Abstract
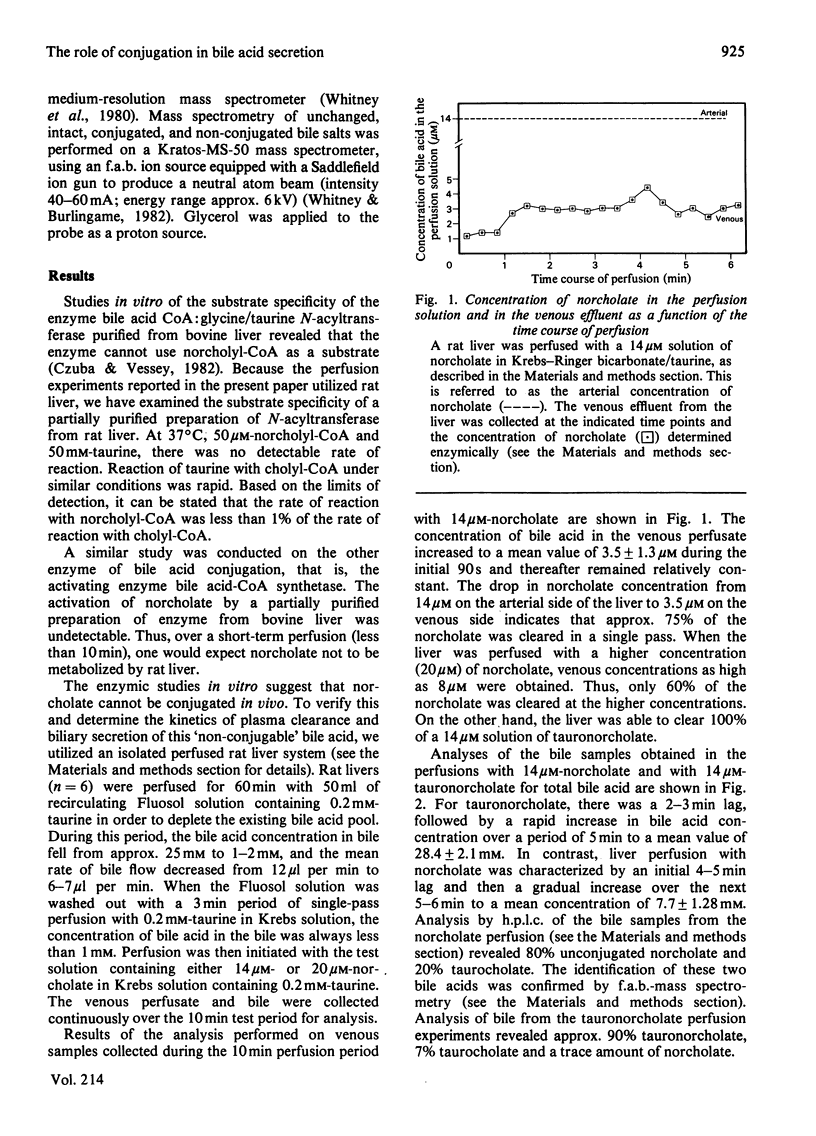
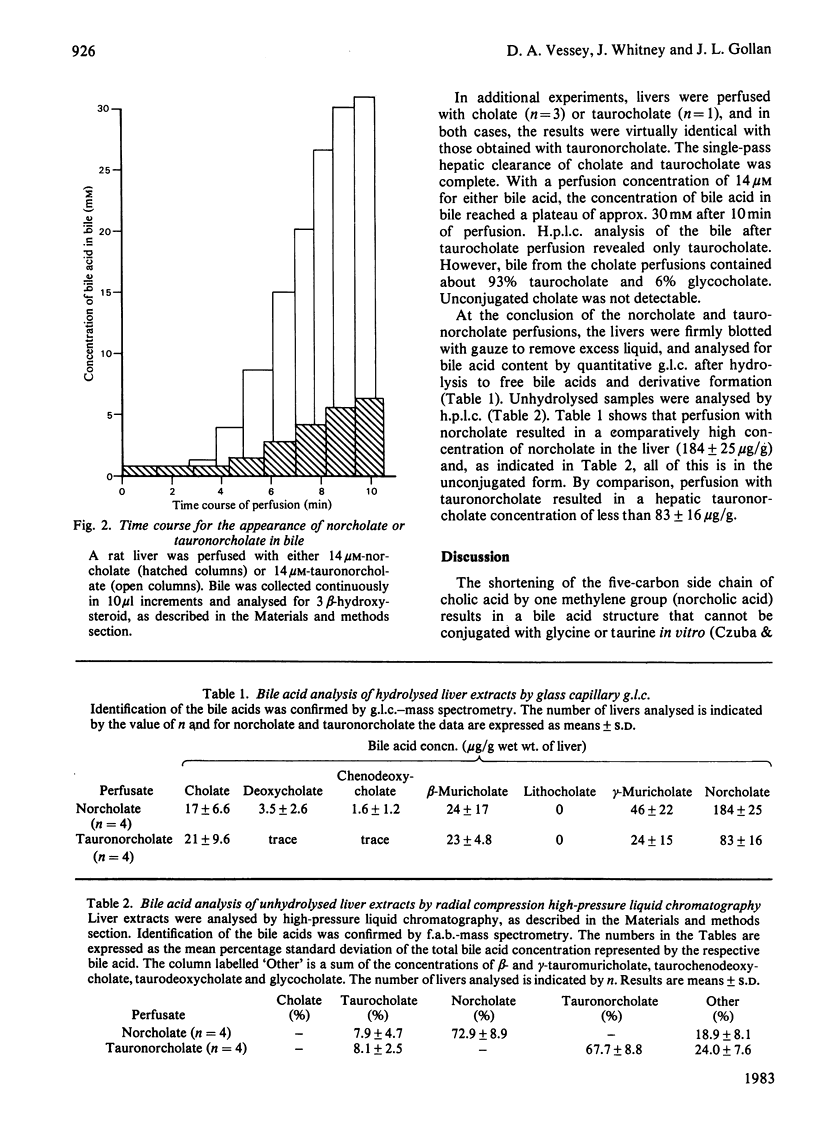
Shortening the five-carbon carboxylic acid side chain of cholic acid by one methylene group gave rise to a bile acid (norcholate) that was not a substrate for the bile acid-conjugating enzymes. The metabolism and biliary secretion of norcholate in intact liver was examined in the isolated perfused rat liver system. When rat livers were perfused with 14-20 microM solutions of norcholate for 10 min, norcholate was found in the unconjugated form in liver, venous effluent and bile. Neither tauronorcholate nor glyconorcholate was detectable by high-pressure liquid chromatography or fast-atom-bombardment mass spectrometry. The kinetics of hepatic uptake and biliary secretion of norcholate was compared with that for cholate, taurocholate and chemically synthesized tauronorcholate. The latter three bile acids were completely cleared from the perfusate and efficiently secreted into the bile. However, norcholate was incompletely extracted from the perfusate, and this was shown to be at least partially due to its relatively lower rate of hepatic uptake. Furthermore, the rate of norcholate secretion into bile was greatly reduced relative to the secretion of cholate or chemically synthesized tauronorcholate, even though the concentration of norcholate in the liver was comparatively high. These data demonstrate that the conjugation of bile acids greatly facilitates their secretion into bile.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Czuba B., Vessey D. A. Kinetic characterization of cholyl-CoA glycine-taurine N-acyltransferase from bovine liver. J Biol Chem. 1980 Jun 10;255(11):5296–5299. [PubMed] [Google Scholar]
- Czuba B., Vessey D. A. The effect of bile acid structure on the activity of bile acid-CoA:glycine/taurine-N-acetyltransferase. J Biol Chem. 1982 Aug 10;257(15):8761–8765. [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Gollan J., Hammaker L., Licko V., Schmid R. Bilirubin kinetics in intact rats and isolated perfused liver. Evidence for hepatic deconjugation of bilirubin glucuronides. J Clin Invest. 1981 Apr;67(4):1003–1015. doi: 10.1172/JCI110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslewood G. A. Bile salt evolution. J Lipid Res. 1967 Nov;8(6):535–550. [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. Influence of bile acids on bile canalicular membrane morphology and the lobular gradient in canalicular size. Lab Invest. 1978 Aug;39(2):110–119. [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G., Short A. H. Acute taurine depletion and maximal rates of hepatic conjugation and secretion of cholic acid in the dog. J Physiol. 1965 Sep;180(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- Osuga T., Mitamura K., Mashige F., Imai D. Evaluation of fluorimetrically estimated serum bile acid in liver disease. Clin Chim Acta. 1977 Feb 15;75(1):81–90. doi: 10.1016/0009-8981(77)90502-2. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Hachey D. L., Klein P. D. An improved procedure for the synthesis of glycine and taurine conjugates of bile acids. J Lipid Res. 1977 May;18(3):404–407. [PubMed] [Google Scholar]
- Vessey D. A. The co-purification and common identity of cholyl CoA:glycine- and cholyl CoA:taurine-N-acyltransferase activities from bovine liver. J Biol Chem. 1979 Mar 25;254(6):2059–2063. [PubMed] [Google Scholar]



