Abstract
Background
Insurance companies have adopted variable and inconsistent approval criteria for chronic venous disease (CVD) treatment. Although vein ablation (VA) is accepted as the standard of care for venous ulcers, the treatment criteria for patients with milder forms of CVD remain controversial. This study aims to identify factors associated with a lack of clinical improvement (LCI) in patients with less severe CVD without ulceration undergoing VA to improve patient selection for treatment.
Methods
We performed a retrospective analysis of patients undergoing VA for CEAP C2 to C4 disease in the Vascular Quality Initiative varicose veins database from 2014 to 2023. Patients who required intervention in multiple veins, had undergone prior interventions, or presented with CEAP C5 to C6 disease were excluded. The difference (Δ) in venous clinical severity score (VCSS; VCSS before minus after the procedure) was used to categorize the patients. Patients with a ΔVCSS of ≤0 were defined as having LCI after VA, and patients with ≥1 point decrease in the VCSS after VA (ΔVCSS ≥1) as having some benefit from the procedure and, therefore, “clinical improvement.” The characteristics of both groups were compared, and multivariable regression analysis was performed to identify factors independently associated with LCI. A second analysis was performed based on the VVSymQ instrument, which measures patient-reported outcomes using five specific symptoms (ie, heaviness, achiness, swelling, throbbing pain, and itching). Patients with LCI showed no improvement in any of the five symptoms, and those with clinical improvement had a decrease in severity of at least one symptom.
Results
A total of 3544 patients underwent initial treatment of CVD with a single VA. Of the 3544 patients, 2607 had VCSSs available before and after VA, and 420 (16.1%) had LCI based on the ΔVCSS. Patients with LCI were more likely to be significantly older and African American and have CEAP C2 disease compared with patients with clinical improvement. Patients with clinical improvement were more likely to have reported using compression stockings before treatment. The vein diameters were not different between the two groups. The incidence of complications was overall low, with minor differences between the two groups. However, the patients with LCI were significantly more likely to have symptoms after intervention than those with improvement. Patients with LCI were more likely to have technical failure, defined as vein recanalization. On multivariable regression, age (odds ratio [OR], 1.01; 95% confidence interval [CI], 1.00-1.02) and obesity (OR, 1.47; 95% CI, 1.09-2.00) were independently associated with LCI, as was treatment of less severe disease (CEAP C2; OR, 1.82; 95% CI, 1.30-2.56) compared with more advanced disease (C4). The lack of compression therapy before intervention was also associated with LCI (OR, 6.05; 95% CI, 4.30-8.56). The analysis based on the VVSymQ showed similar results.
Conclusions
LCI after VA is associated with treating patients with a lower CEAP class (C2 vs C4) and a lack of compression therapy before intervention. Importantly, no significant association between vein size and clinical improvement was observed.
Keywords: Chronic venous disease, Clinical effectiveness, Quality of care, Venous ablation
Article Highlights.
-
•
Type of Research: An analysis of the Vascular Quality Initiative varicose veins database
-
•
Key Findings: This study provides factors to improve the selection of patients with mild chronic venous disease for vein ablation (VA). Patients with milder forms of disease (CEAP [Clinical-Etiological-Anatomical-Pathophysiological] C2) were more likely to experience a lack of clinical improvement (LCI) compared with patients with more advanced disease (CEAP C4). Furthermore, the gradual decrease in the frequency of use of compression therapy was associated with the incremental likelihood of LCI after VA, supporting the use of compression therapy as a treatment modality for chronic venous disease before VA and possibly as a tool to improve the selection of patients who would benefit from VA.
-
•
Take Home Message: LCI after VA is associated with treating patients with a less severe CEAP clinical classification (C2) and lack of compression therapy.
Chronic venous disease (CVD) affects approximately 25% of the adult population in the United States, with an annual incidence of 2.6% in women and 1.9% in men.1 The associated economic burden is estimated to exceed $3 billion annually.2 The effect of CVD on patients' quality of life has been well documented,3 and a progressive decline in overall quality of life as the CEAP (Clinical-Etiological-Anatomical-Pathophysiological) clinical class severity increases has been described.2 Vein ablation (VA) is accepted as the standard of care for patients with CEAP class C5 and C6 based on level 1 evidence with high success rates and proven cost-effectiveness.4, 5, 6, 7 However, the criteria for selecting patients for VA for those with less advanced CVD remain controversial, as reflected by the inconsistencies in the coverage policies of various insurance carriers.8 In contrast, VA is a safe procedure that vascular and nonvascular specialists have overused.9, 10, 11 This exponential growth in the overall volume of services related to CVD treatment has led to the development of restrictive policies, which lead to delays in care and potentially negative patient outcomes.8,12, 13, 14, 15
Although VA is a common treatment modality for all patients with CVD, the criteria for selecting patients with milder forms of disease who would most benefit from treatment remain elusive. As healthcare in the United States has increasingly focused on the value of care, a consensus on what constitutes “valuable” treatment of CVD is needed to guide physicians and payers in optimizing patient outcomes and allocating resources. Notably, the standard for outcome assessment after venous procedures relies not only on technical success but also on disease-specific and psychometric evaluation of patient-reported outcomes (PRO), such as the venous clinical severity score (VCSS) and VVSymQ.16, 17, 18 To better understand the value of VA for patients with less advanced CVD, this study aims to identify the factors associated with the lack of clinical improvement (LCI) after VA in a large national database.
Methods
Database
A retrospective analysis of patients undergoing VA in the Vascular Quality Initiative (VQI) varicose veins database from 2014 to 2023 was performed. The Society for Vascular Surgery – Patient Safety Organization VQI is a prospective, national clinical registry collaboration between regional quality groups designed to improve vascular healthcare's quality, safety, effectiveness, and cost.19 The database captures patient and procedural details regarding VA and early patient follow-up to 3 months after each procedure and then late follow-up for visits after >3 months. The Yale University institutional review board exempted this study, and no patient consent was required.
Definitions and patient selection
Only patients with CEAP class C2 to C4 disease without prior venous treatment undergoing VA of one vein were included. Patients with missing VCSSs and VVSymQ scores before or after intervention that prevented calculating the difference (delta [Δ]) in the scores to assess clinical improvement were excluded from the respective analysis. The VCSS is a validated, physician-reported outcome tool used to measure the severity of venous disease. It consists of a 30-point score based on 10 common descriptors (ie, pain, varicose veins, edema, pigmentation, inflammation, induration, number of active ulcers, ulcer duration, active ulcer size, and compression therapy) scored from 0 to 3 for a total possible score of 30. The VCSS is a dynamic and quantitative evaluation sensitive to treatment effects. It complements the CEAP classification, which primarily relies on descriptive and qualitative categorization.20 A LCI was defined as a lack of decrease in the VCSS after the procedure (VCSS before minus VCSS after the procedure of ≤0).
The VVSymQ instrument, which queries for five specific symptoms, including heaviness, achiness, swelling, throbbing pain, and itching, was used as a PRO measurement, and the results were compared before and after treatment. Patients with LCI were defined as those who reported no improvement in any of the five symptoms of the VVSymQ instrument. In contrast, patients were classified as having clinical improvement if they reported improvement in at least one of the symptoms (ie, heaviness, achiness, swelling, throbbing pain, and itching). Treatment failure was defined as LCI and recanalization of the treated vein on follow-up ultrasound. Two separate analyses were performed in this study. The first analysis focused on patients with LCI based on the VCSS. Thus, patients with ΔVCSS of ≤0 were compared with patients with ΔVCSS of ≥1. The second analysis focused on patients with LCI based on the VVSymQ PRO.
Patient characteristics
The demographic variables included age, sex, race, and ethnicity. Race was divided as White, African American, and other. The comorbidities were reviewed and included prior phlebitis, prior deep vein thrombosis (DVT), and prior pulmonary embolism. Information related to the patients’ initial CEAP classification, VCSS, use of compression stockings, and quality-of-life survey (ie, heaviness, achiness, swelling, throbbing, itching, appearance, and work impact) were analyzed. Each parameter in the quality-of-life survey was rated on a scale from 0 (indicating not severe) to 5 (indicating severe), except for the appearance parameter, which used a scale from 0 (indicating not severe) to 4 (indicating severe). In the present analysis, the proportion of patients with LCI was compared statistically with those with clinical improvement.
Procedural characteristics
The procedural characteristics included the use of general anesthesia and treatment types such as radiofrequency ablation, endovenous laser ablation, and others. The types of veins treated were grouped as the great saphenous vein, anterior accessory saphenous vein, small saphenous vein, and others. Other characteristics, such as vein length and diameter and deep venous reflux in the ipsilateral lower extremity, were also compared between the two groups.
Postoperative outcomes
The postoperative outcomes included changes in the VCSS and VVSymQ, technical success (defined as the absence of vein recanalization), and the occurrence of various postoperative complications, including postoperative bleeding, blistering, DVT, hematoma, paresthesia, pigmentation, phlebitis, ulcer formation, and thrombus extension. Furthermore, the study analyzed compliance with compression therapy, PRO, and symptoms, which included assessments of heaviness, achiness, swelling, throbbing pain, itching, perceived appearance, and the impact of these symptoms on work.
Statistical analysis
Descriptive statistics were calculated as frequencies for categorical variables and the mean ± standard deviation for continuous variables. Differences between categorical variables were assessed using a χ2 test or the Fischer exact test and the Wilcoxon rank sum test as the nonparametric test for ordinal variables. A P value of < .05 was defined as statistically significant. Multivariable regression analysis was performed for both groups to determine the factors independently associated with LCI. In the analysis for LCI based on the VCSS, the model incorporated the following variables: age, race, body mass index, prior DVT, CEAP classification, use of compression therapy, and general anesthesia. Similarly, in the model for LCI based on VVSymQ, the variables considered were sex, prior DVT, CEAP classification, use of compression therapy, and the type of anesthesia administered. R statistical software, version 4.0.4, was used for statistical analysis.
Results
Definitions and patient selection
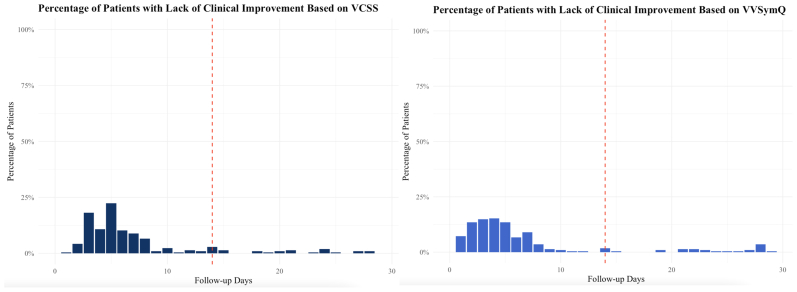
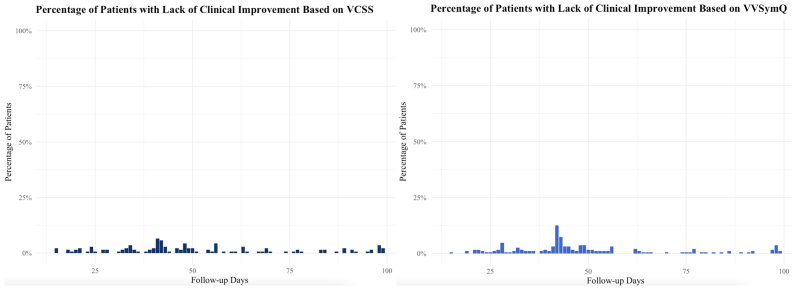
A total of 6342 patients underwent initial VA and presented for follow-up between day 1 and 180 days after treatment. Because the patients could still be complaining of phlebitis and discomfort related to the procedure in the early postoperative period and might need more time to experience clinical improvement, the proportions of patients with LCI during the first month after treatment were derived. A higher proportion of LCI in the first 2 weeks after the procedure and >20% in the first week is demonstrated in the Fig. However, the proportion of patients with LCI significantly decreases after the first 2 weeks and remains lower ≤100 days of follow-up (Supplementary Fig, online only). Thus, the analysis was limited to patients with follow-up between 2 weeks and 6 months. After excluding patients with follow-up only during the initial 2 weeks, a total of 3544 patients underwent initial treatment with VA. Of the 3544 patients, 2607 had complete VCSS data available before and after treatment in the first analysis based on the VCSSs and 2841 patients for the second analysis based on the analyzed VVSymQ scores. Thus, 937 patients with missing VCSSs were excluded from the analysis focusing on the VCSSs and 703 patients with missing VVSymQ scores were excluded from the analysis focusing on the VVSymQ scores.
Fig.
Percentage of patients reporting a lack of clinical improvement (LCI) during the first month after treatment based on the venous clinical severity score (VCSS) and VVSymQ (red line represents the 2-week mark).
Supplementary Fig (online only).
Percentage of patients presenting with lack of clinical improvement (LCI) during the first 100 days based on venous clinical severity score (VCSS) and VVSymQ.
Analysis based on VCSS
Patient characteristics
A total of 420 patients (16.1%) had LCI based on the VCSS. Patients with LCI were more likely to be older (58 vs 56 years; P = .04), White, and obese (51% vs 42%; P = .004; Table I). They were also more likely to report symptoms at baseline, such as pain, varicosities, and edema. In contrast, they were less likely to present with certain symptoms assessed by the VVSymQ, such as throbbing pain and itchiness. Also, patients with LCI were more likely to have CEAP class C2 than were patients with clinical improvement. Patients with clinical improvement were more likely to have reported using compression stockings before treatment (P < .001). Additionally, the median VCSS before treatment was lower for patients with LCI than for those with clinical improvement (P < .001).
Table I.
Baseline patient characteristics stratified by clinical improvement based on venous clinical severity score (VCSS)
| Characteristic | Clinical improvement (n = 2187; 83.9%) | LCI (n = 420; 16.1%) | P value |
|---|---|---|---|
| Age, years | 56 (45-66) | 58 (47-67) | .043 |
| Female sex | 1480 (68) | 286 (68) | .9 |
| Race | .001 | ||
| White | 1720 (79) | 345 (82) | |
| African American | 76 (3.5) | 25 (6.0) | |
| Other | 391 (18) | 50 (12) | |
| Ethnicity (Latino) | 115 (5.3) | 19 (4.5) | .5 |
| BMI | .004 | ||
| Underweight (<18.5 kg/m2) | 18 (0.8) | 4 (1.0) | |
| Normal (18.5-24.9 kg/m2) | 538 (25) | 87 (21) | |
| Overweight (25-30 kg/m2) | 716 (33) | 114 (27) | |
| Obese (>30 kg/m2) | 912 (42) | 215 (51) | |
| Anticoagulation | 217 (9.9) | 51 (12) | .2 |
| Prior phlebitis | 250 (11) | 41 (9.8) | .3 |
| Prior DVT | 123 (5.6) | 26 (6.2) | 0.6 |
| Prior PE | 19 (2.7) | 1 (0.9) | .5 |
| Compression therapy before intervention | < .001 | ||
| Daily | 765 (35) | 84 (20) | |
| Most days | 614 (28) | 111 (26) | |
| Intermittent | 509 (23) | 99 (24) | |
| None | 299 (14) | 126 (30) | |
| CEAP classification | .005 | ||
| C4 | 450 (21) | 85 (20) | |
| C3 | 1127 (52) | 186 (44) | |
| C2 | 610 (28) | 149 (35) | |
| Symptoms | |||
| Pain | 2137 (98) | 383 (91) | < .001 |
| Varicosities | 2128 (97) | 388 (92) | < .001 |
| Edema | 1529 (70) | 252 (60) | < .001 |
| Pigmentation | 385 (18) | 71 (17) | .7 |
| Inflammation | 368 (17) | 58 (14) | .13 |
| Induration | 214 (9.8) | 26 (6.2) | .02 |
| Quality of life | |||
| Heaviness | 1479 (70) | 294 (73) | .2 |
| Achiness | 1892 (89) | 349 (86) | .13 |
| Swelling | 1744 (82) | 329 (81) | .7 |
| Throbbing | 1151 (54) | 262 (65) | < .001 |
| Itching | 919 (43) | 203 (50) | .01 |
| Appearance | 1972 (93) | 371 (92) | .5 |
| Impact on work | 1808 (85) | 339 (83) | .5 |
| Any symptom | 2116 (97) | 401 (95) | .2 |
| VCSS before treatment | 7 (6-9) | 6 (4-7) | < .001 |
BMI, Body mass index; CEAP, Clinical-Etiological-Anatomical-Pathophysiological; DVT, deep vein thrombosis; LCI, lack of clinical improvement; PE, pulmonary embolism.
Data presented as median (interquartile range) or number (%).
Boldface P values represent statistical significance (P < .05).
Procedural characteristics
Patients who showed clinical improvement based on the VCSS were more frequently administered general anesthesia (Table II). The two groups had no significant differences in vein diameters, vein lengths, or the presence of deep venous reflux. However, patients with LCI based on the VCSS were more likely to present with isolated small saphenous vein reflux.
Table II.
Procedural details of patients stratified by clinical improvement based on venous clinical severity score (VCSS)
| Characteristic | Clinical improvement (n = 2187; 83.9%) | LCI (n = 420; 16.1%) | P valuea |
|---|---|---|---|
| Deep venous reflux | 738 (34) | 143 (35) | .8 |
| Type of treatment | < .001 | ||
| RFA | 1056 (48) | 247 (59) | |
| EVLA | 396 (18) | 111 (26) | |
| Other | 735 (34) | 62 (15) | |
| General anesthesia | 376 (17) | 12 (2.9) | < .001 |
| Type of vein treated | .015 | ||
| GSV | 1424 (89) | 330 (89) | |
| AAGSV | 43 (2.7) | 11 (3.0) | |
| SSV | 128 (8.0) | 26 (7.0) | |
| Other | 2 (0.1) | 5 (1.3) | |
| Diameter of vein treated, mm | 7.2 ± 3.5 | 7.3 ± 3.1 | .08 |
| Length of vein treated, mm | 37 ± 15 | 37 ± 15 | > .9 |
AAGSV, Anterior accessory saphenous vein; EVLA, endovenous laser ablation; GSV, great saphenous vein; LCI, lack of clinical improvement; RFA, radiofrequency ablation; SSV, small saphenous vein.
Data presented as number (%) or mean ± standard deviation.
Boldface P values represent statistical significance (P < .05).
P values computed using the Wilcoxon rank sum test or Pearson χ2 test.
Outcomes
After treatment, the median follow-up in days was not significantly different between the two groups (Table III). The median ΔVCSS was significantly lower in the LCI group (0 vs 4; P < .001). The incidence of complications was overall low, with minor differences between the two groups. Patients with LCI based on VCSS were more likely to present with phlebitis and to develop ulcers. Furthermore, patients with LCI were more likely to have technical failure, defined as vein recanalization (1.7% vs 0.4%; P < .001), and were significantly more likely to report symptoms after intervention than were those with improvement.
Table III.
Postoperative outcomes of patients stratified by clinical improvement based on venous clinical severity score (VCSS)
| Characteristic | Clinical improvement (n = 2187; 83.9%) | LCI (n = 420; 16.1%) | P value |
|---|---|---|---|
| Follow-up, days | 49 (42-78) | 49 (39-103) | .14 |
| VCSS after treatment | 2 (1-4) | 6 (5-8) | < .001 |
| VCSS delta | 4.00 (3.00-6.00) | 0.00 (−1.00 to 0.0) | < .001 |
| LCI based on VVSymQ | 137 (6.8) | 89 (23) | < .001 |
| Complications | |||
| Blistering | 2 (0.1) | 0 (0) | > .9 |
| DVT | 7 (0.4) | 3 (1.0) | .2 |
| Hematoma | 7 (0.4) | 2 (0.7) | .4 |
| Paresthesia | 24 (1.4) | 4 (1.4) | > .9 |
| Pigmentation | 23 (1.3) | 2 (0.7) | .6 |
| Phlebitis | 21 (1.2) | 8 (2.7) | .054 |
| Ulcer | 0 (0) | 2 (0.7) | .02 |
| Wound | 8 (0.5) | 1 (0.3) | > .9 |
| Proximal thrombus extension | 40 (2.3) | 2 (0.7) | .078 |
| Any complication | 128 (5.9) | 23 (5.5) | .8 |
| Compression therapy after treatment | < .001 | ||
| Daily | 397 (18) | 193 (46) | |
| Almost daily | 359 (16) | 117 (28) | |
| Intermittent | 604 (28) | 69 (16) | |
| None | 827 (38) | 41 (9.8) | |
| Vein recanalization | 9 (0.4) | 7 (1.7) | < .001 |
| Quality of life | |||
| Heaviness | 541 (26) | 204 (52) | < .001 |
| Achiness | 862 (42) | 272 (69) | < .001 |
| Swelling | 756 (37) | 254 (64) | < .001 |
| Throbbing | 357 (17) | 150 (38) | < .001 |
| Itching | 342 (17) | 136 (34) | < .001 |
DVT, Deep vein thrombosis; LCI, lack of clinical improvement.
Data presented as median (interquartile range) or number (%).
Boldface P values represent statistical significance (P < .05).
Factors associated with LCI
The multivariable regression analysis identified several significant risk factors for LCI after treatment (Table IV). Age was identified as a significant risk factor for LCI after VA (odds ratio [OR], 1.01; 95% confidence interval [CI], 1.00-1.02), as was obesity (OR, 1.47; 95% CI, 1.09-2.00). Patients with CEAP class C2 had a higher likelihood of LCI (OR, 1.82; 95% CI, 1.30-2.56) compared with patients treated for more advanced C4 disease. In addition, the lack of compression therapy before intervention was associated with LCI (OR, 6.05; 95% CI, 4.30-8.56). In contrast, the use of general anesthesia was associated with significantly lower odds of LCI (OR, 0.07; 95% CI, 0.04-0.14).
Table IV.
Regression analysis of factors independently associated with lack of clinical improvement (LCI) based on venous clinical severity score (VCSS)
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.01 | 1.00-1.02 | .015 |
| Race | |||
| White | – | – | |
| African American | 1.15 | 0.68-1.87 | .593 |
| Other | 1.33 | 0.89-1.96 | .162 |
| BMI | |||
| Normal | – | – | |
| Overweight | 0.93 | 0.68-1.29 | .680 |
| Underweight | 1.29 | 0.34-3.95 | .674 |
| Obese | 1.47 | 1.09-2.00 | .012 |
| Prior DVT | 1.34 | 0.82-2.14 | .228 |
| CEAP classification | |||
| C4 | – | – | |
| C3 | 0.92 | 0.68-1.25 | .571 |
| C2 | 1.82 | 1.30-2.56 | < .001 |
| Compression therapy before treatment | |||
| Daily | – | – | |
| Almost daily | 1.83 | 1.33-2.52 | < .001 |
| Intermittent | 3.09 | 2.19-4.37 | < .001 |
| None | 6.05 | 4.30-8.56 | < .001 |
| General anesthesia | 0.07 | 0.04-0.14 | < .001 |
BMI, Body mass index; CEAP, Clinical-Etiological-Anatomical-Pathophysiological; CI, confidence interval; DVT, deep vein thrombosis; OR, odds ratio.
Boldface P values represent statistical significance (P < .05).
Analysis based on VVSymQ
Patient characteristics
A total of 280 patients (9.9%) demonstrated LCI based on the VVSymQ (Table V). No significant differences in baseline characteristics were noted between the two groups, except for a higher rate of prior DVT in patients with LCI. Patients reporting clinical improvement in this group were more likely to wear compression stockings daily than were the LCI group (P < .001). Patients with LCI were also more likely to be treated for CEAP C2 disease than were patients with clinical improvement. Furthermore, patients with LCI were more symptomatic before treatment, experiencing elevated rates of pain, edema, varicosities, inflammation, and induration (P < .001). The median VCSS before treatment was significantly lower for the patients with LCI than for those with clinical improvement (P < .001).
Table V.
Baseline patient characteristics stratified by clinical improvement based on VVSymQ
| Characteristic | Clinical improvement (n = 2561; 90.1%) | LCI (n = 280; 9.9%) | P value |
|---|---|---|---|
| Age, years | 56 (45-66) | 59 (44-67) | .11 |
| Female sex | 1772 (69) | 181 (65) | .12 |
| Race | .2 | ||
| White | 2021 (79) | 233 (83) | |
| African American | 103 (4.0) | 11 (3.9) | |
| Other | 437 (17) | 36 (13) | |
| Ethnicity (Latino) | 120 (4.7) | 13 (4.6) | > .9 |
| BMI | .2 | ||
| Underweight (<18.5 kg/m2) | 19 (0.7) | 5 (1.8) | |
| Normal (18.5-24.9 kg/m2) | 593 (23) | 70 (25) | |
| Overweight (25-30 kg/m2) | 801 (31) | 91 (33) | |
| Obese (>30 kg/m2) | 1144 (45) | 114 (41) | |
| Anticoagulation | 304 (12) | 27 (9.6) | .3 |
| Prior phlebitis | 273 (11) | 35 (13) | .3 |
| Prior DVT | 133 (5.2) | 25 (8.9) | .01 |
| Prior PE | 12 (1.6) | 1 (0.9) | > .9 |
| Compression therapy before intervention | .029 | ||
| Daily | 875 (34) | 72 (26) | |
| Most days | 684 (27) | 91 (33) | |
| Intermittent | 566 (22) | 64 (23) | |
| None | 423 (17) | 51 (18) | |
| CEAP classification | .008 | ||
| C4 | 527 (21) | 44 (16) | |
| C3 | 1316 (51) | 134 (48) | |
| C2 | 718 (28) | 102 (36) | |
| Symptoms | |||
| Pain | 2472 (97) | 255 (92) | < .001 |
| Varicosities | 2460 (97) | 258 (93) | .002 |
| Edema | 1763 (69) | 165 (59) | < .001 |
| Pigmentation | 447 (17) | 39 (14) | .13 |
| Inflammation | 420 (16) | 29 (10) | .009 |
| Induration | 245 (9.6) | 18 (6.5) | .087 |
| Quality of life | |||
| Heaviness | 1901 (74) | 99 (35) | < .001 |
| Achiness | 2360 (92) | 158 (56) | < .001 |
| Swelling | 2157 (85) | 137 (49) | < .001 |
| Throbbing | 1513 (59) | 98 (35) | < .001 |
| Itching | 1189 (47) | 105 (38) | .005 |
| Appearance | 2392 (94) | 234 (84) | < .001 |
| Impact on work | 2217 (87) | 173 (62) | < .001 |
| VCSS before treatment | 7 (6-9) | 6 (5-8) | < .001 |
BMI, Body mass index; CEAP, Clinical-Etiological-Anatomical-Pathophysiological; DVT, deep vein thrombosis; LCI, lack of clinical improvement; PE, pulmonary embolism.
Data presented as median (interquartile range) or number (%).
Boldface P values represent statistical significance (P < .05).
Procedural characteristics
Patients who showed clinical improvement based on the VVSymQ were more likely to undergo intervention under general anesthesia (Table VI). Both groups had no significant differences in vein diameters, vein lengths, or deep venous reflux. Patients with LCI were more commonly treated for isolated great saphenous vein reflux.
Table VI.
Procedural details of patients stratified by clinical improvement based on VVSymQ
| Characteristic | Clinical improvement (n = 2561; 90.1%) | LCI (n = 280; 9.9%) | P value |
|---|---|---|---|
| Deep venous reflux | 937 (37) | 106 (38) | .7 |
| Type of treatment | < .001 | ||
| RFA | 1321 (52) | 154 (55) | |
| EVLA | 525 (20) | 30 (11) | |
| Other | 715 (28) | 96 (34) | |
| General anesthesia | 390 (15) | 15 (5.4) | < .001 |
| Type of vein treated | .036 | ||
| GSV | 1773 (89) | 189 (94) | |
| AAGSV | 61 (3.1) | 3 (1.5) | |
| SSV | 154 (7.7) | 7 (3.5) | |
| Other | 9 (0.5) | 2 (1.0) | |
| Diameter of vein treated, mm | 7.3 ± 3.4 | 6.8 ± 2.8 | .2 |
| Length of vein treated, mm | 37 ± 15 | 37 ± 16 | .7 |
AAGSV, Anterior accessory saphenous vein; EVLA, endovenous laser ablation; GSV, great saphenous vein; LCI, lack of clinical improvement; RFA, radiofrequency ablation; SSV, small saphenous vein.
Data presented as number (%) or mean ± standard deviation.
Boldface P values represent statistical significance (P < .05).
Outcomes
The median long-term follow-up in days for patients evaluated with the VVSymQ was not significantly different between the two groups (Table VII). The median ΔVCSS was significantly lower in the LCI VVSymQ group (VCSS, 2 vs 4; P < .001). No differences between postoperative complications were observed between the two groups, with very low rates of complications. Patients reporting clinical improvement were more likely to be noncompliant with compression therapy after VA (32% vs 24%; P < .001). Technical failure due to vein recanalization was significantly different between both groups and significantly higher in the LCI group (0.5% vs 1.1%; P < .001). Patients with LCI were more likely to be symptomatic after the procedure for all the variables analyzed in this database.
Table VII.
Postoperative outcomes of patients stratified by clinical improvement based on VVSymQ
| Characteristic | Clinical improvement (n = 2561; 90.1%) | LCI (n = 280; 9.9%) | P value |
|---|---|---|---|
| Follow-up, days | 53 (42-100) | 53 (42-106) | 0.5 |
| VCSS after treatment | 3 (1-5) | 4 (2-6) | < .001 |
| VCSS delta | 4 (2-6) | 2 (0-4) | < .001 |
| LCI based on VCSS | 293 (13) | 89 (39) | < .001 |
| Complications | |||
| Blistering | 2 (0.1) | 0 (0) | > .9 |
| DVT | 9 (0.5) | 1 (0.6) | .6 |
| Hematoma | 9 (0.5) | 0 (0) | > .9 |
| Paresthesia | 22 (1.2) | 1 (0.6) | .7 |
| Pigmentation | 23 (1.3) | 1 (0.6) | .7 |
| Phlebitis | 22 (1.2) | 3 (1.7) | .5 |
| Ulcer | 2 (0.1) | 0 (0) | > .9 |
| Wound | 9 (0.5) | 0 (0) | > .9 |
| Proximal thrombus extension | 35 (1.9) | 3 (1.7) | > .9 |
| Any complication | 128 (5.0) | 9 (3.2) | .2 |
| Compression therapy after treatment | .028 | ||
| Daily | 599 (23) | 67 (24) | |
| Almost daily | 468 (18) | 62 (22) | |
| Intermittent | 668 (26) | 83 (30) | |
| None | 820 (32) | 66 (24) | |
| Vein recanalization | 12 (0.5) | 3 (1.1) | .001 |
| Quality of life | |||
| Heaviness | 754 (30) | 132 (47) | < .001 |
| Achiness | 1145 (45) | 188 (67) | < .001 |
| Swelling | 1012 (40) | 159 (57) | < .001 |
| Throbbing | 488 (19) | 132 (47) | < .001 |
| Itching | 472 (18) | 109 (39) | < .001 |
DVT, Deep vein thrombosis; LCI, lack of clinical improvement.
Data presented as median (interquartile range) or number (%).
Boldface P values represent statistical significance (P < .05).
Factors associated with LCI
The multivariable regression analysis for patients with LCI based on the VVSymQ revealed that female sex was associated with a greater risk of LCI (OR, 1.37; 95% CI, 1.00-1.87), as was a history of prior DVT (OR, 1.95; 95% CI, 1.10-3.31; Table VIII). Similar to the first analysis, patients treated for CEAP class C2 had a higher risk of LCI after treatment (OR, 1.7; 95% CI, 1.09-2.67). There was a trend for a lack of compression therapy before VA to result in a higher likelihood of LCI; however, this finding did not reach statistical significance (OR, 1.47; 95% CI, 0.95-2.24). Conversely, general anesthesia (OR, 0.3; 95% CI, 0.17-0.51) was associated with clinical improvement.
Table VIII.
Regression analysis of factors independently associated with lack of clinical improvement (LCI) based on VVSymQ
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Female sex | 1.37 | 1.00-1.87 | .047 |
| Prior DVT | 1.95 | 1.10-3.31 | .017 |
| CEAP classification | |||
| C4 | – | – | |
| C3 | 1.28 | 0.85-1.97 | .240 |
| C2 | 1.70 | 1.09-2.67 | .020 |
| Compression therapy before treatment | |||
| Daily | – | – | |
| Almost daily | 1.20 | 0.81-1.76 | .364 |
| Intermittent | 1.27 | 0.81-1.95 | .289 |
| None | 1.47 | 0.95-2.24 | .078 |
| General anesthesia | 0.30 | 0.17-0.51 | < .001 |
BMI, Body mass index; CEAP, Clinical-Etiological-Anatomical-Pathophysiological; CI, confidence interval; DVT, deep vein thrombosis; OR, odds ratio.
Boldface P values represent statistical significance (P < .05).
Discussion
This study provides factors to improve the selection of patients for VA and highlights the challenges in studying the value of such procedures in a population with less severe CVD (CEAP class C2-C4). Patients with milder forms of disease (CEAP class C2) were consistently more likely to experience LCI than were patients with more advanced disease (CEAP class C4). However, the gradual decrease in the frequency of use of compression therapy was associated with an incremental likelihood of LCI after VA, supporting the use of compression therapy as a treatment modality for CVD before VA and possibly as a tool to improve the selection of patients who would benefit from VA. Interestingly, age, sex, obesity, and a history of DVT demonstrated some trends toward an association with LCI, but the findings were not consistent. Finally, failure of VA, defined in our report as LCI, in combination with technical failure demonstrated by treated vein recanalization, was extremely rare, affecting only 0.3% of all cases.
The need to quantify and measure the benefits after vein procedures has driven the development of several outcomes assessment instruments for evaluating CVD severity or post-treatment clinical outcomes. However, a clear consensus regarding the choice of psychometric instruments for accurately evaluating treatment outcomes is yet to be determined. The validated VCSS consistently mirrors the severity of venous disease and strongly correlates with treatment outcomes.21,22 Other instruments based on PRO, such as the VVSymQ, have been validated to capture patients' disease experiences.18 In this study, patients were more likely to show LCI based on the evaluation of the VCSS than based on the VVSymQ. This underscores the significance of selecting the appropriate assessment tool to standardize the evaluation of these procedures. This is especially important in the context of comparing VCSSs before and after treatment, because compliance with compression therapy, including stockings, is a major contributing factor that can elevate the VCSS. Because patients who adhere to compression therapy after VA might exhibit a higher VCSS, this could potentially lead to an erroneous clinical classification of LCI if the patient becomes more compliant after VA. Regardless of the tool used for the assessment of clinical outcomes, failure of VA was extremely rare, confirming that it is safe and effective and provides some benefit for most patients, even those with milder forms of CVD, consistent with prior literature.23, 24, 25, 26, 27, 28
The 2022 practice guidelines for managing varicose veins recommend superficial VA over long-term compression therapy for symptomatic patients with axial reflux.5,11 Numerous studies, including randomized trials, have demonstrated the superiority of VA over compression therapy regarding health-related quality of life.29, 30, 31 Therefore, these results underscore the importance of not relying solely on the qualitative CEAP classification for determining the clinical necessity of VA for individuals with mild CVD. Instead, our findings highlight the need to incorporate clinical scoring assessments such as the VCSS or PRO instruments into the evaluation process.
Alleviating venous hypertension with compression therapy before treatment was a factor associated with clinical improvement after VA in this analysis. Prior studies have shown that compression therapy has proven beneficial for decreasing inflammatory cytokines and improving microcirculation and is recommended to decrease symptoms.32,33 Furthermore, prospective studies have reported an association between compliance with compression stockings and improvements in the VCSS.34 A previous study indicated that improvement with compression stockings should not be used to withhold surgical therapy. Instead, this improvement should serve as an indicator for patients who will improve further with VA.31 Therefore, compliance with preoperative compression therapy seems to be an indicator of success when VA is pursued. However, definitive recommendations on the duration of compression therapy before VA are still lacking.
In addition, obesity was found to be a significant factor associated with LCI. Obese individuals were 47% more likely to experience LCI compared with patients with a normal body mass index. Prior studies have reported changes in vein biomechanics due to venous return obstruction secondary to increased abdominal adipose tissue as the basis for an increased risk of CVD in patients with obesity.35,36 Furthermore, patients with obesity often face challenges in adhering to compression therapy due to the difficulty associated with donning or wearing compression stockings due to body habitus, which might contribute to poor adherence to compression therapy and a decreased clinical response after VA. Moreover, the body mass index has been shown to negatively affect the CEAP clinical category, pain, and quality of life, independently of venous reflux.35 The body mass index has also been shown to be a significant factor associated with less improvement in the revised VCSS after VA.34,37
Vein diameter has been used as a coverage criterion for VA in approximately 50% of insurance policies in the United States.8 Nonetheless, practice guidelines do not include this parameter as a standalone indicator for treatment because a high level of evidence supporting treatment decisions solely based on this factor is still lacking.5 We found no association between vein size and clinical improvement in the present study. Prior studies have also demonstrated the great saphenous vein diameter to be a poor surrogate marker for evaluating the effects of CVD on patients' quality of life38 and to correlate poorly with VCSS improvement.39,40
Furthermore, to better understand the effects of VA, it is essential to assess patients' symptoms and their impact on quality of life because some treatment effects are not directly observable by clinicians. Vein recanalization has been reported in <10% of patients after 1 year of follow-up.41 However, recanalization found by duplex ultrasound does not necessarily lead to clinical recurrence or a return of symptoms and, therefore, is not always of clinical relevance.42 A recent study that evaluated recanalization and the reappearance of venous symptoms reported the discrepancy between these two variables.43 Although the incidence of vein recanalization was higher in patients presenting with LCI (1.1% vs 0.4%), the rates were very low overall. As such, its impact on assessing the clinical response after VA appears to be minimal. In contrast, an analysis to determine the factors associated with the failure of VA that incorporated LCI and technical failure was not possible due to the very low number of patients who experienced both.
Study limitations
The limitations of this study include those inherent to the retrospective analysis of an observational database. Specifically, there could be variations in data entry practices and follow-up protocols across different centers contributing to the registry, potentially introducing biases or inconsistencies in the dataset. Additionally, the analysis focused on patients undergoing isolated VA, excluding other concomitant procedures such as phlebectomy, which have shown potential to enhance outcomes. Furthermore, the study presents outcomes evaluated at discrete time points without a cumulative assessment. Moreover, the analysis was confined to a 6-month period, without examining patient outcomes beyond this period and without evaluating the effect of further interventions for patients with CVD recurrence. Finally, patients with CEAP class C1 disease were not included in this study cohort, and the value of VA for these patients was not studied.
Conclusions
LCI after VA is associated with treating patients with a less severe CEAP clinical classification (C2) and the lack of compression therapy. Importantly, no significant association between vein size and clinical improvement was observed.
Author Contributions
Conception and design: PP, AO, NO, SR, BJ, FA, KN, AG, LR, EF, US, CO
Analysis and interpretation: PP, MF, AO, NO, SR, BJ, FA, KN, AG, LR, EF, US, CO
Data collection: Not applicable
Writing the article: PP, AO, NO, SR, BJ, FA, KN, AG, LR, EF, US, CO
Critical revision of the article: PP, MF, AO, NO, SR, BJ, FA, KN, AG, LR, EF, US, CO
Final approval of the article: PP, MF, AO, NO, SR, BJ, FA, KN, AG, LR, EF, US, CO
Statistical analysis: PP
Obtained funding: Not applicable
Overall responsibility: CO
Disclosures
None.
Footnotes
Additional material for this article may be found online at www.jvsvenous.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
Additional material for this article may be found online at www.jvsvenous.org.
Supplementary Material
References
- 1.Brand F.N., Dannenberg A.L., Abbott R.D., Kannel W.B. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4:96–101. [PubMed] [Google Scholar]
- 2.Kim Y., Png C.Y.M., Sumpio B.J., DeCarlo C.S., Dua A. Defining the human and health care costs of chronic venous insufficiency. Semin Vasc Surg. 2021;34:59–64. doi: 10.1053/j.semvascsurg.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Farah M.H., Nayfeh T., Urtecho M., et al. A systematic review supporting the society for vascular surgery, the American venous forum, and the American vein and lymphatic society guidelines on the management of varicose veins. J Vasc Surg Venous Lymphat Disord. 2022;10:1155–1171. doi: 10.1016/j.jvsv.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Gloviczki P., Lawrence P.F., Wasan S.M., et al. The 2023 Society for Vascular Surgery American venous forum, and American vein and lymphatic society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II: endorsed by the society of interventional radiology and the society for vascular medicine. J Vasc Surg Venous Lymphat Disord. 2024;12 doi: 10.1016/j.jvsv.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Gloviczki P., Lawrence P.F., Wasan S.M., et al. The 2022 society for vascular surgery, American venous forum, and American vein and lymphatic society clinical practice guidelines for the management of varicose veins of the lower extremities. Part I. Duplex scanning and treatment of superficial truncal reflux: endorsed by the society for vascular medicine and the international union of phlebology. J Vasc Surg Venous Lymphat Disord. 2023;11:231–261.e6. doi: 10.1016/j.jvsv.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Epstein D., Bootun R., Diop M., Ortega-Ortega M., Lane T.R.A., Davies A.H. Cost-effectiveness analysis of current varicose veins treatments. J Vasc Surg Venous Lymphat Disord. 2022;10:504–513.e7. doi: 10.1016/j.jvsv.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Gohel M.S., Heatley F., Liu X., et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378:2105–2114. doi: 10.1056/NEJMoa1801214. [DOI] [PubMed] [Google Scholar]
- 8.Pinto P., Fukaya E., Rodriguez L.E., et al. Variations and inconsistencies in venous ablation coverage policies between single-state and multistate carriers in the United States. J Vasc Surg Venous Lymphat Disord. 2024;12 doi: 10.1016/j.jvsv.2023.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Gabel J., O'Dell T., Masuda E., et al. Who is treating venous disease in America today? J Vasc Surg Venous Lymphat Disord. 2019;7:610–614. doi: 10.1016/j.jvsv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar A.M., Misono A.S., Sheth R.A., et al. Changing medicare utilization of minimally invasive procedures for the treatment of chronic venous insufficiency. J Vasc Interv Radiol. 2017;28:818–824. doi: 10.1016/j.jvir.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Masuda E., Ozsvath K., Vossler J., et al. The 2020 appropriate use criteria for chronic lower extremity venous disease of the American venous forum, the society for vascular surgery, the American vein and lymphatic society, and the society of interventional radiology. J Vasc Surg Venous Lymphat Disord. 2020;8:505–525.e4. doi: 10.1016/j.jvsv.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Schul M.W., King T., Kabnick L.S. Inequalities of health insurance guidelines for the treatment of symptomatic varicose veins. Phlebology. 2014;29:236–246. doi: 10.1177/0268355513479589. [DOI] [PubMed] [Google Scholar]
- 13.Welch H.J., Schul M.W., Monahan D.L., Iafrati M.D. Private payers' varicose vein policies are inaccurate, disparate, and not evidence based, which mandates a proposal for a reasonable and responsible policy for the treatment of venous disease. J Vasc Surg Venous Lymphat Disord. 2021;9:820–832. doi: 10.1016/j.jvsv.2020.12.076. [DOI] [PubMed] [Google Scholar]
- 14.Ochoa Chaar C.I., Aurshina A., Zhang Y., et al. The effect of commercial insurance policies on outcomes of venous ablation. J Vasc Surg Venous Lymphat Disord. 2018;6:331–337.e1. doi: 10.1016/j.jvsv.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Welch H.J., Kabnick L., Vasquez M.A., Monahan D.L., Lurie F., Jacobowitz G. Proposal for a national coverage determination for the treatment of varicose veins and venous disease due to disparate Centers for Medicare and Medicaid Services local coverage determination policies. J Vasc Surg Venous Lymphat Disord. 2017;5:453–459. doi: 10.1016/j.jvsv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Catarinella F.S., Nieman F.H.M., Wittens C.H.A. An overview of the most commonly used venous quality of life and clinical outcome measurements. J Vasc Surg. 2015;3:333–340. doi: 10.1016/j.jvsv.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Vasquez M.A., Munschauer C.E. Revised venous clinical severity score: a facile measurement of outcomes in venous disease. Phlebology. 2012;27(Suppl 1):119–129. doi: 10.1258/phleb.2012.012s16. [DOI] [PubMed] [Google Scholar]
- 18.Paty J., Elash C.A., Turner-Bowker D.M. Content validity for the VVSymQ((R)) instrument: a new patient-reported outcome measure for the assessment of varicose veins symptoms. Patient. 2017;10:51–63. doi: 10.1007/s40271-016-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronenwett J.L., Kraiss L.W., Cambria R.P. The society for vascular surgery vascular quality initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez M.A., Rabe E., McLafferty R.B., et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387–1396. doi: 10.1016/j.jvs.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 21.Kakkos S.K., Rivera M.A., Matsagas M.I., et al. Validation of the new venous severity scoring system in varicose vein surgery. J Vasc Surg. 2003;38:224–228. doi: 10.1016/s0741-5214(03)00323-9. [DOI] [PubMed] [Google Scholar]
- 22.Passman M.A., McLafferty R.B., Lentz M.F., et al. Validation of venous clinical severity score (VCSS) with other venous severity assessment tools from the American venous forum, national venous screening program. J Vasc Surg. 2011;54(6 Suppl):2S–9S. doi: 10.1016/j.jvs.2011.05.117. [DOI] [PubMed] [Google Scholar]
- 23.Brown C.S., Obi A.T., Cronenwett J.L., Kabnick L., Wakefield T.W., Osborne N.H. Outcomes after truncal ablation with or without concomitant phlebectomy for isolated symptomatic varicose veins (C2 disease) J Vasc Surg Venous Lymphat Disord. 2021;9:369–376. doi: 10.1016/j.jvsv.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheirelseid E.A.H., Crowe G., Sehgal R., et al. Systematic review and meta-analysis of randomized controlled trials evaluating long-term outcomes of endovenous management of lower extremity varicose veins. J Vasc Surg: Venous and Lymphatic Disorders. 2018;6:256–270. doi: 10.1016/j.jvsv.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen L., Lawaetz M., Bjoern L., Blemings A., Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg. 2013;58:421–426. doi: 10.1016/j.jvs.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Lawaetz M., Serup J., Lawaetz B., et al. Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT. Int Angiol. 2017;36:281–288. doi: 10.23736/S0392-9590.17.03827-5. [DOI] [PubMed] [Google Scholar]
- 27.Vähäaho S., Halmesmäki K., Albäck A., Saarinen E., Venermo M. Five-year follow-up of a randomized clinical trial comparing open surgery, foam sclerotherapy and endovenous laser ablation for great saphenous varicose veins. Br J Surg. 2018;105:686–691. doi: 10.1002/bjs.10757. [DOI] [PubMed] [Google Scholar]
- 28.Wallace T., El-Sheikha J., Nandhra S., et al. Long-term outcomes of endovenous laser ablation and conventional surgery for great saphenous varicose veins. Br J Surg. 2018;105:1759–1767. doi: 10.1002/bjs.10961. [DOI] [PubMed] [Google Scholar]
- 29.Michaels J.A., Brazier J.E., Campbell W.B., MacIntyre J.B., Palfreyman S.J., Ratcliffe J. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006;93:175–181. doi: 10.1002/bjs.5264. [DOI] [PubMed] [Google Scholar]
- 30.Sell H., Vikatmaa P., Albäck A., et al. Compression therapy versus surgery in the treatment of patients with varicose veins: a RCT. Eur J Vasc Endovasc Surg. 2014;47:670–677. doi: 10.1016/j.ejvs.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Lurie F., Kistner R.L. Trends in patient reported outcomes of conservative and surgical treatment of primary chronic venous disease Contradict current practices. J Vasc Surg. 2011;54:1536. doi: 10.1097/SLA.0b013e31821d4a5f. [DOI] [PubMed] [Google Scholar]
- 32.Attaran R.R., Ochoa Chaar C.I. Compression therapy for venous disease. Phlebology. 2017;32:81–88. doi: 10.1177/0268355516633382. [DOI] [PubMed] [Google Scholar]
- 33.Kakkos S.K., Timpilis M., Patrinos P., et al. Acute effects of graduated elastic compression stockings in patients with symptomatic varicose veins: a randomised double blind placebo controlled trial. Eur J Vasc Endovasc Surg. 2018;55:118–125. doi: 10.1016/j.ejvs.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kobata T., Kasamaki Y., Kanda T. Personal factors and postoperative changes in the revised Venous Clinical Severity Score of varicose veins. J Vasc Surg Venous Lymphat Disord. 2023;11:31–38. doi: 10.1016/j.jvsv.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Donbaloğlu M.O. Does body mass index have an effect on clinical and pain after endovenous therapy? A retrospective study. Vascular. 2022;30:969–976. doi: 10.1177/17085381211036556. [DOI] [PubMed] [Google Scholar]
- 36.Wall M.L. University of Birmingham; 2011. The effect of obesity on venous impedance and outflow measured by ultrasound. [Google Scholar]
- 37.Deol Z.K., Lakhanpal S., Franzon G., Pappas P.J. Effect of obesity on chronic venous insufficiency treatment outcomes. J Vasc Surg Venous Lymphat Disord. 2020;8:617–628.e1. doi: 10.1016/j.jvsv.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Gibson K., Meissner M., Wright D. Great saphenous vein diameter does not correlate with worsening quality of life scores in patients with great saphenous vein incompetence. J Vasc Surg. 2012;56:1634–1641. doi: 10.1016/j.jvs.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 39.Attaran R.R., Bhalla A., Mena-Hurtado C.I., Ochoa Chaar C.I. Correlation between great saphenous length of treatment zone and diameter with improvement in symptoms after ablation. J Vasc Surg Venous Lymphat Disord. 2021;9:1443–1450. doi: 10.1016/j.jvsv.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Bendix S.D., Peterson E.L., Kabbani L.S., Weaver M.R., Lin J.C. Effect of endovenous ablation assessment stratified by great saphenous vein size, gender, clinical severity, and patient-reported outcomes. J Vasc Surg Venous Lymphat Disord. 2021;9:128–136. doi: 10.1016/j.jvsv.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Rass K., Frings N., Glowacki P., et al. Comparable effectiveness of endovenous laser ablation and high ligation with stripping of the great saphenous vein: two-year results of a randomized clinical trial (RELACS study) Arch Dermatol. 2012;148:49–58. doi: 10.1001/archdermatol.2011.272. [DOI] [PubMed] [Google Scholar]
- 42.Lomazzi C., Bissacco D., Logan M.S., et al. Risk factors for saphenous vein recanalization after endovenous radiofrequency ablation. J Cardiovasc Surg. 2021;62:427–434. doi: 10.23736/S0021-9509.21.11908-1. [DOI] [PubMed] [Google Scholar]
- 43.Bissacco D., Stegher S., Calliari F., Casana R., Trimarchi S., Viani M.P. Relationship between great saphenous vein recanalization, venous symptoms reappearance, and varicose veins recurrence rates after endovenous radiofrequency ablation. Phlebology. 2022;37:686–688. doi: 10.1177/02683555221114537. [DOI] [PubMed] [Google Scholar]