
- 96. Radmanić L, Zidovec-Lepej S. The role of stem cell factor, epidermal growth factor and angiopoietin-2 in HBV, HCV, HCC and NAFLD. Life 2022; 12(12): 20221209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Romero-Gallo J, Sozmen EG, Chytil A, et al. Inactivation of TGF-β signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene 2005; 24(18): 3028–3041. [DOI] [PubMed] [Google Scholar]
- 98. Thenappan A, Li Y, Kitisin K, et al. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology 2010; 51(4): 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Karkampouna S, Ten Dijke P, Dooley S, et al. TGFβ signaling in liver regeneration. Curr Pharm Des 2012; 18(27): 4103–4113. [DOI] [PubMed] [Google Scholar]
- 100. Chen Z, Wan L, Jin X, et al. Transforming growth factor-β signaling confers hepatic stellate cells progenitor features after partial hepatectomy. J Cell Physiol 2020; 235(3): 2655–2667. [DOI] [PubMed] [Google Scholar]
- 101. Simpson K, Hogaboam CM, Kunkel SL, et al. Stem cell factor attenuates liver damage in a murine model of acetaminophen-induced hepatic injury. Laboratory Investigation 2003; 83(2): 199–206. [DOI] [PubMed] [Google Scholar]
- 102. Mahla RS. Stem Cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016; 2016: 6940283–20160719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 2002; 30(9): 973–981. [DOI] [PubMed] [Google Scholar]
- 104. Rauth S, Karmakar S, Batra SK, et al. Recent advances in organoid development and applications in disease modeling. Biochim Biophys Acta Rev Cancer 2021; 1875(2): 188527–20210226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yin X, Mead BE, Safaee H, et al. Engineering stem cell organoids. Cell Stem Cell 2016; 18(1): 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Takematsu E, Massidda M, Auster J, et al. Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation. Nat Commun 2022; 13(1): 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cottler-Fox MH, Lapidot T, Petit I, et al. Stem cell mobilization. Hematology 2003; 2003: 419–437. [DOI] [PubMed] [Google Scholar]
- 108. Heyworth CM, Whetton AD, Nicholls S, et al. Stem cell factor directly stimulates the development of enriched granulocyte-macrophage colony-forming cells and promotes the effects of other colony-stimulating factors. Blood 1992; 80(9): 2230–2236. [PubMed] [Google Scholar]
- 109. Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med 2010; 5(1): 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kang Y, Na J, Karima G, et al. Mesenchymal stem cell spheroids: a promising tool for vascularized tissue regeneration. TERM 2024; 21(5): 673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012; 26(6): 1166–1173. [DOI] [PubMed] [Google Scholar]
- 112. Bouland C, Philippart P, Dequanter D, et al. Cross-talk between mesenchymal stromal Cells (MSCs) and endothelial progenitor Cells (EPCs) in bone regeneration. Front Cell Dev Biol 2021; 9: 674084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shen N, Maggio M, Woods I, et al. Mechanically activated mesenchymal-derived bone cells drive vessel formation via an extracellular vesicle mediated mechanism. J Tissue Eng 2023; 14: 20417314231186918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kwon M, Kim BS, Yoon S, et al. Hematopoietic stem cells and their niche in bone marrow. Int J Mol Sci 2024; 25(13): 6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yu VW, Scadden DT. Hematopoietic stem cell and its bone marrow niche. Curr Top Dev Biol 2016; 118: 21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 2019; 20(5): 303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tang X, Wang Z, Wang J, et al. Functions and regulatory mechanisms of resting hematopoietic stem cells: a promising targeted therapeutic strategy. Stem Cell Res Ther 2023; 14(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang Z, Wen S, Zhong M, et al. Epigenetics: novel crucial approach for osteogenesis of mesenchymal stem cells. J Tissue Eng 2023; 14: 20417314231175364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shah M, Li H, Harris M, et al. Role of SCF-Expressing Bone Marrow Populations in Hematopoietic and Leukemic Stem Cell Regulation. Blood 2017; 130(22): 2439–2439. [Google Scholar]
- 120. Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration: current concepts and future directions. BMC Med 2011; 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. DiMascio L, Voermans C, Uqoezwa M, et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol 2007; 178(6): 3511–3520. [DOI] [PubMed] [Google Scholar]
- 122. Matsushita Y, Ono W, Ono N. Skeletal stem cells for bone development and repair: diversity Matters. Curr Osteoporos Rep 2020; 18(3): 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yogambha R, Khoon S, Hala Z, et al. Stem cells for bone regeneration: role of trophic factors. In: Alessandro Rozim Z, Joao Batista de M. (eds) Advanced techniques in bone regeneration. Rijeka: IntechOpen, 2016, p. 15. [Google Scholar]
- 124. Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, et al. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther 2020; 11(1): 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang H, Liesveld JL, Calvi LM, et al. The roles of bone remodeling in normal hematopoiesis and age-related hematological malignancies. Bone Res 2023; 11(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Papa L, Djedaini M, Hoffman R. Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis. Ann N Y Acad Sci 2020; 1466(1): 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cheng H, Zheng Z, Cheng T. New paradigms on hematopoietic stem cell differentiation. Protein Cell 2020; 11(1): 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Raghav PK, Singh AK, Gangenahalli G. Stem cell factor and NSC87877 synergism enhances c-Kit mediated proliferation of human erythroid cells. Life Sci 2018; 214: 84–97. [DOI] [PubMed] [Google Scholar]
- 129. Raghav PK, Singh AK, Gangenahalli G. Correction: stem cell factor and NSC87877 combine to enhance c-kit mediated proliferation of human megakaryoblastic cells. PLoS One 2018; 13(12): e0206364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Saraya K, Reid C. Synergistic interaction between c-kit Ligand (SCF), GM-CSF and TNF promotes optimal dendritic Langerhans cell proliferation from primitive progenitors in human bone marrow. In: Banchereau J, Schmitt D. (eds) Dendritic cells in fundamental and clinical immunology. Boston, MA: Springer US, 1995, pp.13–16, Vol. 2. [DOI] [PubMed] [Google Scholar]
- 131. Duarte RF, Frank DA. SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood 2000; 96(10): 3422–3430. [PubMed] [Google Scholar]
- 132. Miner X, Shanshan Z, Fang D. Granulocyte colony-stimulating factor directly acts on mouse lymphoid-biased but not myeloid-biased hematopoietic stem cells. Haematologica 2020; 106(6): 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cardier JE, Erickson-Miller CL, Murphy Mj., Jr. Differential effect of erythropoietin and GM-CSF on Megakaryocytopoiesis from primitive bone marrow cells in serum-free conditions. Stem Cells 1997; 15(4): 286–290. [DOI] [PubMed] [Google Scholar]
- 134. Barroeta Seijas AB, Simonetti S, Vitale S, et al. GM-CSF inhibits c-Kit and SCF expression by bone marrow-derived dendritic cells. Front Immunol 2017; 8: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Molineux G, Migdalska A, Szmitkowski M, et al. The effects on hematopoiesis of recombinant stem cell factor (Ligand for c-kit) administered in vivo to mice either alone or in combination with granulocyte colony-stimulating factor. Blood 1991; 78(4): 961–966. [PubMed] [Google Scholar]
- 136. Kurzrock R, et al. Stem cell factor. In: Kufe DP, Weichselbaum R. (eds) Holland-Frei Cancer Medicine. Hamilton, ON: BC Decker, 2003. 6th ed, pp. 858–859. [Google Scholar]
- 137. Shpall EJ, Wheeler CA, Turner SA, et al. A randomized phase 3 study of peripheral blood progenitor cell mobilization with stem cell factor and filgrastim in high-risk breast cancer patients. Blood 1999; 93(8): 2491–2501. [PubMed] [Google Scholar]
- 138. Glaspy J. Clinical applications of stem cell factor. Curr Opin Hematol 1996; 3(3): 223–229. [DOI] [PubMed] [Google Scholar]
- 139. Matsui Y, Zsebo KM, Hogan BLM. Embryonic expression of a haematopoietic growth factor encoded by the SI locus and the ligand for c-kit. Nature 1990; 347(6294): 667–669. [DOI] [PubMed] [Google Scholar]
- 140. Costa JJ, Demetri GD, Harrist TJ, et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med 1996; 183(6): 2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Grichnik JM, Crawford J, Jimenez F, et al. Human recombinant stem-cell factor induces melanocytic hyperplasia in susceptible patients. J Am Acad Dermatol 1995; 33(4): 577–583. [DOI] [PubMed] [Google Scholar]
- 142. Wang W, Li E, Campbell K, et al. Economic analysis on adoption of biosimilar granulocyte colony-stimulating factors in patients with nonmyeloid cancer at risk of febrile neutropenia within the Oncology Care Model Framework. JCO Oncol Pract 2021; 17(8): e1139–e1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Usuki K, Iki S, Arai S, et al. Stable response after administration of stem cell factor combined with granulocyte colony-stimulating factor in aplastic anemia. Int J Hematol 2006; 83(5): 404–407. [DOI] [PubMed] [Google Scholar]
- 144. Foo JB, Looi QH, Chong PP, et al. Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int 2021; 2021: 2616807–20210819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 2014; 15(2): 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Da Silva CA, Reber L, Frossard N. Stem cell factor expression, mast cells and inflammation in asthma. Fundam Clin Pharmacol 2006; 20(1): 21–39. [DOI] [PubMed] [Google Scholar]
- 147. Ishikawa K, Fish K, Aguero J, et al. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ Heart Fail 2015; 8(1): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Herberts CA, Kwa MS, Hermsen HPHPH, Risk factors in the development of stem cell therapy. J Transl Med 2011; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Liao HT, Chen CT. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 2014; 6(3): 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yoshida Y, Matsubara H, Fang X, et al. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS One 2019; 14(3): e0214488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Amaroli A, Pasquale C, Zekiy A, et al. Steering the multipotent mesenchymal cells towards an anti-inflammatory and osteogenic bias via photobiomodulation therapy: how to kill two birds with one stone. J Tissue Eng 2022; 13: 20417314221110192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gao X, Ma S, Xing X, et al. Microvessels derived from hiPSCs are a novel source for angiogenesis and tissue regeneration. J Tissue Eng 2022; 13: 20417314221143240–20417314320221226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40(5): 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Banfi A, Holnthoner W, Martino MM, et al. Editorial: vascularization for regenerative medicine. Front Bioeng Biotechnol 2018; 6: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Masson-Meyers DS, Tayebi L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J Tissue Eng Regen Med 2021; 15(9): 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kengelbach-Weigand A, Thielen C, Bäuerle T, et al. Personalized medicine for reconstruction of critical-size bone defects: a translational approach with customizable vascularized bone tissue. NPJ Regen Med 2021; 6(1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bonany M, Del-Mazo-Barbara L, Espanol M, et al. Microsphere incorporation as a strategy to tune the biological performance of bioinks. J Tissue Eng 2022; 13: 20417314221119895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Oliveira É R, Nie L, Podstawczyk D, et al. Advances in growth factor delivery for bone tissue engineering. Int J Mol Sci 2021; 22(2): 20210118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med 2011; 15(4): 718–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Luginbuehl V, Meinel L, Merkle HP, et al. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm 2004; 58(2): 197–208. [DOI] [PubMed] [Google Scholar]
- 161. Ren X, Zhao M, Lash B, et al. Growth factor engineering strategies for regenerative medicine applications. Front Bioeng Biotechnol 2019; 7: 469–20200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Iaquinta MR, Mazzoni E, Bononi I, et al. Adult stem cells for bone regeneration and Repair. Front Cell Dev Biol 2019; 7: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010; 31(3): 461–466. [DOI] [PubMed] [Google Scholar]
- 164. Decambron A, Fournet A, Bensidhoum M, et al. Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J Orthop Res 2017; 35(12): 2637–2645. [DOI] [PubMed] [Google Scholar]
- 165. Cross LM, Thakur A, Jalili NA, et al. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater 2016; 42: 2–17. [DOI] [PubMed] [Google Scholar]
- 166. Hao Z, Song Z, Huang J, et al. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci 2017; 5(8): 1382–1392. [DOI] [PubMed] [Google Scholar]
- 167. Ort C, Dayekh K, Xing M, et al. Emerging strategies for stem cell lineage commitment in tissue engineering and regenerative medicine. ACS Biomater Sci Eng 2018; 4(11): 3644–3657. [DOI] [PubMed] [Google Scholar]
- 168. Matsumoto T, Ii M, Nishimura H, et al. Lnk-dependent axis of SCF-ckit signal for osteogenesis in bone fracture healing. J Exp Med 2010; 207(10): 2207–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Simon C, Dondi E, Chaix A, et al. Lnk adaptor protein down-regulates specific kit-induced signaling pathways in primary mast cells. Blood 2008; 112(10): 4039–4047. [DOI] [PubMed] [Google Scholar]
- 170. Kwon S-M, Suzuki T, Kawamoto A, et al. Pivotal role of lnk adaptor protein in endothelial progenitor cell biology for vascular regeneration. Circ Res 2009; 104(8): 969–977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tej-10.1177_20417314241287491 for Unlocking the regenerative key: Targeting stem cell factors for bone renewal by Gul Karima and Hwan D. Kim in Journal of Tissue Engineering