Abstract
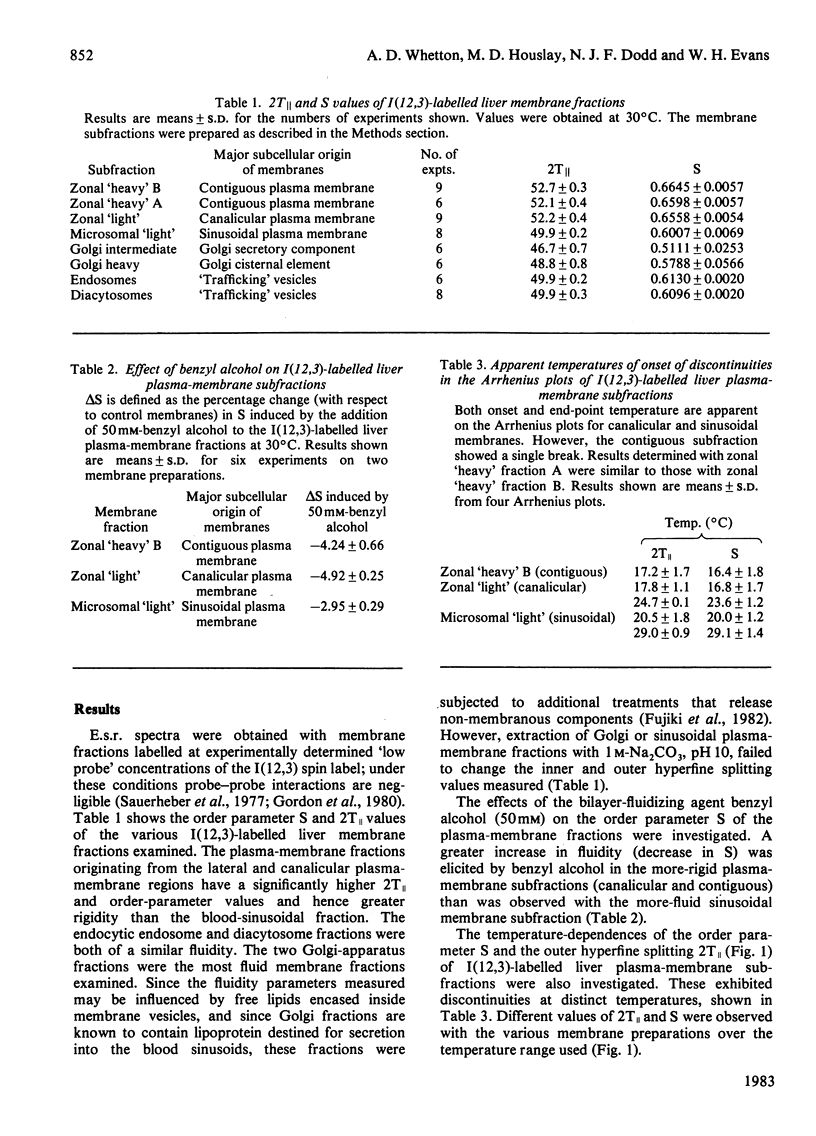
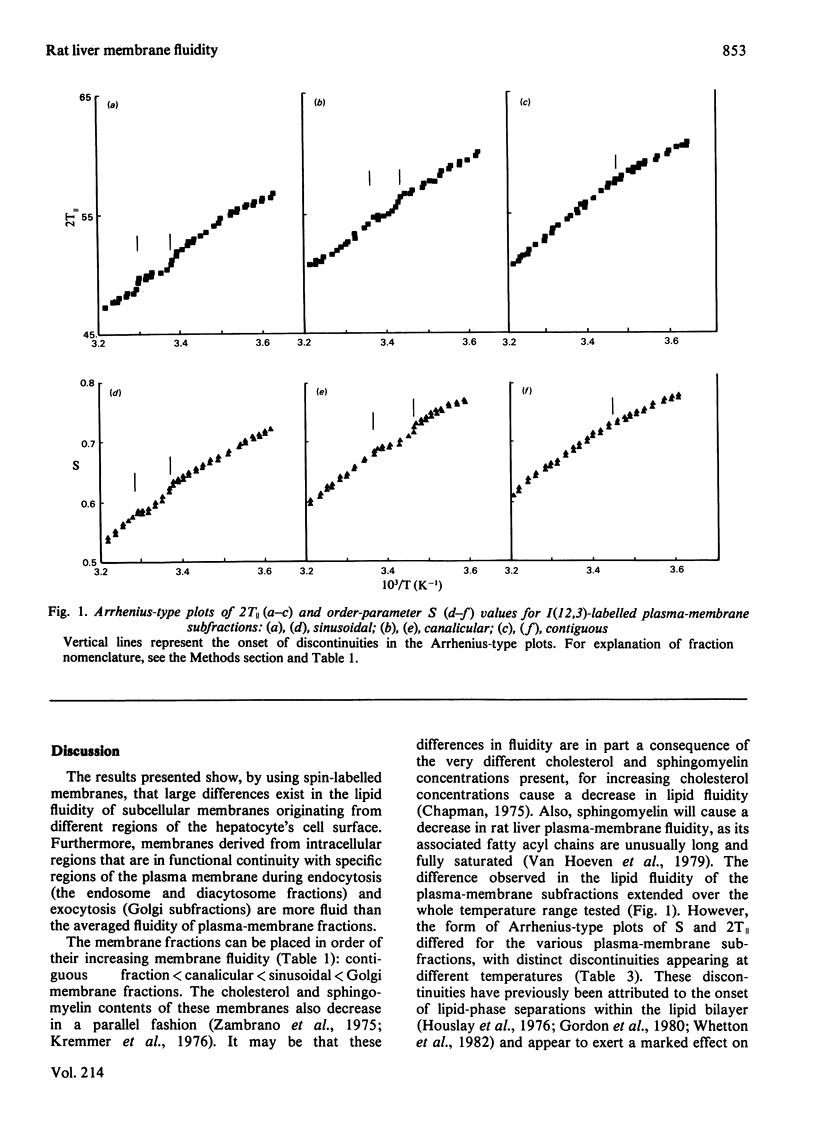
1. The lipid fluidity of three major rat liver plasma-membrane subfractions, as well as Golgi apparatus and endocytic fractions, was assessed with a fatty acid spin probe by using e.s.r. techniques. 2. The sinusoidal (blood-facing) plasma-membrane subfraction was the most fluid of the three plasma-membrane regions. Fractions originating from the bile-canalicular and contiguous (lateral) regions were most rigid. Endocytic fractions isolated (endosomes and diacytosomes) were of a similar fluidity to fractions originating from the sinusoidal plasma-membrane region. By far the most fluid fractions examined were derived from the Golgi-apparatus complex. 3. The three plasma-membrane subfractions each showed a different response to the bilayer-fluidizing effect of benzyl alcohol. 4. Arrhenius-type plots of the order parameter S and outer hyperfine splitting, 2T∥, identified lipid-phase separations in the plasma-membrane subfractions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Evans W. H., Flint N., Regoeczi E. Receptor-rich intracellular membrane vesicles transporting asialotransferrin and insulin in liver. Nature. 1982 Jul 22;298(5872):398–400. doi: 10.1038/298398a0. [DOI] [PubMed] [Google Scholar]
- Dipple I., Gordon L. M., Houslay M. D. The activity of 5'-nucleotidase in liver plasma membranes is affected by the increase in bilayer fluidity achieved by anionic drugs but not by cationic drugs. J Biol Chem. 1982 Feb 25;257(4):1811–1815. [PubMed] [Google Scholar]
- Evans W. H. A biochemical dissection of the functional polarity of the plasma membrane of the hepatocyte. Biochim Biophys Acta. 1980 May 27;604(1):27–64. doi: 10.1016/0005-2736(80)90584-2. [DOI] [PubMed] [Google Scholar]
- Evans W. H. Membrane traffic at the hepatocyte's sinusoidal and canalicular surface domains. Hepatology. 1981 Sep-Oct;1(5):452–457. doi: 10.1002/hep.1840010515. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A., Dipple I., Marchmont R. J., Houslay M. D. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J Biol Chem. 1980 May 25;255(10):4519–4527. [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D. Studies on spin-labelled egg lecithin dispersions. Biochim Biophys Acta. 1977 Apr 1;466(1):34–43. doi: 10.1016/0005-2736(77)90206-1. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. The lipid environment of the glucagon receptor regulates adenylate cyclase activity. Biochim Biophys Acta. 1976 Jun 17;436(2):495–504. doi: 10.1016/0005-2736(76)90211-x. [DOI] [PubMed] [Google Scholar]
- Kremmer T., Wisher M. H., Evans W. H. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim Biophys Acta. 1976 Dec 14;455(3):655–664. doi: 10.1016/0005-2736(76)90039-0. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Functional properties of biological membranes: a physical-chemical approach. Prog Biophys Mol Biol. 1975;29(1):3–56. doi: 10.1016/0079-6107(76)90019-5. [DOI] [PubMed] [Google Scholar]
- Sauerheber R. D., Gordon L. M., Crosland R. D., Kuwahara M. D. Spin-label studies on rat liver and heart plasma membranes: do probe-probe interactions interfere with the measurement of membrane properties? J Membr Biol. 1977 Feb 24;31(1-2):131–169. doi: 10.1007/BF01869402. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Gordon L. M., Houslay M. D. Adenylate cyclase is inhibited upon depletion of plasma-membrane cholesterol. Biochem J. 1983 May 15;212(2):331–338. doi: 10.1042/bj2120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Gordon L. M., Houslay M. D. Elevated membrane cholesterol concentrations inhibit glucagon-stimulated adenylate cyclase. Biochem J. 1983 Feb 15;210(2):437–449. doi: 10.1042/bj2100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Johannsson A., Wilson S. R., Wallace A. V., Houslay M. D. The thermodependence of the activity of integral enzymes in liver plasma membranes: evidence consistent with a functionally asymmetric lipid bilayer. FEBS Lett. 1982 Jun 21;143(1):147–152. doi: 10.1016/0014-5793(82)80293-7. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Needham L., Dodd N. J., Heyworth C. M., Houslay M. D. Forskolin and ethanol both perturb the structure of liver plasma membranes and activate adenylate cyclase activity. Biochem Pharmacol. 1983 May 15;32(10):1601–1608. doi: 10.1016/0006-2952(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano F., Fleischer S., Fleischer B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim Biophys Acta. 1975 Mar 24;380(3):357–369. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]
- van Hoeven R. P., van Blitterswijk W. J., Emmelot P. Fluorescence polarization measurements on normal and tumour cells and their corresponding plasma membranes. Biochim Biophys Acta. 1979 Feb 20;551(1):44–54. doi: 10.1016/0005-2736(79)90351-1. [DOI] [PubMed] [Google Scholar]


