Abstract
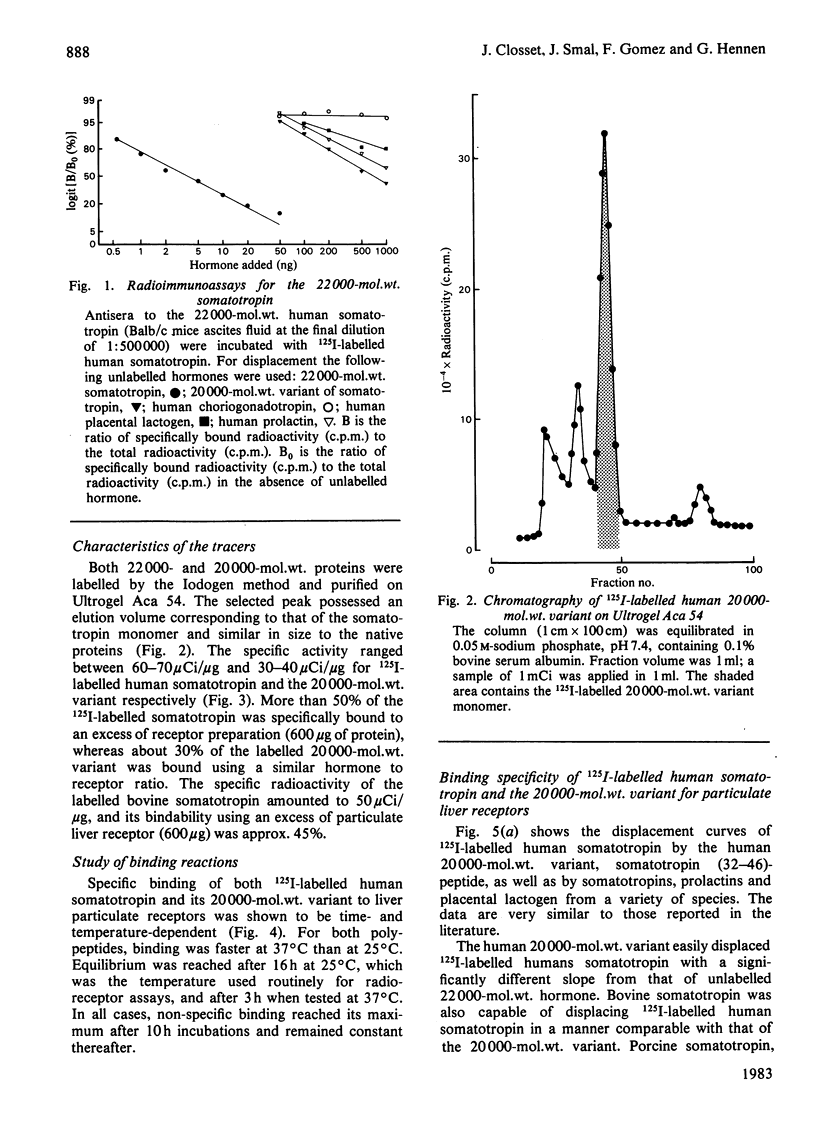
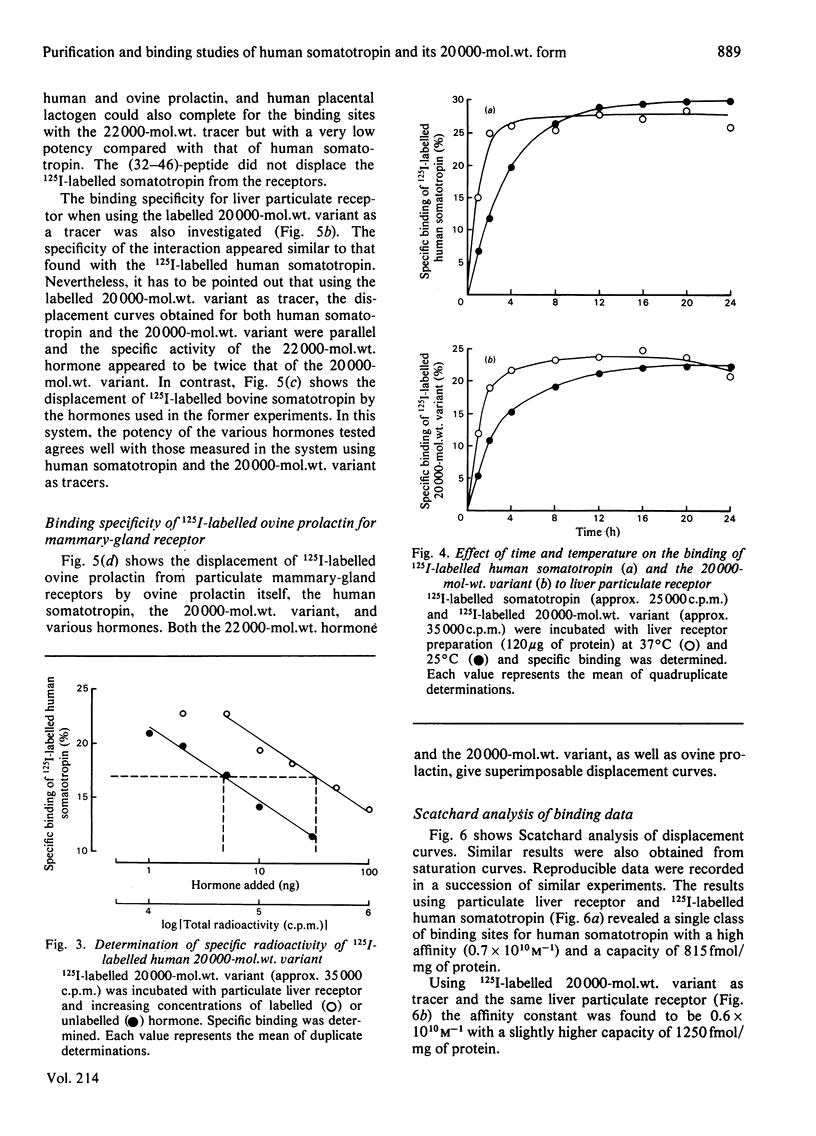
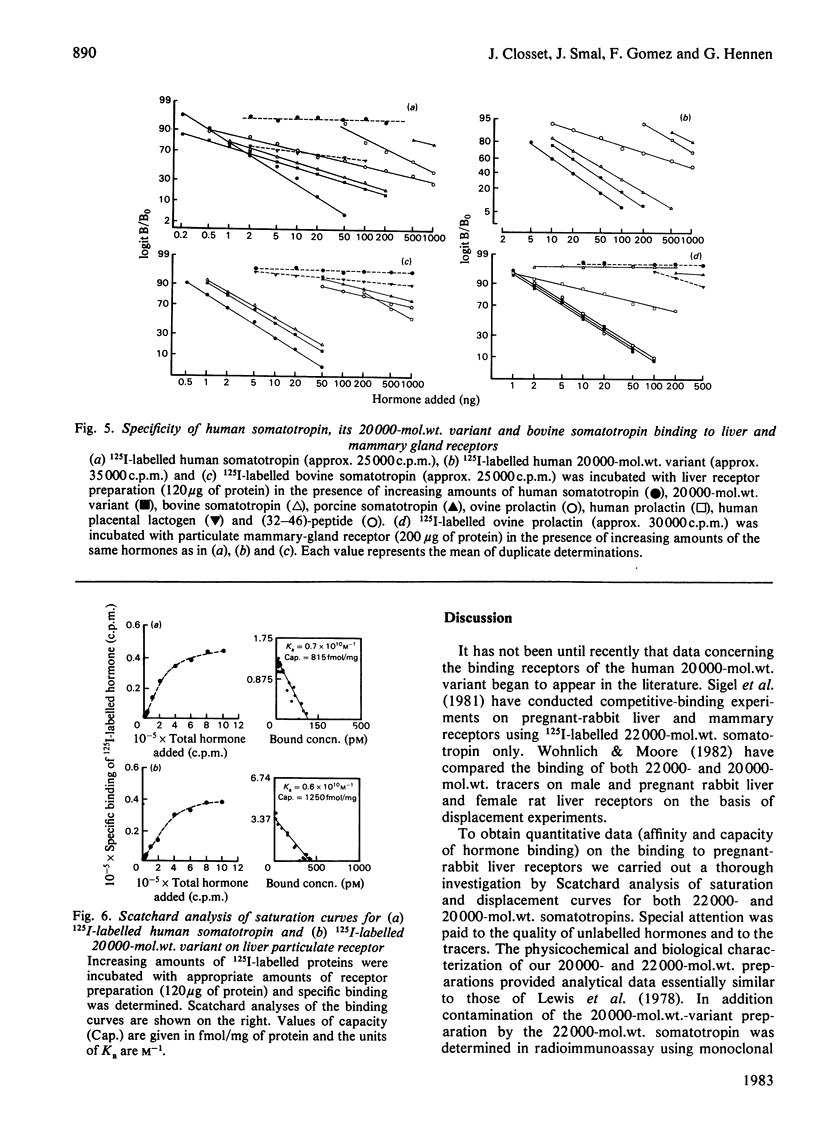
Quantitative data concerning the binding of 22000-mol.wt. human somatotropin and its 20000-mol.wt. variant are described using pregnant-rabbit liver and mammary-gland receptors. The purification and the complete chemical characterization of both human somatotropin and its 20000-mol.wt. variant is also presented. Contamination of the 20000-mol.wt.-variant preparation by 22000-mol.wt. hormone was found to be 0.5% by weight as measured in radioimmunoassay using monoclonal antibody. Labelling of human somatotropin and its 20000-mol.wt. variant using the Iodogen method is described as well as the characterization of the binding to pregnant-rabbit liver and mammary-gland receptor preparations. The maximum binding capacity of the 125I-labelled human somatotropin was between 50 and 60% to liver particulate receptor, whereas that of the 20000-mol.wt. variant was 30%. The specificity of binding of both forms to rabbit hepatic and mammary-gland receptor was found to be similar for both proteins in the same system. The affinity constants and capacity were respectively 0.7 X 10(10)M-1 and 815 fmol/mg of protein for human somatotropin and 0.6 X 10(10)M-1 and 1.250 fmol/mg of protein for the 20000-mol.wt. variant. These data suggest that both proteins behave as partial agonists to the receptors studied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cadman H. F., Wallis M. An investigation of sites that bind human somatotropin (growth hormone) in the liver of the pregnant rabbit. Biochem J. 1981 Sep 15;198(3):605–614. doi: 10.1042/bj1980605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closset J., Hennen G. Porcine thyrotropin. Isolation and characterization of the hormone and its alpha and beta subunits. Eur J Biochem. 1974 Aug 1;46(3):595–602. doi: 10.1111/j.1432-1033.1974.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Closset J., Maghuin-Rogister G., Hennen G., Strosberg A. D. Porcine follitropin. The amino-acid sequence of the beta subunit. Eur J Biochem. 1978 May;86(1):115–120. doi: 10.1111/j.1432-1033.1978.tb12290.x. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Frigeri L. G., Peterson S. M., Lewis U. J. The 20,000-dalton structural variant of human growth hormone: lack of some early insulin-like effects. Biochem Biophys Res Commun. 1979 Dec 14;91(3):778–782. doi: 10.1016/0006-291x(79)91947-8. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Posner B. I., Tsushima T., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: ontogenesis, effects of sex and pregnancy. Endocrinology. 1974 Aug;95(2):532–539. doi: 10.1210/endo-95-2-532. [DOI] [PubMed] [Google Scholar]
- Ketelslegers J. M., Knott G. D., Catt K. J. Kinetics of gonadotropin binding by receptors of the rat testis. Analysis by a nonlinear curve-fitting method. Biochemistry. 1975 Jul 15;14(14):3075–3083. doi: 10.1021/bi00685a006. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Bonewald L. F., Lewis L. J. The 20,000-dalton variant of human growth hormone: location of the amino acid deletions. Biochem Biophys Res Commun. 1980 Jan 29;92(2):511–516. doi: 10.1016/0006-291x(80)90363-0. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Dunn J. T., Bonewald L. F., Seavey B. K., Vanderlaan W. P. A naturally occurring structural variant of human growth hormone. J Biol Chem. 1978 Apr 25;253(8):2679–2687. [PubMed] [Google Scholar]
- McConaghey P., Sledge C. B. Production of "sulphation factor" by the perfused liver. Nature. 1970 Mar 28;225(5239):1249–1250. doi: 10.1038/2251249b0. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Sigel M. B., Thorpe N. A., Kobrin M. S., Lewis U. J., Vanderlaan W. P. Binding characteristics of a biologically active variant of human growth hormone (20K) to growth hormone and lactogen receptors. Endocrinology. 1981 Apr;108(4):1600–1603. doi: 10.1210/endo-108-4-1600. [DOI] [PubMed] [Google Scholar]
- Spencer E. M., Lewis L. J., Lewis U. J. Somatomedin generating activity of the 20,000-dalton variant of human growth hormone. Endocrinology. 1981 Oct;109(4):1301–1302. doi: 10.1210/endo-109-4-1301. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Friesen H. G. Radioreceptor assay for growth hormone. J Clin Endocrinol Metab. 1973 Aug;37(2):334–337. doi: 10.1210/jcem-37-2-334. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Sasaki N., Imai Y., Matsuzaki F., Friesen H. G. Characteristics of solubilized human-somatotropin-binding protein from the liver of pregnant rabbits. Biochem J. 1980 May 1;187(2):479–492. doi: 10.1042/bj1870479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Waters M. J., Friesen H. G. Purification and partial characterization of a nonprimate growth hormone receptor. J Biol Chem. 1979 Jul 25;254(14):6815–6825. [PubMed] [Google Scholar]
- Wohnlich L., Moore W. V. Binding of a variant of human growth hormone to liver plasma membranes. Horm Metab Res. 1982 Mar;14(3):138–141. doi: 10.1055/s-2007-1018948. [DOI] [PubMed] [Google Scholar]



