Graphical abstract
Keywords: Double-chambered right ventricle, Subinfundibular stenosis, Subaortic membrane, Aortic stenosis, Aortic regurgitation
Highlights
-
•
DCRV is a rare finding in older adults.
-
•
DCRV can be seen in association with a wide range of cardiac abnormalities.
-
•
Cardiac imaging characterizes anatomy, hemodynamics, and associated cardiac lesions.
Introduction
Double-chambered right ventricle (DCRV) is a rare congenital cardiac condition caused by an accessory muscle bundle (AMB) in the right ventricle that divides the chamber during systole into two compartments with different compartmental pressures, leading to an outflow gradient. DCRV has been associated most often with ventricular septal defects (VSDs) but less commonly can also occur in association with left heart disease in the absence of a VSD. Although DCRV is usually detected in children or younger adults, it can occasionally present in late adulthood as a cause of subpulmonic obstruction.
Case Presentation
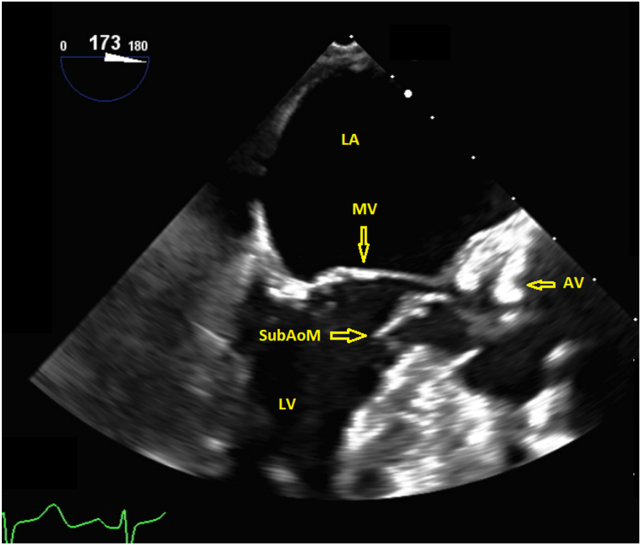
A 67-year-old woman with history of ascending thoracic aneurysm, aortic stenosis (AS) and aortic regurgitation (AR) presented with a history of a recent near-syncopal event. The patient was referred to our institution for transesophageal echocardiography (TEE) for assessment of the AR, as well as right and left heart cardiac catheterization. Right heart catheterization demonstrated a systolic pressure gradient of 55 mm Hg between the pulmonary artery and right ventricle, with systolic right ventricular (RV) and pulmonary artery pressures of 71 and 16 mm Hg, respectively. Coronary angiography demonstrated no coronary artery disease. TEE demonstrated a left ventricular ejection fraction of 65% to 70%, asymmetric thickening of the proximal interventricular septum, aortic root diameter of 4.5 cm at the sinus of Valsalva, and ascending aortic diameter of 4.1 cm. The aortic valve (AV) was trileaflet and thickened, with restricted systolic leaflet excursion. Color flow Doppler imaging of the AR demonstrated a vena contracta width of 0.61 cm (blood pressure 150/69 mm Hg). There was a redundant undulating mobile linear echodensity in the subaortic region that appeared to have an attachment to the basal septum, with an appearance suggestive of an atypical subaortic membrane (Figure 1, Video 1).
Figure 1.
Two-dimensional TEE, midesophageal long-axis view (173°), demonstrates a redundant mobile linear membrane in the LVOT. AV, Aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve; SubAoM, subaortic membrane
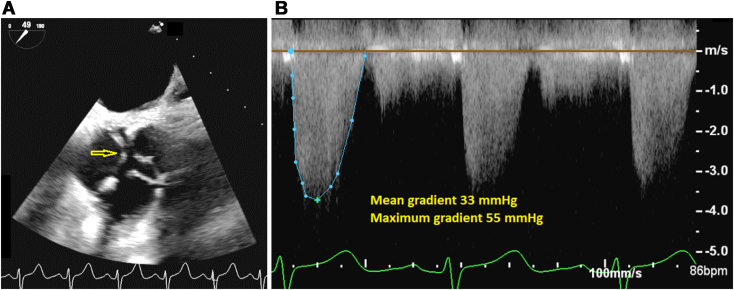
Flow acceleration was seen on color flow Doppler imaging in the region of the membrane, but no hemodynamically significant subaortic gradient was detected on spectral Doppler interrogation. The AV area by three-dimensional and two-dimensional planimetry was 1.2 cm2, indicating moderate valvular AS (Figure 2A). The maximum and mean gradients across the AV region were 55 and 33 mm Hg, respectively (Figure 2B).
Figure 2.
(A) Two-dimensional TEE, midesophageal short-axis systolic view (49°), demonstrates a trileaflet AV with restricted leaflet excursion (arrow). (B) Continuous-wave Doppler imaging across the AV (from a deep transgastric view) demonstrates the pathologic gradients.
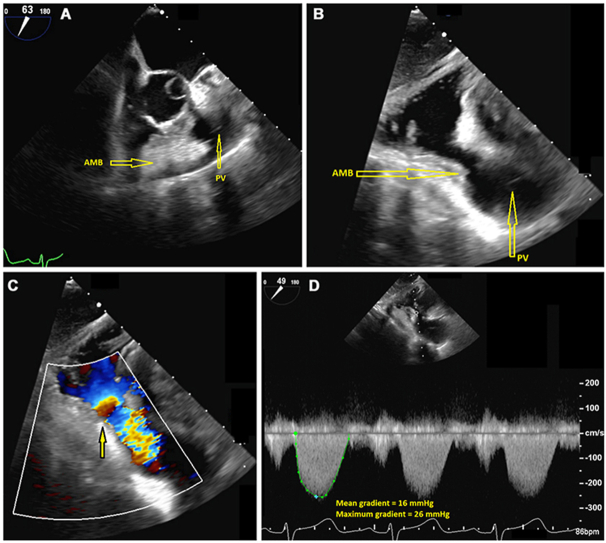
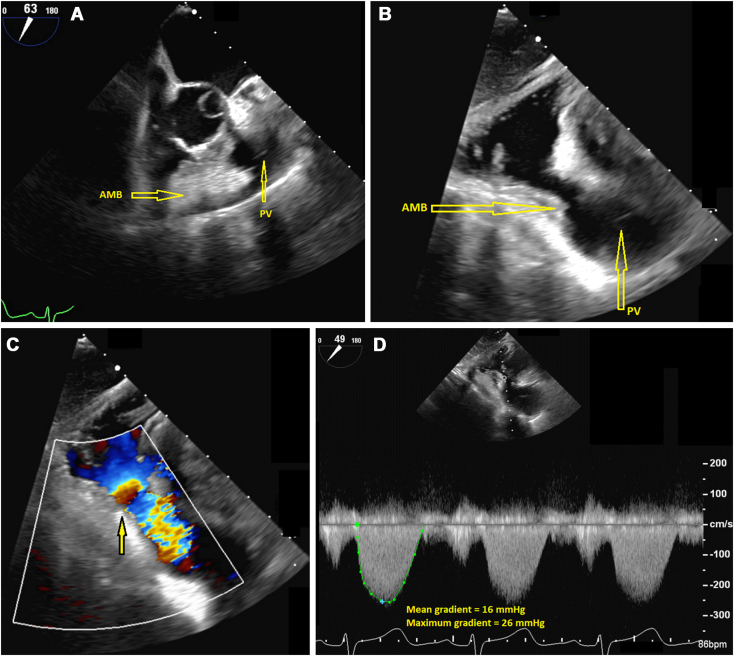
The pulmonic valve leaflets appeared thin and pliable, with normal systolic excursion. There was narrowing of the subinfundibular region on two-dimensional imaging, and flow acceleration was seen in the RV outflow tract (RVOT) on color flow Doppler imaging (Figure 3, Video 2, Video 3, Video 4). The mean and peak gradients across the RVOT were 16 and 26 mm Hg, respectively, indicating subpulmonic stenosis. No evidence of VSD was seen.
Figure 3.
(A) Two-dimensional TEE, midesophageal short-axis systolic view (63°), demonstrates an AMB within the RVOT that narrows the subinfundibular region. Two-dimensional TEE, transgastric long-axis systolic view (94°) without (B) and with (C) color flow Doppler, demonstrates the subinfundibular narrowing in the region of the AMB with mosaic-appearing turbulence consistent with flow acceleration (arrow) and the pathologic gradients with continuous-wave Doppler (D). AMB, Accessory muscle bundle; PV, pulmonic valve.
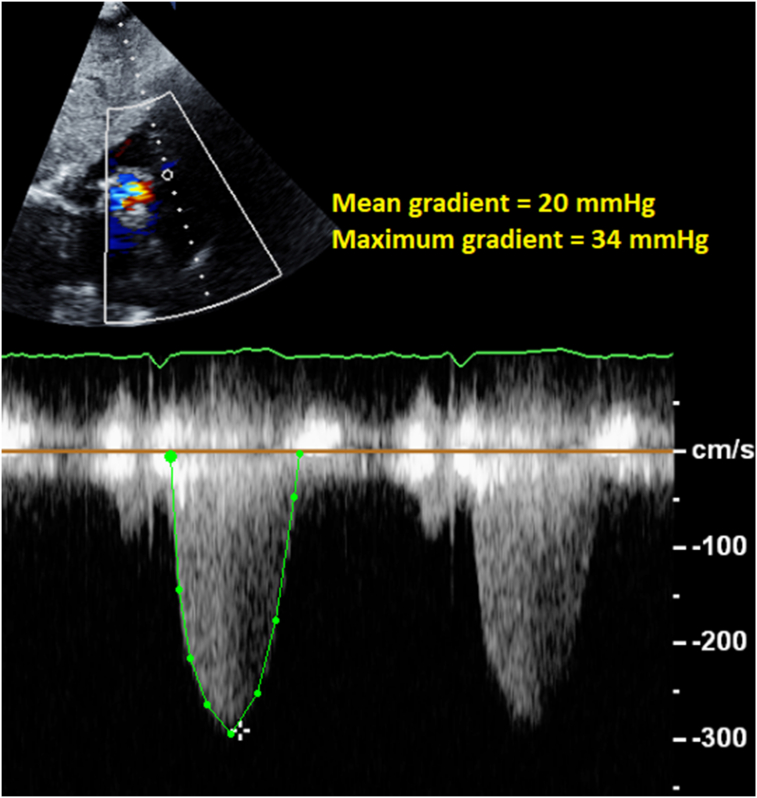
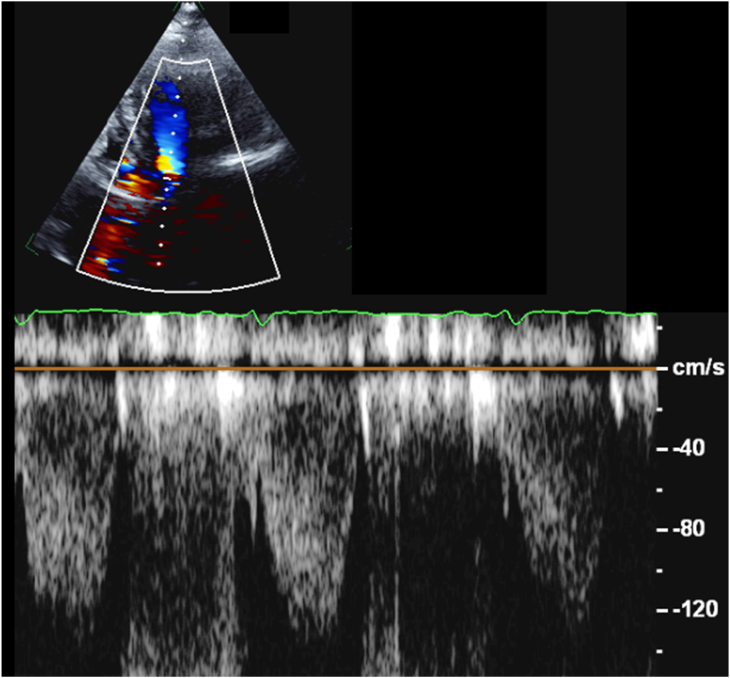
Transthoracic echocardiography (TTE) was performed for further evaluation of the Doppler gradients, and was able to demonstrate the flow acceleration across the RV subinfundibular region, with a maximum gradient of 34 mm Hg and a mean gradient of 20 mm Hg (Figure 4). Although the RVOT is commonly evaluated in the parasternal short-axis view on transthoracic imaging, in this case, the optimal window for spectral Doppler evaluation of the obstruction was the subcostal view. Pulsed-wave Doppler imaging in the left ventricular outflow tract (LVOT) confirmed the lack of significant resting gradient in the region of the subaortic membrane (Figure 5).
Figure 4.
Continuous-wave spectral Doppler display across the RVOT obtained in the subcostal view demonstrates the pathologic subinfundibular gradient.
Figure 5.
Two-dimensional TTE, apical five-chamber view with color flow Doppler-guided pulsed-wave spectral display (sample volume placed in the region of the subaortic membrane), demonstrates the absence of a hemodynamically significant resting gradient in that region.
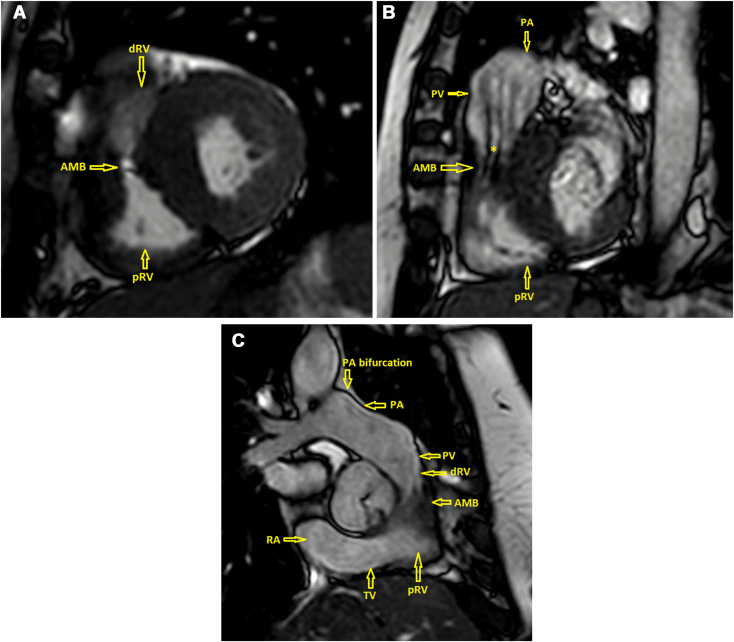
The patient was referred for cardiovascular magnetic resonance imaging (CMR) for more detailed anatomic assessment of the subpulmonic region as well as determining the ratio of the pulmonic flow (Qp) to systemic flow (Qs) to further evaluate for intracardiac shunt. The CMR demonstrated an AMB encircling the distal right ventricle proximal to the infundibular RVOT, causing narrowing of the outflow and dividing the RV cavity during systole into two chambers, a morphologic appearance suggestive of DCRV due to subinfundibular stenosis (Figures 6A-C, Video 5, Video 6, Video 7). The Qp/Qs ratio was 1.07, consistent with the absence of intracardiac shunt. Proximal interventricular septal thickening was also noted, with the basal septum measuring 15 mm in thickness. There was no evidence of late gadolinium enhancement. Aortic regurgitant volume was 41 mL and regurgitant fraction was 44% by the volumetric method. The patient was referred for surgical evaluation for resection of the subinfundibular AMB and subaortic membrane and AV replacement.
Figure 6.
CMR, balanced steady-state free precession sequence, midventricular (A), basal RVOT (B), and AV (C) short-axis systolic views, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region creating a linear loss in signal artifact due to flow acceleration (asterisk). AMB, Accessory muscle bundle; dRV, distal infundibular (low pressure) right ventricle; pRV, proximal (high pressure) right ventricle; PA, pulmonary artery; PV, pulmonic valve; RA, right atrium; TV, tricuspid valve.
Discussion
DCRV is a rare cardiac anomaly, accounting for 0.5% to 2% of all congenital heart diseases. DCRV is caused by an AMB in the right ventricle that divides the chamber during systole into two compartments, with a proximal high-pressure compartment and a distal low-pressure compartment.1 The anatomy of the right ventricle in DCRV can vary and may include an anomalous septoparietal band, anomalous apical shelf, or abnormal moderator band.
DCRV is typically associated with other congenital anomalies, most commonly perimembranous VSDs with restrictive shunts in 75% of cases, and less commonly tetralogy of Fallot, double-outlet right ventricle, and Ebstein’s anomaly.2,3 The pathologic mechanism responsible for the anomaly is not clear, though it is believed that some patients develop postnatal hypertrophic changes in the crista supraventricularis or other RV muscular structures.2 It has been postulated that in patients with VSD, the shunting of blood causes hypertrophy of muscular bands, with the portion of the RV cavity proximal to the hypertrophy experiencing a higher pressure compared with the distal infundibulum.4 In the present case, CMR confirmed the absence of associated VSD both by calculating the Qp/Qs ratio and thorough examination of the interventricular septum in many stacked thin-slice cine views in different planes. Although no VSD was found in this patient, it has been observed that DCRV can develop years after VSD closure.5 It has been speculated that the impact of a high-velocity VSD jet hitting the RV free wall for years may activate a genetic predisposition for cellular growth factor production and tissue hypertrophy even after the resolution of the shunt.6 Thus, we cannot exclude the possibility that this patient had a VSD earlier in life that may have spontaneously closed.
There is no known genetic pattern of inheritance for DCRV. In one study of 32 patients, 29 patients had an associated subaortic membrane.7 DCRV has also been reported in the setting of hypertrophic cardiomyopathy.8 Although our patient had basal septal thickening, there was no evidence of late gadolinium enhancement and other morphologic criteria of hypertrophic cardiomyopathy. In the present case, the patient was found to have an unusual appearing membrane in the LVOT, but no evidence of hemodynamically significant subaortic obstruction was detected with pulsed-wave Doppler interrogation of the LVOT. On review of the literature, we found one reported case of DCRV in an elderly patient with AS in the absence of other lesions.9 It should be noted that subaortic membranes can be asymptomatic for years if the left ventricular outflow gradient is low, but the membrane-created alteration in flow may impair normal AV anatomy, ultimately leading to progressive AR or, less commonly, AS, as seen in this patient.
Patients with DCRV present frequently as infants or children, but later presentation in early adulthood has been reported.2 Diagnosis in late adulthood is extremely rare, but it is important that this condition be recognized in older adults who present with DCRV. Presenting symptoms usually include dyspnea and dizziness, with syncope occurring occasionally in patients with severe obstruction. In patients with hemodynamically significant obstruction, the RV obstruction can result in a decrease in left heart filling and reduced cardiac output, which may be a mechanism for syncope. A left-sided parasternal systolic murmur may be auscultated in patients with DCRV. Although DCRV can be detected on TTE, the anatomy may not always be optimally visualized.10 In this case, the patient was referred specifically for TEE, which was therefore performed first; however, TTE is generally the first imaging modality used in the detection of DCRV. When performing TTE in patients with DCRV, sonographers should obtain imaging from the subcostal short-axis view in order to optimize visualization of the subinfundibular obstruction as well as the alignment of the Doppler cursor with the subinfundibular obstruction. Color flow Doppler imaging is helpful to visualize flow acceleration in the right ventricle, which can then be confirmed with spectral Doppler imaging. Additionally, care should be taken to evaluate for any other associated cardiac lesions. When TTE is insufficient, TEE can provide visualization of the subpulmonic gradient, pulmonic valve leaflet excursion, and subinfundibular narrowing. CMR is valuable for determining the diagnosis and for assessment for associated anomalies.11 In the present case, the spectral Doppler gradients across the DCRV by both TTE and TEE were substantially lower than those obtained on invasive hemodynamics, likely because of the difficulty in obtaining an optimal Doppler angle for interrogation.
Management of clinically significant obstruction usually involves surgical resection of the AMB. As obstruction is dynamic, β-blockers and calcium channel blockers may be used, although data regarding their efficacy are limited.11 The current American Heart Association/American College of Cardiology guideline for the management of adults with congenital heart disease has recommended surgical intervention for symptomatic patients with moderate RVOT obstruction (Doppler velocity ≥ 3.0 m/sec, peak gradient ≥ 36 mm Hg) and consideration of surgical repair in asymptomatic patients with severe obstruction (Doppler velocity > 4.0 m/sec, peak gradient > 64 mm Hg, or mean gradient > 35 mm Hg).12 Other publications have recommended consideration of surgical intervention in the setting of a cardiac catheterization–derived peak gradient of >40 mm Hg2 or >50 mm Hg.11 Data supporting the role of stress echocardiography for evaluating patients with DCRV are limited, but the use of dobutamine stress echocardiography to determine provocable gradient in a symptomatic patient with a resting gradient of <50 mm Hg has been reported.13
Conclusion
DCRV is a rare entity and, although more common in children, can present in adulthood and, rarely, in late adulthood. Although this condition is most typically associated with VSD, it can also be seen in the setting of left heart lesions in the absence of VSD. Careful assessment using echocardiography can identify evidence of RVOT obstruction and features suggestive of DCRV. If this condition is suspected, CMR is useful to evaluate RV anatomy and associated anomalies and to evaluate for intracardiac shunt, as VSD is a commonly associated condition. For patients with DCRV in whom clinically significant RVOT obstruction is suspected but who demonstrate RV outflow velocity < 3.0 m/sec on echocardiography, stress echocardiography or invasive hemodynamics should be considered for further evaluation. Surgical management is usually needed for symptomatic patients with at least moderate obstruction and in asymptomatic patients with severe obstruction.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2024.05.011.
Supplementary Materials
Two-dimensional TEE, midesophageal long-axis view (173°), demonstrates a redundant, highly mobile linear membrane in the LVOT.
Two-dimensional TEE, midesophageal short-axis view (63°), demonstrates an AMB within the RVOT that narrows the subinfundibular region. Also seen coming in and out of the image is the LVOT linear membrane.
Two-dimensional TEE, transgastric long-axis view (94°), demonstrates the subinfundibular narrowing in the region of the AMB.
Two-dimensional TEE, transgastric long-axis view (94°) with color flow Doppler, demonstrates the subinfundibular narrowing in the region of the AMB with mosaic-appearing turbulence consistent with flow acceleration.
CMR, balanced steady-state free precession sequence, midventricular display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region.
CMR, balanced steady-state free precession sequence, basal RVOT display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region creating a linear loss in signal artifact due to flow acceleration across the area of maximal narrowing.
CMR, balanced steady-state free precession sequence, AV display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region creating a linear loss in signal artifact due to flow acceleration across the area of maximal narrowing.
References
- 1.Kucher N., Seiler C., Allemann Y., Eberli F.R. Double-chambered right ventricle. Circulation. 2001;103:e105–e106. doi: 10.1161/01.cir.103.21.e105. [DOI] [PubMed] [Google Scholar]
- 2.Loukas M., Housman B., Blaak C., Kralovic S., Tubbs R.S., Anderson R.H. Double-chambered right ventricle: a review. Cardiovasc Pathol. 2013;22:417–423. doi: 10.1016/j.carpath.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Malone R.J., Henderson E.R., Wilson Z.R., McMullan M.R., Skelton T.N., Campbell W.F., et al. Double-chambered right ventricle in adulthood: a case series. CASE (Phila) 2024;8:202–209. doi: 10.1016/j.case.2023.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luna-López R., Segura de la Cal T., Sarnago Cebada F., Solís J., López-Guarch C.J. From double-chambered right ventricle to double-chambered left ventricle: unusual evolution of a ventricular septal defect. J Am Coll Cardiol Case Rep. 2022 Oct;4:1384–1386. doi: 10.1016/j.jaccas.2022.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato R., Hosoba S., Fukumi D., Ito T. Totally 3D endoscopic repair of double-chambered right ventricle. Ann Thorac Surg Short Reports. 2023;1:88–90. [Google Scholar]
- 6.Oliver J.M., Garrido A., Gonzalez A., Benito F., Mateos M., Aroca A., et al. Rapid progression of mid-ventricular obstruction in adults with double-chambered right ventricle. J Thorac Cardiovasc Surg. 2003;126:711–717. doi: 10.1016/s0022-5223(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M., Smallhorn J.F., Freedom R.M., Coles J., Williams W.G., Trusler G.A. An echocardiographic study of the association of ventricular septal defect and right ventricular muscle bundles with a fixed subaortic abnormality. Am J Cardiol. 1988;61:857–860. doi: 10.1016/0002-9149(88)91079-x. [DOI] [PubMed] [Google Scholar]
- 8.Ge J., Hu T., Liu Y., Wang Q., Fan G., Liu C., et al. Case report: double-chambered right ventricle diagnosed in a middle-aged female with hypertrophic cardiomyopathy and atrial flutter: a rare case. Front Cardiovasc Med. 2022 Jul 22;9 doi: 10.3389/fcvm.2022.937758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasumoto Y., Nakamura Y., Ushijima M., Yoshiyama D., Kuroda M., Sawa S., et al. An elderly case of aortic stenosis associated with a double-chambered right ventricle. J Cardiol Cases. 2023;27:159–161. doi: 10.1016/j.jccase.2022.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman P., Wójcik A.W., Różański J., Siudalska H., Jakubowska E., Włodarska E.K., et al. The role of echocardiography in diagnosing double chambered right ventricle in adults. Heart. 2004;90:789–793. doi: 10.1136/hrt.2003.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashore T.M. Adult congenital heart disease: right ventricular outflow tract lesions. Circulation. 2007;115:1933–1947. doi: 10.1161/CIRCULATIONAHA.105.592345. [DOI] [PubMed] [Google Scholar]
- 12.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;139:e698–e800. doi: 10.1161/CIR.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 13.Ito A., Iwata S., Ehara S., Izumiya Y., Yoshiyama M. Clinical usefulness of dobutamine stress echocardiography for the assessment of double-chambered right ventricle. Eur Heart J Cardiovasc Imaging. 2019;20:120. doi: 10.1093/ehjci/jey158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TEE, midesophageal long-axis view (173°), demonstrates a redundant, highly mobile linear membrane in the LVOT.
Two-dimensional TEE, midesophageal short-axis view (63°), demonstrates an AMB within the RVOT that narrows the subinfundibular region. Also seen coming in and out of the image is the LVOT linear membrane.
Two-dimensional TEE, transgastric long-axis view (94°), demonstrates the subinfundibular narrowing in the region of the AMB.
Two-dimensional TEE, transgastric long-axis view (94°) with color flow Doppler, demonstrates the subinfundibular narrowing in the region of the AMB with mosaic-appearing turbulence consistent with flow acceleration.
CMR, balanced steady-state free precession sequence, midventricular display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region.
CMR, balanced steady-state free precession sequence, basal RVOT display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region creating a linear loss in signal artifact due to flow acceleration across the area of maximal narrowing.
CMR, balanced steady-state free precession sequence, AV display, demonstrates the morphologic features of DCRV with an AMB in the subinfundibular region creating a linear loss in signal artifact due to flow acceleration across the area of maximal narrowing.