Abstract
Background
Data on complications after upper extremity vein thrombosis (UEVT) are limited and heterogeneous.
Methods
The aim of the present study was to evaluate the pooled proportions of venous thromboembolism (VTE) recurrence, bleeding, and post-thrombotic syndrome (PTS) in patients with UEVT. A systematic literature review was conducted of PubMed, Embase, and the Cochrane Library databases from January 2000 to April 2023 in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines. All studies included patients with UEVT and were published in English. Meta-analyses of VTE recurrence, bleeding, and of PTS after UEVT were performed to compute pooled estimates and associated 95% confidence intervals (CIs). Subgroup analyses of cancer-associated UEVT and catheter-associated venous thrombosis were conducted. Patients with Paget-Schroetter syndrome or effort thrombosis were excluded.
Results
A total of 55 studies with 15,694 patients were included. The pooled proportions for VTE recurrence, major bleeding, and PTS were 4.8% (95% CI, 3.8%-6.2%), 3.0% (95% CI, 2.2%-4.0%), and 23.8% (95% CI, 17.0%-32.3%), respectively. The pooled proportion of VTE recurrence was 2.7% (95% CI, 1.6%-4.6%) for patients treated with direct oral anticoagulants (DOACs), 1.7% (95% CI, 0.8%-3.7%) for patients treated with low-molecular-weight heparin (LMWH), and 4.4% (95% CI, 1.5%-11.8%) for vitamin K antagonists (VKAs; P = .36). The pooled proportion was 6.3% (95% CI, 4.3%-9.1%) for cancer patients compared with 3.1% (95% CI, 2.1%-4.6%) for patients without cancer (P = .01). The pooled proportion of major bleeding for patients treated with DOACs, LMWH, and VKAs, was 2.1% (95% CI, 0.9%-5.1%), 3.2% (95% CI, 1.4%-7.2%), and 3.4% (95% CI, 1.4%-8.4%), respectively (P = .72). The pooled proportion of PTS for patients treated with DOACs, LMWH, and VKAs was 11.8% (95% CI, 6.5%-20.6%), 27.9% (95% CI, 20.9%-36.2%), and 24.5% (95% CI, 17.6%-33.1%), respectively (P = .02).
Conclusions
The results from this study suggest that UEVT is associated with significant rates of PTS and VTE recurrence. Treatment with DOACs might be associated with lower PTS rates than treatment with other anticoagulants.
Keywords: Bleeding, Cancer, Meta-analysis, Post-thrombotic syndrome, Upper extremity vein thrombosis, Venous catheter
Article Highlights.
-
•
Type of Research: A systematic review and meta-analysis
-
•
Key Findings: After upper extremity vein thrombosis, the venous thromboembolism (VTE) recurrence, major bleeding complications, and post-thrombotic syndrome rates were 4.8%, 3.0%, and 23.8%, respectively.
-
•
Take Home Message: The pooled proportion of VTE recurrence was 2.7%, 1.7%, and 4.4% for patients treated with direct oral anticoagulants, low-molecular-weight heparin, and vitamin K antagonists, respectively. The rate of VTE recurrence was higher in cancer patients than in patients without cancer (6.3% vs 3.1%). The pooled proportion of post-thrombotic syndrome was lower for patients treated with direct oral anticoagulants than for patients treated with low-molecular-weight heparin or vitamin K antagonists (11.8% vs 27.9% vs 24.5%).
The incidence of upper extremity venous thrombosis (UEVT) has been increasing in recent years, accounting for ≤10% of all cases of venous thromboembolism (VTE).1, 2, 3, 4 UEVT occurs in very different clinical contexts. Most UEVT cases can be attributed to secondary causes, such as venous catheter placement and malignancy5; however, UEVT can also occur suddenly after physical exercise, such as in Paget-Schroetter syndrome.
Patients with UEVT secondary to venous thoracic outlet syndrome require specific management that combines surgery, fibrinolysis, and anticoagulant therapy.6 This management is very different from that for other patients with UEVT for whom treatment is poorly codified. Few data are available regarding the risk of thrombosis recurrence and bleeding and the prevalence of post-thrombotic syndrome (PTS) after treatment of UEVT. In addition, anticoagulant treatments have evolved, with the increasing use of direct oral anticoagulants (DOACs).
For patients with UEVT, the modalities and duration of anticoagulant treatment must be balanced against the risk of bleeding complications, especially in specific high-risk groups such as those with cancer, for whom major bleeding events were reported in ≤10% of cases.7 To better determine the prognosis of patients with UEVT and adapt patient management, it is important to first evaluate the frequency of UEVT complications. To the best of our knowledge, no randomized controlled trials have evaluated the efficacy and safety of anticoagulant treatment for UEVT.
The objective of this systematic review and meta-analysis was to evaluate the pooled proportions of PTS, VTE recurrence, and bleeding complications in a population of patients with UEVT. An analysis of high-risk patients with cancer and patients with thrombosis associated with central venous catheter (CVC) was also performed.
Methods
Search strategy
A systematic literature search of the PubMed, Embase, and Cochrane electronic databases were conducted with restrictions to adults aged >18 years and studies published in English. The search strategies were adapted for each database to include database-specific thesaurus terms and field names.
The search queries were developed using combinations of subject headings and free text words, including upper extremity vein thrombosis, upper extremity deep vein thrombosis, thoracic outlet syndrome, upper extremity, axillary vein, subclavian vein, vascular access device, catheterization, peripheral, venous thromboembolism, post-thrombotic syndrome, prevalence, bleeding, and recurrence. The search strategy is shown in the Appendix (online only). To identify additional relevant studies that met our inclusion criteria, the references of the relevant retrieved reports were also examined.
Criteria for study inclusion
Observational studies (cohort and case-control studies), randomized control trials, and case series were included if they included >10 patients. The included studies must have objectively diagnosed UEVT via venography, computed tomography, magnetic resonance imaging, and/or ultrasound and included only patients aged ≥18 years. UEVT was defined via ultrasound as incompressible material in the venous lumen or the lack of venous flow modulation for proximally located thromboses. For computed tomography and magnetic resonance imaging, UEVT was defined as endovenous material of a clot nature. Tumor occlusions were excluded. During venography, UEVT was considered present if the contrast medium stopped, indicating venous thrombus. Eligible studies reported from January 1, 2000, to April 30, 2023, were analyzed.
Letters to the editor, review articles, editorials, and commentaries were excluded. Studies reporting UEVT due to Paget-Schroetter syndrome or exertional venous thrombosis were also excluded.
Study selection and data extraction
Two reviewers (O.E. and B.P.) independently examined the retrieved studies for possible inclusion by assessing the study title and abstract. Any disagreements were resolved by consensus or consulting a third reviewer (O.S.). The potentially relevant studies were marked for full-text review.
The studies selected for further review were evaluated in detail in accordance with the inclusion criteria and outcome measures. Using a standardized data extraction form, three reviewers (O.E., B.P., A.R.) independently collected data on the number of included patients with UEVT, the association of UEVT with secondary causes (ie, venous catheter [CVC], malignancy), choice of treatment (ie, anticoagulation, thrombolytic therapy), and the occurrence of PTS, recurrent VTE, and bleeding. Study data quality, including enrollment of patients, authors, year of publication, country, study design, and duration of follow-up for each study were also noted. All studies selected for final inclusion in the systematic review were also evaluated by the lead author, and any disagreements regarding data were resolved by discussion and consensus. Upper extremity deep vein thrombosis was defined according to the following thrombosis locations: jugular vein, innominate vein, subclavian vein, axillary vein, and brachial vein.
Outcome measures
The objective of this study was to evaluate the pooled proportions of PTS, VTE recurrence, and bleeding complications in a population of patients with UEVT stratified by the type of anticoagulant treatment, presence of cancer, and presence of an upper limb CVC. Unprovoked UEVT was defined by the absence of a catheter, cancer, or recent surgery. The outcome measures were recurrent VTE objectively confirmed by imaging, PTS, and bleeding events.
PTS in the individual studies was defined by the investigators according to the clinical assessment and using the modified Villalta scale (score ≥4) when it was reported. PTS was evaluated at the completion of follow-up. VTE recurrence was defined as a new UEVT in a previously unaffected venous segment, a new pulmonary embolism, or new lower limb deep vein thrombosis proven by computed tomography and/or ultrasound. Major, minor, and clinically relevant nonmajor bleeding events were analyzed when reported using the criteria of the International Society on Thrombosis and Haemostasis.8,9
The following data were extracted using predesigned forms: number of patients, study characteristics, follow-up duration, UEVT etiology, cancer, presence of a CVC, type of treatment, recurrent VTE, PTS, and bleeding complications during anticoagulant therapy. All clinical outcomes were extracted for the overall study population and for patient subgroups.
Assessment of study quality
The risk of bias was assessed by two independent investigators (O.E. and A.R.). The Cochrane Collaboration risk of bias tool, version 2, and an adapted version of the Newcastle-Ottawa scale were used for the randomized control trials and observational studies, respectively10,11 For observational cohort studies, the risk of bias was determined for three categories: selection, comparability, and outcome. For observational case-control studies, three similar categories—selection, comparability, and exposure—were assessed.
Group definitions
The outcomes of VTE recurrence, bleeding, and PTS were analyzed sequentially according to the presence of cancer and the presence of a CVC. In a population of patients with UEVT, this study compared VTE recurrence, bleeding, and PTS stratified by the type of anticoagulant treatment (DOACs, VKAs, LMWH), presence of cancer, presence of a CVC, and duration of anticoagulant treatment (<3 months vs ≥ 3 months).
For the subgroup analyses of UEVT associated with cancer or a CVC, only studies with 100% of patients with cancer or a CVC were analyzed. When multiple patient subgroups were included in the same study, each individualized subgroup was analyzed independently.
Statistical analysis
Pooled estimates and the associated 95% confidence intervals (CIs) were calculated using random effects (RE) models for all three outcomes: VTE recurrence, bleeding, and PTS. Statistical heterogeneity was assessed using the χ2 Cochran Q test and I2 index, which measures the percentage of variation between trials due to heterogeneity.12 An I2 value of 25% corresponds to low, 25% to 50% to moderate, and 50% to high heterogeneity. Subgroup analyses of the treatment strategy, presence of cancer, and presence of a CVC were also conducted. Publication bias was evaluated using funnel plots of the logit event rate against standard error and an Egger's test at a 5% significance threshold.13 Asymmetry patterns and estimate distortion were explored. All analyses were conducted in R with the {meta} package (R Foundation for Statistical Computing).
Ethics and registration
This study is reported in agreement with the PRISMA (preferred reporting items for a systematic review and meta-analysis) statement. No ethical approval or patient informed consent was required. This meta-analysis was performed in accordance with the Cochrane's Handbook guidelines and MOOSE (meta-analysis of observational studies in epidemiology).14 The protocol for this systematic review was registered in PROSPERO (International Prospective Register of Systematic Reviews; CRD 42021249053).
Results
Study selection and characteristics
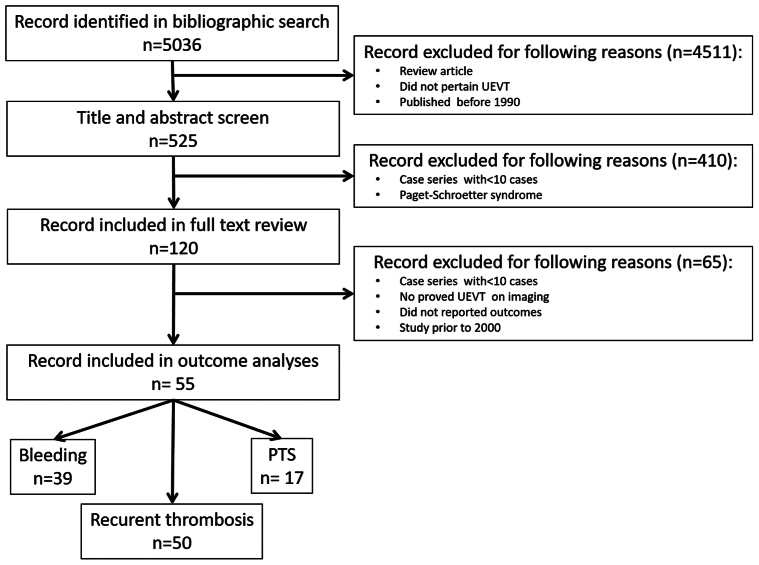
We identified 5036 citations (Fig 1) through our initial literature search and included 120 studies in our full-text review. A total of 55 studies met our inclusion criteria with relevant data on our outcomes and were included in the final meta-analysis. The 55 studies included 15,594 patients diagnosed with UEVT; 16 studies were prospective and 15 were multicenter studies. The characteristics of the included studies are presented in Supplementary Table I (online only). Of these, 50 studies reported data on the occurrence of VTE recurrence,7,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 17 reported PTS,18, 19, 20, 21, 22,26,29,32,34,39,41,45,48,58,62,64,65 and 39 reported bleeding.7,15,18,23,26, 27, 28,30, 31, 32,35,36,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48,51, 52, 53, 54, 55, 56, 57, 58, 59,61, 62, 63,66, 67, 68, 69 Only 10 studies evaluated VTE recurrence, bleeding, and PTS.18,26,32,39,41,45,48,58,62 We did not identify any randomized controlled trials. All included studies were observational cohort studies. Unprovoked thrombosis accounted for 16.7% of UEVT in 42 studies.7,16,17,19, 20, 21,23, 24, 25, 26, 27,29, 30, 31,33,34,36, 37, 38,40, 41, 42, 43, 44, 45, 46, 47, 48,51, 52, 53, 54, 55,59, 60, 61, 62, 63,65, 66, 67,69 Of the 15,594 patients, 51% were women. The mortality rate was 14.1% (range, 0%-78.1%).
Fig 1.
Flow diagram displaying the number of studies screened, assessed in-depth for eligibility, and included in the review and specific outcome analyses. UEVT, Upper extremity vein thrombosis.
The duration of anticoagulation therapy was available from 45 studies, and 88.9% of these studies reported an anticoagulant duration of ≥3 months (Supplementary Table I, online only). For the five studies with shorter durations, three were of thrombosis associated with CVCs and two reported superficial venous thrombosis. Of the 55 studies, 45 reported the location of UEVT, 41 had included only patients with upper extremity deep vein thrombosis, and 1 series presented 57 patients with only superficial UEVT.
Study quality
Key quality features and the quality assessment of each study are presented in Supplementary Tables I and II and Supplementary Fig 1 (online only).
VTE recurrence
VTE recurrence was described in 50 studies with 13,342 patients. The pooled proportion of VTE recurrence using a RE model was 4.8% (95% CI, 3.8-6.2).
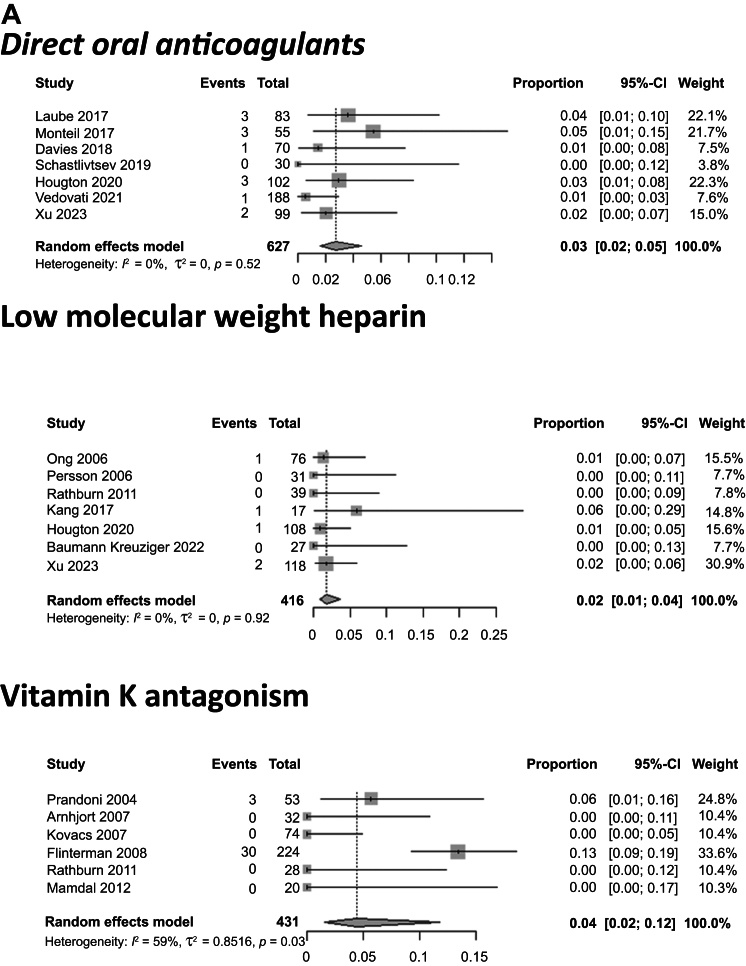
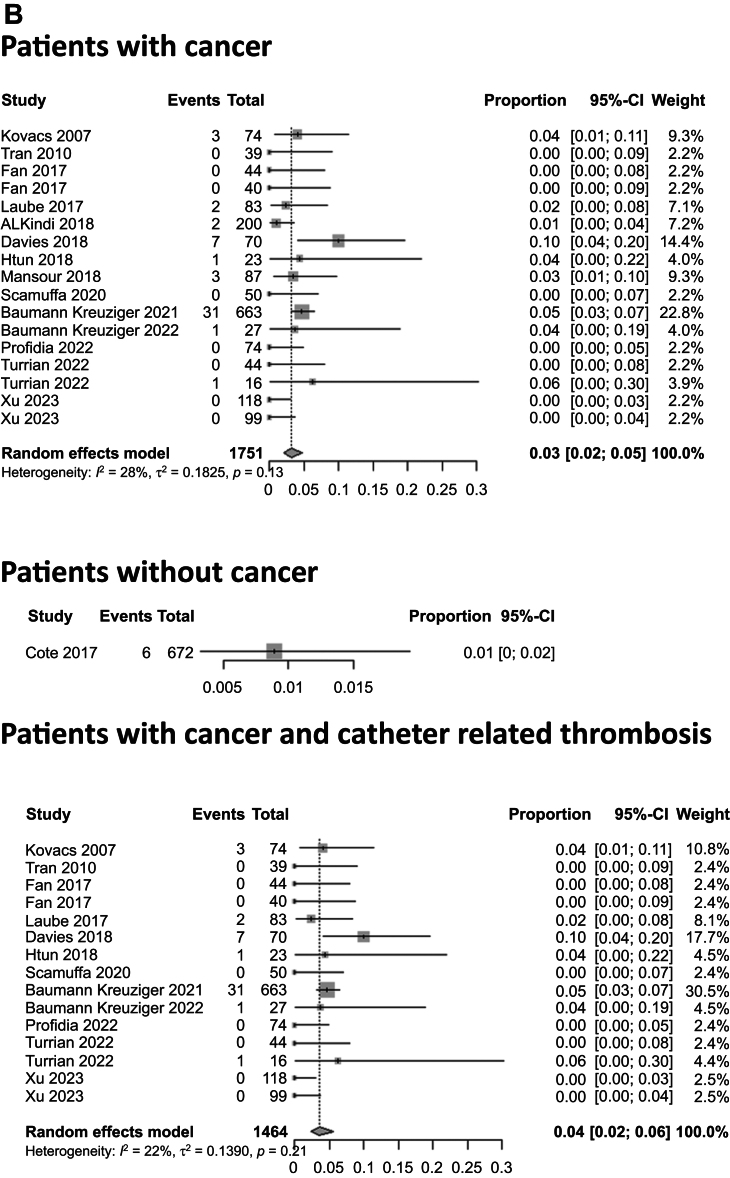
Stratified by the type of anticoagulant treatment, the pooled proportions for VTE recurrence for patients treated with DOACs, LMWH, and VKAs were 2.7% (95% CI, 1.6%-4.6%), 1.7% (95% CI, 0.8%-3.7%), and 4.4% (95% CI, 1.5%-11.8%), respectively (P = .36; Fig 2). The pooled proportions of patients with VTE recurrence also differed when stratified by the clinical context. For patients with cancer, the VTE recurrence rate was 6.3% (95% CI, 4.3%-9.1%) vs 3.1% (95% CI, 2.1%-4.6%) for those without cancer (P = .01; Fig 2). For patients with CVC-related thrombosis, the VTE recurrence rate was 5.3% (95% CI, 3.6%-7.9%) vs 5.5% (95% CI, 3.5%-8.6%) for patients with cancer and CVC-related thrombosis (P = .07) and 2.9% (95% CI, 2.1%-3.9%) for those without a CVC (P = .02; Fig 2 and Supplementary Fig 2, online only). Regarding the treatment duration, patients with anticoagulation treatment for <3 months had a recurrence rate of 1.5% (95% CI, 0.2%-9.9%) vs 4.5% (95% CI, 2.6%-7.8%) for patients with anticoagulation treatment for >3 months (P = .28). The median follow-up in these studies was 12.6 months (range, 0.23-60 months).
Fig 2.
Pooled proportions of patients with venous thromboembolism (VTE) recurrence after upper extremity vein thrombosis (UEVT). A, VTE recurrence rate for patients treated with direct oral anticoagulants (DOACs), low-molecular-weight heparin (LMWH), and vitamin K antagonists (VKAs) was 3%, 2%, and 4%, respectively. B, VTE recurrence rate for patients with cancer, patients without cancer, and patients with cancer and central venous catheter (CVC)-related thrombosis was 6%, 3%, and 5%, respectively. CI, Confidence interval.
Bleeding complications
The overall pooled proportion of patients with major bleeding was calculated from 39 studies (12,811 patients) and was estimated at 3.0% (95% CI, 2.2%-4.0%) using an RE model.
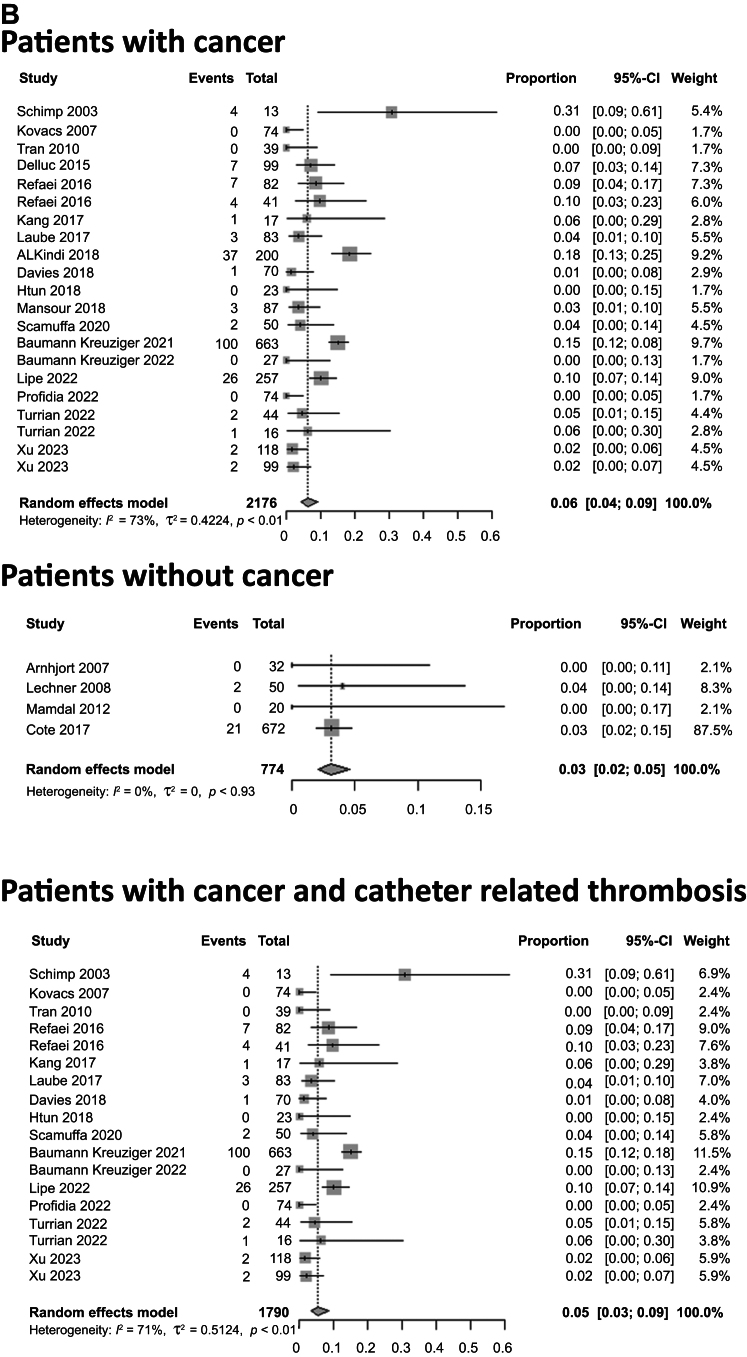
The pooled proportion of major bleeding for patients treated with DOACs, LMWH, and VKAs was 2.1% (95% CI, 0.9%-5.1%), 3.2% (95% CI, 1.4%-7.2%), and 3.4% (95% CI, 1.4%-8.4%), respectively (P = .72; Fig 3). The pooled proportion of major bleeding was 3.2% (95% CI, 2.1%-4.8%) for patients with cancer and 0.9% (95% CI, 0.4%-2.0%) for patients without cancer (P = .006). The pooled proportion of major bleeding for patients with CVC-related thrombosis was 3.8% (95% CI, 2.3%-6.2%) and was 3.6% (95% CI, 2.3%-5.6%) for patients with cancer and CVC-related thrombosis and 1.8% (95% CI, 0.5%-6.0%) for patients without cancer and with non–CVC-related thrombosis (P = .002; Fig 3 and Supplementary Fig 3, online only). For patients with anticoagulation treatment for <3 months, the pooled proportion of major bleeding was 1.9% (95% CI, 0.5%-6.3%) compared with 1.9% (95% CI, 0.6%-6.5%) for patients with anticoagulation >3 months (P = .98). The pooled proportion for minor and clinically relevant non–major bleeding was 6.3% (95% CI, 4.7%-8.3%). The pooled proportion of minor and major bleeding for those with cancer was 6.1% (95% CI, 4.0%-9.1%; Supplementary Table III, online only). The median follow-up for these studies was 11.7 months (range, 0.23-42 months).
Fig 3.
Pooled proportions of patients with major bleeding after upper extremity vein thrombosis (UEVT). A, Incidence of bleeding in patients treated with direct oral anticoagulants (DOACs), low-molecular-weight heparin (LMWH), and vitamin K antagonists (VKAs) was 2%, 3%, and 3%, respectively. B, Incidence of bleeding in patients with cancer, patients without cancer, and patients with cancer and central venous catheter (CVC)-related thrombosis was 3%, 1%, and 4%, respectively. CI, Confidence interval.
Post-thrombotic syndrome
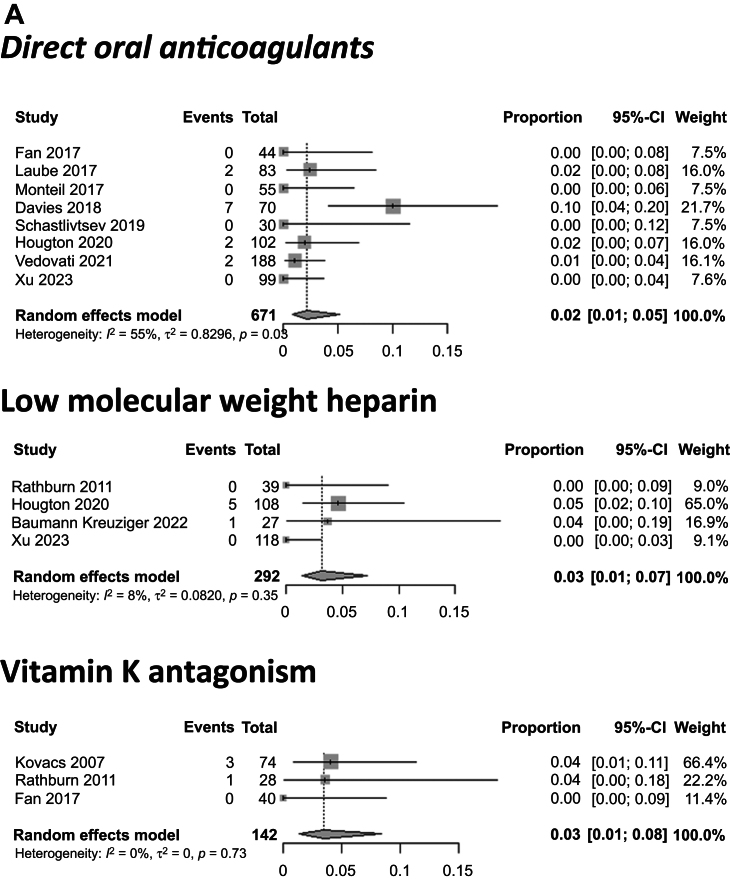
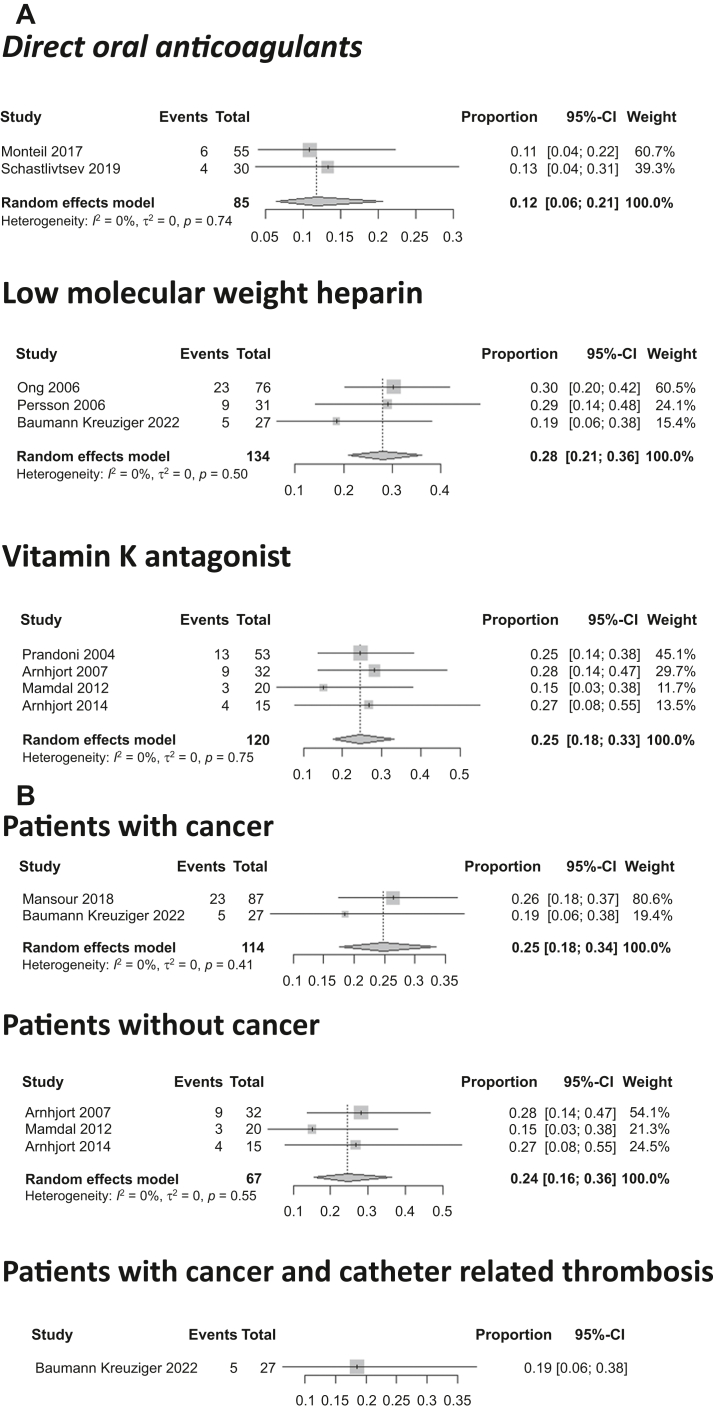
The occurrence of PTS was described in 17 studies with 692 patients. Of these studies, 13 (75.6%) used the modified Villalta score, 3 did not specify the scale used and reported physical and functional signs of PTS manifestation in the upper limbs, and 1 used the DASH (disabilities of the arm, shoulder, and hand) score. The pooled proportion for the overall population using an RE model was 23.3% (95% CI, 16.6%-31.7%). The pooled proportion of PTS for patients treated with DOACs, LMWH, and VKAs was 11.8% (95% CI, 6.5%-20.6%), 27.9% (95% CI, 20.9%-36.2%), and 24.5% (95% CI, 17.6%-33.1%), respectively (P = .02; Fig 4). The median follow-up for these studies was 21 months (range, 3.25-120 months).
Fig 4.
Pooled proportions of patients with post-thrombotic syndrome (PTS) after upper extremity vein thrombosis (UEVT). A, PTS rate for patients treated with direct oral anticoagulants (DOACs), low-molecular-weight heparin (LMWH), and vitamin K antagonists (VKAs) was 12%, 28%, and 25%, respectively. B, PTS rate for patients with cancer, patients without cancer, and patients with cancer and central venous catheter (CVC)-related thrombosis was 25%, 24%, and 19%, respectively. CI, Confidence interval.
Exploration for publication bias
Contour-enhanced funnel plots for each outcome are presented in Supplementary Fig 1, A (online only). The funnel plots contain three shaded regions outlining the significance level of each study. The examination of the occurrence of major bleeding suggested the absence of a publication bias and small-study effect (confirmed by Egger's test; P = .27). A statistical analysis of the occurrence of PTS was not feasible owing to the small number of included studies.
Publication bias was suspected for VTE recurrence, and Egger's test was close to significant (P = .06). The associated I2 was 84.3%, indicating relatively high heterogeneity for this outcome. Two trim-and-fill analyses were conducted to evaluate such effects70: one for all studies and a sensitivity analysis that excluded outliers. The estimates of the corrected effect (6.9% [95% CI, 5.4%-8.6%] and 6.2% [95% CI, 5.0%-7.6%]) were close but were slightly higher than the pooled effect from the principal analysis owing to small-study effects (Supplementary Fig 1, B and C, online only).
Discussion
To the best of our knowledge, the present study is the largest systematic review of UEVT complications and the first to specifically investigate patients with cancer and a CVC associated with thrombosis. The main findings of this study are (1) the pooled proportion of VTE recurrence was 2.7%, 1.7%, and 4.4% for patients treated with DOACs, LMWH, and VKAs, respectively; (2) the rate of VTE recurrence was higher for cancer patients than for patients without cancer (6.3% vs 3.1%); (3) the rate of major bleeding complications stratified by the type of anticoagulant were close (DOACs, 2.1%; LMWH, 3.2%; VKAs, 3.4%); and (4) the pooled proportion of PTS for patients treated with DOACs was lower (11.8%) than that for patients treated with LMWH or VKAs (27.9% and 24.5%, respectively).
This study specifies the effects of complications of anticoagulant treatment in a specific well-selected population of UEVT. Unlike Paget-Schroetter syndrome, for which surgical decompression of the thoracic outlet as adjunctive therapy could reduce the risk of thromboembolism recurrence and PTS in patients with venous thoracic outlet syndrome,71,72 surgical management could not be evaluated in our population because of the very low frequency of surgery for UEVT. In this study, we found no negative indicators for the use of DOACs to treat UEVT, with a low risk of VTE recurrence, major bleeding, and PTS. However, randomized controlled trials comparing DOACs with other anticoagulants are lacking. This difference could be explained by better patient compliance and treatment efficacy instead of a better therapeutic index. DOACs appeared to be similar to other anticoagulant therapies in terms of both effectiveness and safety.73
A higher pooled proportion of VTE recurrence was observed in the cancer population, similar to that for other venous thrombosis locations.74 However, this study is the first to show that patients with UEVT secondary to a CVC have a greater VTE recurrence rate than do those without a CVC. It is possible that the catheters induce sequelae in the venous wall that favor recurrence, just as the underlying disease that led to placement of the CVC could also favor VTE recurrence, such as active malignancy. In addition, CVC reinsertion could present an ongoing risk for VTE recurrence. However, the rate of catheter removal, which reduces vessel injury and minimizes the risk of developing venous sequelae, was not available in many studies or was reported using different statistical approaches not appropriate for meta-analysis.
In this study, patients with an anticoagulation duration of >3 months had more VTE recurrence than did patients with a shorter duration, which can be explained by the persistence of thrombosis-promoting factors that prolonged anticoagulation therapy and, thus, increased the risk of recurrence. Moreover, the presence of an active malignancy or CVC reinsertion, both risk factors for recurrent VTE, could not be evaluated owing to a lack of information.
Regarding the risk of bleeding in this population, patients treated with DOACs seemed to have lower bleeding rates than patients treated with other anticoagulants. For patients with cancer and those with CVC-associated thrombosis, the risk of bleeding appears to be increased, which probably results from the presence of multiple comorbidities and aggressive cancer management.
Only six studies were performed of non–Western patient cohorts. Most studies did not specify the race of the patients; however, there could be variability, such as a higher incidence of VTE in African-American patients.75 UEVT was not more frequent in women (51%), unlike other VTE locations. It is possible that the role of cancer and the presence of a CVC are more important than gender in the occurrence of UEVT.75
Study limitations
These results should be interpreted with caution because this study has some limitations. The quality of the studies is poor, with no randomized controlled trials. Most studies are cohort studies with heterogeneous follow-up methods. The large number of different clinical situations favors heterogeneity in therapeutic management with various types and durations of anticoagulation. In addition, we found great variability in the incidence of CVC removal. Fifteen studies appeared to be the most informative because of their prospective method, quality of the selection and analyses, or their large patient numbers.7,23,32,36,38,40,43,44,46,51,52,56,58,62,68 The higher recurrence rate for patients with anticoagulation therapy for >3 months is most likely related to a bias secondary to the presence of persistent thrombosis recurrence risk factors that caused anticoagulation to be maintained. Concerning follow-up, most of the studies had ≥3 months of follow-up; however, several studies did not specify the follow-up duration and/or patients lost to follow-up, limiting their validity. For bleeding, several studies did not report events using the International Society on Thrombosis and Haemostasis criteria. Moreover, no definition of PTS in the upper limb has been established, which limits comparisons. Regarding the meta-analysis, pooled estimates from all three outcomes were associated with the presence of relatively high statistical heterogeneity. The results from the subgroup analyses seemed to suggest such heterogeneity was due to cancer and/or CVC status. An assessment of the outliers' effect was explored and led to close estimates compared with the pooled effects from the principal analysis, with lower heterogeneity. Also, information for the subgroups was not always available and led to the exclusion of studies owing to the lack of evidence. For UEVT, because of the lack of randomized studies, the difference in the outcomes between different treatment modalities could not be directly compared because the difference could have resulted from a confounding bias. Studies using one treatment might have had patients with different characteristic than studies using different treatment strategies. These factors include CVC removal and anticoagulation duration. Thus, it was difficult to compare studies and to compare data within studies, and caution should prevail when interpreting these results.
Conclusions
The results from the current study suggest that UEVT is associated with a 4.8% rate of VTE recurrence, 3.0% rate of major bleeding, and 23.3% rate of PTS. Patients treated with DOACs seem to develop PTS less often than patients treated with LMWH or VKAs. The observed rate of major bleeding was 2.1% for patients treated with DOACs. Further prospective studies of UEVT are needed to better investigate the association of PTS, bleeding complications, and VTE recurrence.
Author Contributions
Conception and design: OE, SK, JM
Analysis and interpretation: OE, AR, BP, OS, AB, JM
Data collection: OE, AR, BP, OS, BE, AB
Writing the article: OE, JM
Critical revision of the article: OE, AR, BP, SK, OS, BE, AB, JM
Final approval of the article: OE, AR, BP, SK, OS, BE, AB, JM
Statistical analysis: SK, JM
Obtained funding: Not applicable
Overall responsibility: OE
Disclosures
None.
Acknowledgments
We thank Sophie Guiquerro for her help in bibliographic research.
Footnotes
O.E. received a mobility grant for 2020 to 2021 from the Société Française de Médecine Vasculaire for this study.
Clinical Trail Registration: CRD 42021249053.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix
Additional material for this article may be found online at www.jvsvenous.org.
Appendix
References
- 1.Isma N., Svensson P.J., Gottsäter A., Lindblad B. Upper extremity deep venous thrombosis in the population-based Malmö thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res. 2010;125:e335–e338. doi: 10.1016/j.thromres.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz F.J., Mismetti P., Poggio R., et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133:143–148. doi: 10.1378/chest.07-1432. [DOI] [PubMed] [Google Scholar]
- 3.Delluc A., Le Mao R., Tromeur C., et al. Incidence of upper-extremity deep vein thrombosis in western France: a community-based study. Haematologica. 2019;104:e29–e31. doi: 10.3324/haematol.2018.194951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ageno W., Haas S., Weitz J.I., et al. Characteristics and management of patients with venous thromboembolism: the GARFIELD-VTE registry. Thromb Haemost. 2019;119:319–327. doi: 10.1055/s-0038-1676611. [DOI] [PubMed] [Google Scholar]
- 5.Ploton G., Pistorius M., Raimbeau A., et al. A STROBE cohort study of 755 deep and superficial upper-extremity vein thrombosis. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun T.T., O’Connell J.B., Rigberg D.A., et al. Preoperative thrombolysis is associated with improved vein patency and functional outcomes after first rib resection in acute Paget-Schroetter syndrome. J Vasc Surg. 2022;76:806–813.e1. doi: 10.1016/j.jvs.2022.03.893. [DOI] [PubMed] [Google Scholar]
- 7.Davies G.A., Lazo-Langner A., Gandara E., et al. A prospective study of Rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2) Thromb Res. 2018;162:88–92. doi: 10.1016/j.thromres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S., Angerås U., Bergqvist D., Eriksson B., Lassen M.R., Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Savović J., Page M.J., Elbers R.G., Sterne J.A.C. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) Higgins J.P.T., Thomas J., J Chandler, et al., editors. Cochrane; 2023. Chapter 8: Assessing risk of bias in a randomized trial.www.training.cochrane.org/handbook Available at: [Google Scholar]
- 11.Wells G., Sheal B., O’Connelll D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Available at:
- 12.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A., Egger M., Smith G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabeti S., Schillinger M., Mlekusch W., Haumer M., Ahmadi R., Minar E. Treatment of subclavian-axillary vein thrombosis: long-term outcome of anticoagulation versus systemic thrombolysis. Thromb Res. 2002;108:279–285. doi: 10.1016/s0049-3848(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 16.Schimp V.L., Munkarah A.R., Morris R.T., Deppe G., Malone J. Upper extremity deep vein thrombosis associated with indwelling peripheral venous catheters in gynecology oncology patients. Gynecol Oncol. 2003;89:301–305. doi: 10.1016/s0090-8258(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli I., Battaglioli T., Bucciarelli P., Passamonti S.M., Mannucci P.M. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation. 2004;110:566–570. doi: 10.1161/01.CIR.0000137123.55051.9B. [DOI] [PubMed] [Google Scholar]
- 18.Karabay O., Yetkin U., Onol H. Upper extremity deep vein thrombosis: clinical and treatment characteristics. J Int Med Res. 2004;32:429–435. doi: 10.1177/147323000403200413. [DOI] [PubMed] [Google Scholar]
- 19.Prandoni P., Bernardi E., Marchiori A., et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484–485. doi: 10.1136/bmj.38167.684444.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong B., Gibbs H., Catchpole I., Hetherington R., Harper J. Peripherally inserted central catheters and upper extremity deep vein thrombosis. Australas Radiol. 2006;50:451–454. doi: 10.1111/j.1440-1673.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 21.Persson L.M., Arnhjort T., Lärfars G., Rosfors S. Hemodynamic and morphologic evaluation of sequelae of primary upper extremity deep venous thromboses treated with anticoagulation. J Vasc Surg. 2006;43:1230–1235. doi: 10.1016/j.jvs.2006.02.045. discussion: 1235. [DOI] [PubMed] [Google Scholar]
- 22.Arnhjort T., Persson L.M., Rosfors S., Ludwigs U., Lärfars G. Primary deep vein thrombosis in the upper limb: a retrospective study with emphasis on pathogenesis and late sequelae. Eur J Intern Med. 2007;18:304–308. doi: 10.1016/j.ejim.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs M.J., Kahn S.R., Rodger M., et al. A pilot study of central venous catheter survival in cancer patients using low-molecular-weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study) J Thromb Haemost. 2007;5:1650–1653. doi: 10.1111/j.1538-7836.2007.02613.x. [DOI] [PubMed] [Google Scholar]
- 24.Flinterman L.E., van Hylckama Vlieg A., Rosendaal F.R., Doggen C.J.M. Recurrent thrombosis and survival after a first venous thrombosis of the upper extremity. Circulation. 2008;118:1366–1372. doi: 10.1161/CIRCULATIONAHA.107.748699. [DOI] [PubMed] [Google Scholar]
- 25.Lechner D., Wiener C., Weltermann A., Eischer L., Eichinger S., Kyrle P.A. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost. 2008;6:1269–1274. doi: 10.1111/j.1538-7836.2008.02998.x. [DOI] [PubMed] [Google Scholar]
- 26.Vik A., Holme P.A., Singh K., et al. Catheter-directed thrombolysis for treatment of deep venous thrombosis in the upper extremities. Cardiovasc Intervent Radiol. 2009;32:980–987. doi: 10.1007/s00270-009-9655-y. [DOI] [PubMed] [Google Scholar]
- 27.Tran H., Arellano M., Chamsuddin A., et al. Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma. 2010;51:1473–1477. doi: 10.3109/10428194.2010.481065. [DOI] [PubMed] [Google Scholar]
- 28.Rathbun S.W., Stoner J.A., Whitsett T.L. Treatment of upper-extremity deep vein thrombosis. J Thromb Haemost. 2011;9:1924–1930. doi: 10.1111/j.1538-7836.2011.04466.x. [DOI] [PubMed] [Google Scholar]
- 29.Mandal S., Pande A., Mandal D., et al. Permanent pacemaker-related upper extremity deep vein thrombosis: a series of 20 cases. Pacing Clin Electrophysiol. 2012;35:1194–1198. doi: 10.1111/j.1540-8159.2012.03467.x. [DOI] [PubMed] [Google Scholar]
- 30.Delluc A., Le Gal G., Scarvelis D., Carrier M. Outcome of central venous catheter associated upper extremity deep vein thrombosis in cancer patients. Thromb Res. 2015;135:298–302. doi: 10.1016/j.thromres.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Rosa-Salazar V., Trujillo-Santos J., Díaz Peromingo J.A., et al. A prognostic score to identify low-risk outpatients with acute deep vein thrombosis in the upper extremity. J Thromb Haemost. 2015;13:1274–1278. doi: 10.1111/jth.13008. [DOI] [PubMed] [Google Scholar]
- 32.Bleker S.M., van Es N., van Gils L., et al. Clinical course of upper extremity deep vein thrombosis in patients with or without cancer: a systematic review. Thromb Res. 2016;140(Suppl 1):S81–S88. doi: 10.1016/S0049-3848(16)30104-9. [DOI] [PubMed] [Google Scholar]
- 33.Refaei M., Fernandes B., Brandwein J., Goodyear M.D., Pokhrel A., Wu C. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol. 2016;95:2057–2064. doi: 10.1007/s00277-016-2798-4. [DOI] [PubMed] [Google Scholar]
- 34.Riera-Mestre A., Buonaro A., Villegas A.R., Corbella X. Primary upper extremity deep vein thrombosis: clinical characteristics and outcome according to the presence of thoracic outlet syndrome. Eur J Intern Med. 2016;30:e19–e20. doi: 10.1016/j.ejim.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Stone R.H., Bress A.P., Nutescu E.A., Shapiro N.L. Upper-extremity deep-vein thrombosis: a retrospective cohort evaluation of thrombotic risk factors at a University Teaching hospital antithrombosis clinic. Ann Pharmacother. 2016;50:637–644. doi: 10.1177/1060028016649601. [DOI] [PubMed] [Google Scholar]
- 36.Cote L.P., Greenberg S., Caprini J.A., et al. Comparisons between upper and lower extremity deep vein thrombosis: a review of the RIETE registry. Clin Appl Thromb. 2017;23:748–754. doi: 10.1177/1076029616663847. [DOI] [PubMed] [Google Scholar]
- 37.Kang J.R., Long L.H., Yan S.W., Wei W.W., Jun H.Z., Chen W. Peripherally inserted central catheter-related vein thrombosis in patients with lung cancer. Clin Appl Thromb. 2017;23:181–186. doi: 10.1177/1076029615595880. [DOI] [PubMed] [Google Scholar]
- 38.Laube E.S., Mantha S., Samedy P., Wills J., Harnicar S., Soff G.A. Treatment of central venous catheter-associated deep venous thrombosis in cancer patients with rivaroxaban. Am J Hematol. 2017;92:E9–E10. doi: 10.1002/ajh.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montiel F.S., Ghazvinian R., Gottsäter A., Elf J. Treatment with direct oral anticoagulants in patients with upper extremity deep vein thrombosis. Thromb J. 2017;15:26. doi: 10.1186/s12959-017-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newton D.H., Monreal Bosch M., Amendola M., et al. Analysis of noncatheter-associated upper extremity deep venous thrombosis from the RIETE registry. J Vasc Surg Venous Lymphat Disord. 2017;5:18–24.e1. doi: 10.1016/j.jvsv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Ozcinar E., Yaman N.D., Cakici M., et al. Pharmacomechanical thrombectomy of upper extremity deep vein thrombosis. Int Angiol J Int Union Angiol. 2017;36:275–280. doi: 10.23736/S0392-9590.16.03749-4. [DOI] [PubMed] [Google Scholar]
- 42.Underhill J., Sherman M.A., Howard R., et al. The natural history and outcomes of line-associated upper extremity deep venous thromboses in critically ill patients. J Vasc Surg Venous Lymphat Disord. 2017;5:630–637. doi: 10.1016/j.jvsv.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 43.ALKindi S.Y., Chai-Adisaksopha C., Cheah M., Linkins L.A. Management of cancer-associated upper extremity deep vein thrombosis with and without venous catheters at a tertiary care center. Thromb Res. 2018;166:92–95. doi: 10.1016/j.thromres.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Baumann Kreuziger L., Cote L., Verhamme P., et al. A RIETE registry analysis of recurrent thromboembolism and hemorrhage in patients with catheter-related thrombosis. J Vasc Surg Venous Lymphat Disord. 2015;3:243–250.e1. doi: 10.1016/j.jvsv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Mansour A., Saadeh S.S., Abdel-Razeq N., Khozouz O., Abunasser M., Taqash A. Clinical course and complications of catheter and non-catheter-related upper extremity deep vein thrombosis in patients with cancer. Clin Appl Thromb. 2018;24:1234–1240. doi: 10.1177/1076029618788177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ageno W., Haas S., Weitz J.I., et al. Upper extremity DVT versus lower extremity DVT: perspectives from the GARFIELD-VTE registry. Thromb Haemost. 2019;119:1365–1372. doi: 10.1055/s-0039-1688828. [DOI] [PubMed] [Google Scholar]
- 47.Htun K.T., Ma M.J.Y., Lee A.Y.Y. Incidence and outcomes of catheter related thrombosis (CRT) in patients with acute leukemia using a platelet-adjusted low molecular weight heparin regimen. J Thromb Thrombolysis. 2018;46:386–392. doi: 10.1007/s11239-018-1711-5. [DOI] [PubMed] [Google Scholar]
- 48.Schastlivtsev I., Lobastov K., Tsaplin S., et al. Rivaroxaban in the treatment of upper extremity deep vein thrombosis: a single-center experience and review of the literature. Thromb Res. 2019;181:24–28. doi: 10.1016/j.thromres.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita Y., Bikdeli B., Monreal M., et al. Difference between Japanese and White patients with acute pulmonary embolism. Thromb Res. 2021;204:52–56. doi: 10.1016/j.thromres.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Beiswenger A.C., Quereshy H.A., Rouabhi M., et al. Midterm outcomes in patients with upper extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8:930–938.e2. doi: 10.1016/j.jvsv.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 51.Houghton D.E., Casanegra A.I., Peterson L.G., et al. Treatment of upper extremity deep vein thrombosis with apixaban and rivaroxaban. Am J Hematol. 2020;95:817–823. doi: 10.1002/ajh.25820. [DOI] [PubMed] [Google Scholar]
- 52.Porfidia A., Agostini F., Giarretta I., et al. Upper extremity deep vein thrombosis treated with direct oral anticoagulants: a multi-center real world experience. J Thromb Thrombolysis. 2020;50:355–360. doi: 10.1007/s11239-020-02044-4. [DOI] [PubMed] [Google Scholar]
- 53.Scamuffa M.C., Morano S.G., Serrao A., et al. PICC-related upper deep venous thrombosis in patients with hematological malignancies. Management of anticoagulant therapy according to the platelet count. J Thromb Thrombolysis. 2020;49:426–430. doi: 10.1007/s11239-020-02040-8. [DOI] [PubMed] [Google Scholar]
- 54.Baumann Kreuziger L., Gaddh M., Onadeko O., et al. Treatment of catheter-related thrombosis in patients with hematologic malignancies: a Venous thromboEmbolism Network U.S. retrospective cohort study. Thromb Res. 2021;202:155–161. doi: 10.1016/j.thromres.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Liu L., Huang J., Wu Z., Ma Y. Effectiveness and safety of catheter removal alone versus standard anticoagulation therapy after catheter removal for peripherally inserted central catheter (PICC)-related thrombosis. Ann Transl Med. 2021;9:1778. doi: 10.21037/atm-21-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vedovati M.C., Tratar G., Mavri A., et al. Upper extremities deep vein thrombosis treated with oral direct anticoagulants: a prospective cohort study. Int J Cardiol. 2021;339:158–163. doi: 10.1016/j.ijcard.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Cires-Drouet R.S., Durham F., Sharma J., et al. Prevalence and clinical outcomes of hospitalized patients with upper extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:102–110. doi: 10.1016/j.jvsv.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosa V., Chaar C., Espitia O., et al. A RIETE registry analysis of patients with upper extremity deep vein thrombosis and thoracic outlet syndrome. Thromb Res. 2022;213:65–70. doi: 10.1016/j.thromres.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 59.Porfidia A., Cammà G., Coletta N., et al. A single center retrospective cohort study comparing different anticoagulants for the treatment of catheter-related thrombosis of the upper extremities in women with gynecologic and breast cancer. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.880698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipe D.N., Qdaisat A., Rajha E., et al. Characteristics and predictors of venous thrombosis recurrence in patients with cancer and catheter-related thrombosis. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turrian U., Lapebie F.X., Bura-Rivière A. Duration of anticoagulation for upper extremity deep vein thrombosis associated with cancer and central venous catheter: outcome of a cohort study. J Med Vasc. 2022;47:11–18. doi: 10.1016/j.jdmv.2022.01.138. [DOI] [PubMed] [Google Scholar]
- 62.Baumann Kreuziger L., Feng M., Bartosic A., Simpson P., Wang T.F. A prospective cohort study of catheter-related thrombosis in cancer patients treated with 1 month of anticoagulation after catheter removal. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2022;33:171–175. doi: 10.1097/MBC.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 63.Xu J., Wang G., Chen X., Shen Y., Wang X., Wang H. Efficacy and safety of rivaroxaban for the treatment of PICC-related upper extremity deep vein thrombosis in cancer patients: a retrospective study. Thromb J. 2023;21:15. doi: 10.1186/s12959-023-00456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnhjort T., Nordberg J., Delle M., Borgis C.J., Rosfors S., Lärfars G. The importance of the costoclavicular space in upper limb primary deep vein thrombosis, a study with magnetic resonance imaging (MRI) technique enhanced by a blood pool agent. Eur J Intern Med. 2014;25:545–549. doi: 10.1016/j.ejim.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Hervé H., Toquet C., Ploton G., et al. Prevalence of post-thrombotic syndrome in a cohort of upper extremity vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:111–117.e3. doi: 10.1016/j.jvsv.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Chang R., Horne M.K., Shawker T.H., et al. Low-dose, once-daily, intraclot injections of alteplase for treatment of acute deep venous thrombosis. J Vasc Interv Radiol. 2011;22:1107–1116. doi: 10.1016/j.jvir.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan F., Zou Y., Zhang S., et al. Rivaroxaban in the treatment of PICC-associated upper extremity venous thrombosis. Clin Ther. 2017;39:1882–1888. doi: 10.1016/j.clinthera.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 68.Yamashita Y., Morimoto T., Amano H., et al. Deep vein thrombosis in upper extremities: clinical characteristics, management strategies and long-term outcomes from the COMMAND VTE Registry. Thromb Res. 2019;177:1–9. doi: 10.1016/j.thromres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 69.Ploton G., Brebion N., Guyomarch B., et al. Predictive factors of venous recanalization in upper-extremity vein thrombosis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 71.Illig K.A., Doyle A.J. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg. 2010;51:1538–1547. doi: 10.1016/j.jvs.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Thiyagarajah K., Ellingwood L., Endres K., et al. Post-thrombotic syndrome and recurrent thromboembolism in patients with upper extremity deep vein thrombosis: a systematic review and meta-analysis. Thromb Res. 2019;174:34–39. doi: 10.1016/j.thromres.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Valeriani E., Di Nisio M., Porceddu E., et al. Anticoagulant treatment for upper extremity deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost. 2022;20:661–670. doi: 10.1111/jth.15614. [DOI] [PubMed] [Google Scholar]
- 74.Farge D., Frere C., Connors J.M., et al. 2022 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23:e334–e347. doi: 10.1016/S1470-2045(22)00160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Banion L.A., Ozsvath K., Cutler B., Kiguchi M. A review of the current literature of ethnic, gender, and socioeconomic disparities in venous disease. J Vasc Surg Venous Lymphat Disord. 2023;11:682–687. doi: 10.1016/j.jvsv.2023.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.