Abstract
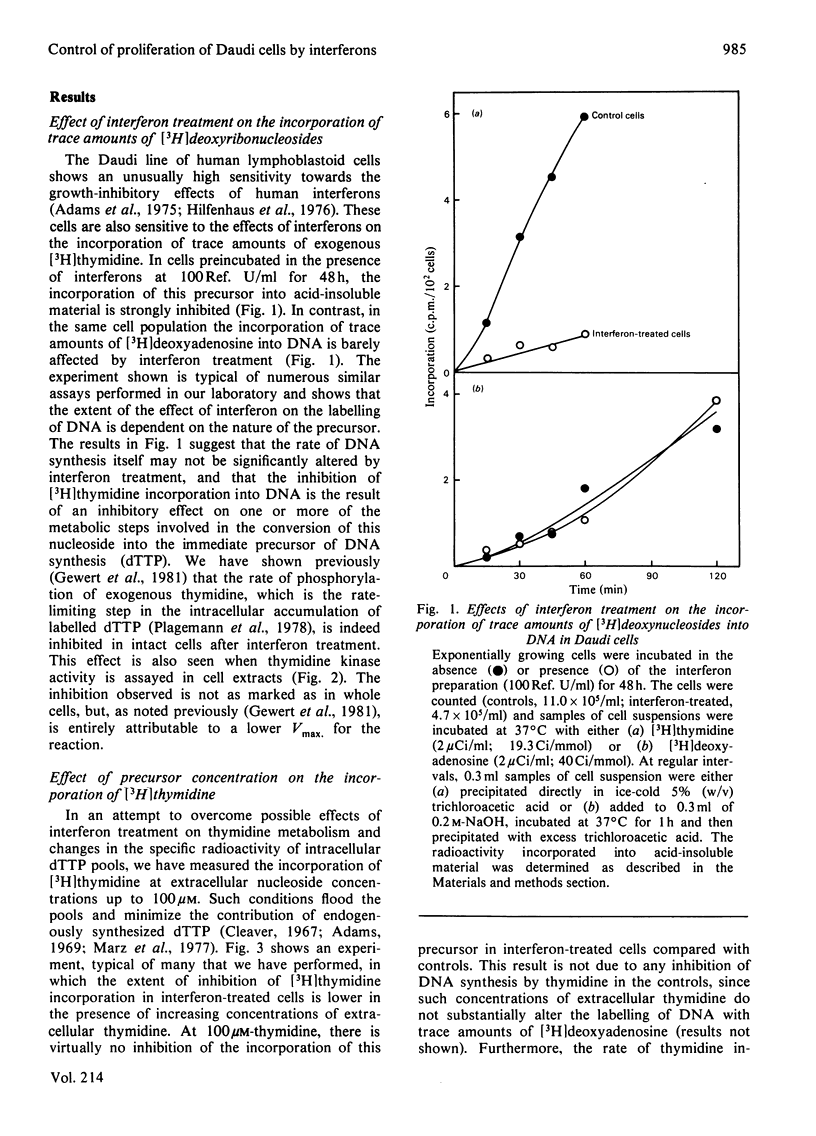
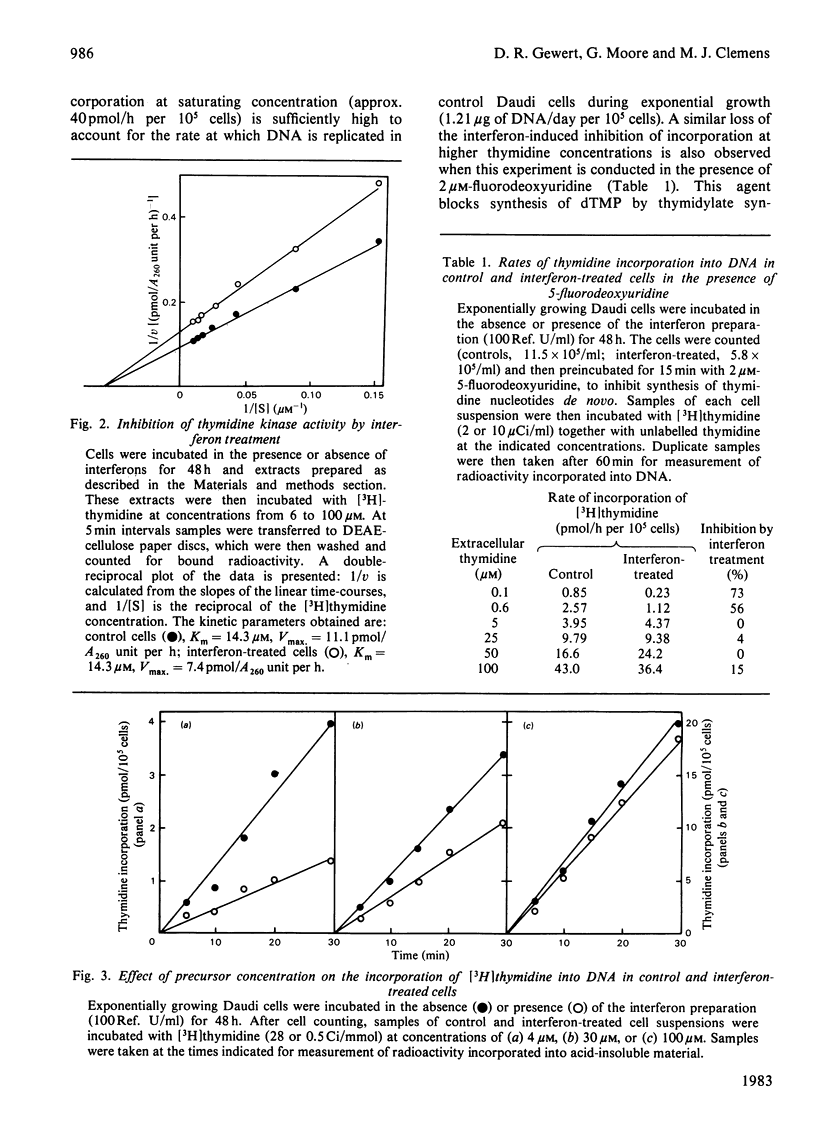
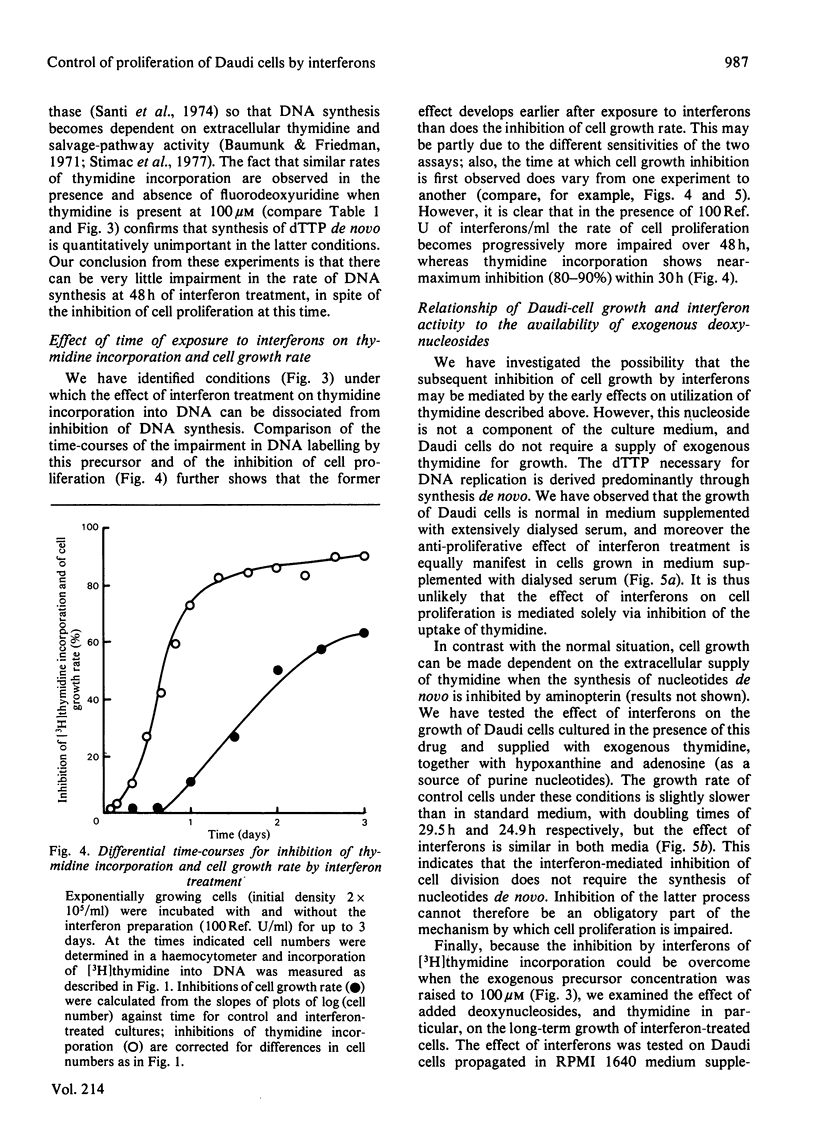
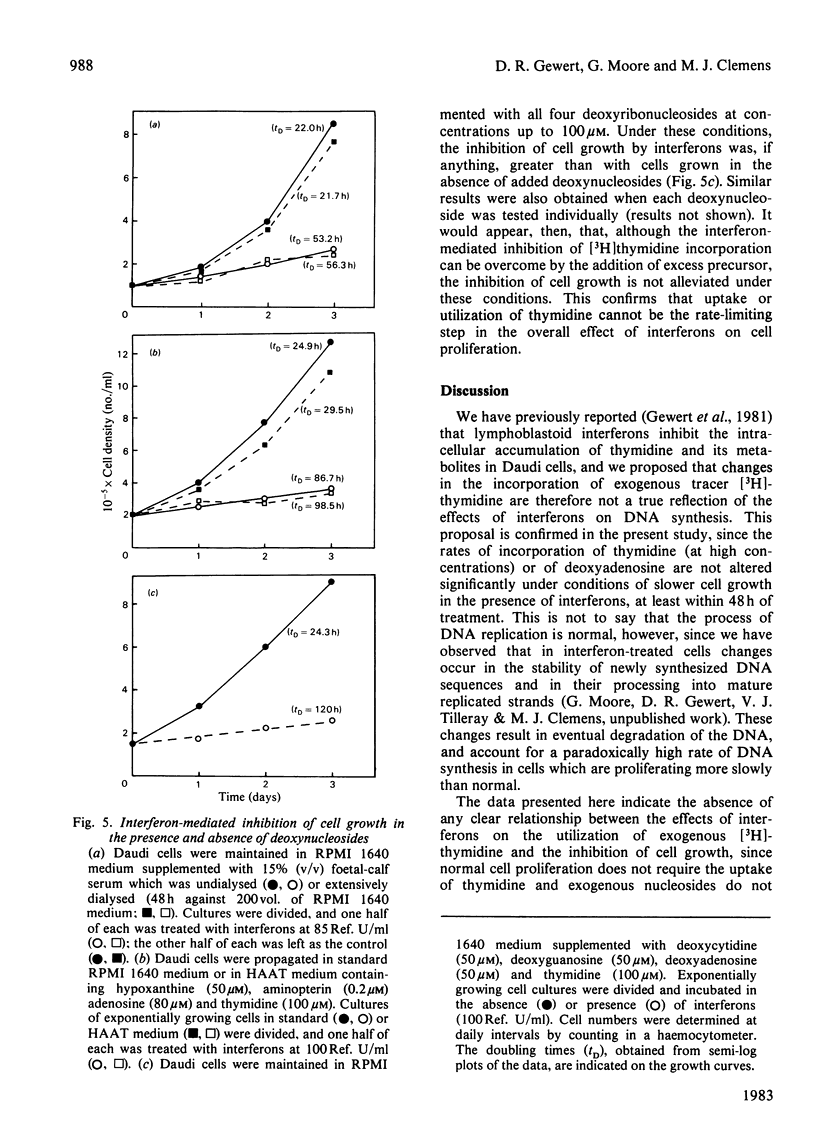
Inhibition of the proliferation of Daudi cells by exposure to human lymphoblastoid interferons is associated with an early and marked decrease in the incorporation into DNA of exogenous [3H]thymidine when cells are incubated with trace amounts of this precursor. In contrast, incorporation of exogenous deoxyadenosine into DNA is unchanged under the same conditions. Interferon treatment results in a lowering of thymidine kinase activity, an effect which may be largely responsible for the inhibition of incorporation of labelled thymidine into DNA. At higher concentrations of exogenous thymidine, which minimize the contribution of intracellular sources to the dTTP pool, the inhibition of thymidine incorporation is abolished. Under conditions in which exogenous thymidine is rigorously excluded from the medium or, conversely, in which cells are entirely dependent on exogenous thymidine for growth, the magnitude of the inhibition of cell proliferation by interferons is the same as under normal culture conditions. We conclude that, even though cell growth is impaired, the rate of DNA synthesis is not grossly inhibited up to 48 h after commencement of interferon treatment. Furthermore, changes in neither the utilization of exogenous thymidine nor the synthesis of nucleotides de novo are responsible for the effect on cell proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Adams R. L. Phosphorylation of tritiated thymidine by L929 mouse fibroblasts. Exp Cell Res. 1969 Jul;56(1):49–54. doi: 10.1016/0014-4827(69)90392-9. [DOI] [PubMed] [Google Scholar]
- Allen G., Fantes K. H. A family of structural genes for human lymphoblastoid (leukocyte-type) interferon. Nature. 1980 Oct 2;287(5781):408–411. doi: 10.1038/287408a0. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Taylor-Papadimitriou J. Interferon affects both G1 and S+G2 in cells stimulated from quiescence to growth. Nature. 1978 Aug 24;274(5673):798–800. doi: 10.1038/274798a0. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Watling D., Taylor-Papadimitriou J. Inhibition by lymphoblastoid interferon of growth of cells derived from the human breast. Int J Cancer. 1978 Sep 15;22(3):258–265. doi: 10.1002/ijc.2910220307. [DOI] [PubMed] [Google Scholar]
- Baumunk C. N., Friedman D. L. Absence of an effect of amethopterin and 5-fluorodeoxyuridine upon levels of thymidine triphosphate in HeLa cells. Cancer Res. 1971 Dec;31(12):1930–1935. [PubMed] [Google Scholar]
- Bello L. J. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974 Dec;89(2):263–274. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- Bourgeade M. F., Chany C. Effect of sodium butyrate on the antiviral and anticellular action of interferon in normal and MSV-transformed cells. Int J Cancer. 1979 Sep 15;24(3):314–318. doi: 10.1002/ijc.2910240307. [DOI] [PubMed] [Google Scholar]
- Brouty-Boyé D., Tovey M. G. Inhibition by interferon of thymidine uptake in chemostat cultures of L1210 cells. Intervirology. 1978;9(4):243–252. doi: 10.1159/000148942. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Bartholomew J. C., Merigan T. C. Role of G0-G1 arrest in the inhibition of tumor cell growth by interferon. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1471–1475. doi: 10.1073/pnas.77.3.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse A., Kuwata T. Inhibition of DNA synthesis and alteration of cyclic adenosine 3',5'-monophosphate levels in RSa cells by human leukocyte interferon. J Natl Cancer Inst. 1978 Jun;60(6):1227–1232. doi: 10.1093/jnci/60.6.1227. [DOI] [PubMed] [Google Scholar]
- Fuse A., Kuwata T. Inhibition of DNA synthesis of synchronized RSa cells by human leukocyte interferon. J Natl Cancer Inst. 1977 Apr;58(4):891–896. doi: 10.1093/jnci/58.4.891. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Shah S., Clemens M. J. Inhibition of cell division by interferons: Changes in the transport and intracellular metabolism of thymidine in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1981 Jun 1;116(3):487–492. doi: 10.1111/j.1432-1033.1981.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Karges H. E., Manthey K. F. Growth inhibition of human lymphoblastoid Daudi cells in vitro by interferon preparations. Arch Virol. 1976;51(1-2):87–97. doi: 10.1007/BF01317837. [DOI] [PubMed] [Google Scholar]
- Hunting D., Hordern J., Henderson J. F. Quantitative analysis of purine and pyrimidine metabolism in Chinese hamster ovary cells. Can J Biochem. 1981 Oct;59(10):838–847. doi: 10.1139/o81-116. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase, DNA synthesis and cancer. Mol Cell Biochem. 1976 Jun 15;11(3):161–182. doi: 10.1007/BF01744997. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Werner R. A model for compartmentation of de novo and salvage thymidine nucleotide pools in mammalian cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3333–3336. doi: 10.1073/pnas.72.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanderson T., Sundström S., Mårtensson I. L., Ny T., Lundgren E. Interferon-specific effects on protein synthesis in P3HR-1 cells. EMBO J. 1982;1(12):1505–1511. doi: 10.1002/j.1460-2075.1982.tb01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. L., Ts'o P. O., Hollenberg M. D. The effects of interferon on epidermal growth factor action. Biochem Biophys Res Commun. 1980 Sep 16;96(1):168–174. doi: 10.1016/0006-291x(80)91196-1. [DOI] [PubMed] [Google Scholar]
- Lundblad D., Lundgren E. Changes in DNA-polymerase and thymidine kinase activity during interferon treatment. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1569–1576. doi: 10.1016/0006-291x(82)90967-6. [DOI] [PubMed] [Google Scholar]
- Lundgren E., Larsson I., Miörner H., Strannegård O. Effects of leukocyte and fibroblast interferon on events in the fibroblast cell cycle. J Gen Virol. 1979 Mar;42(3):589–595. doi: 10.1099/0022-1317-42-3-589. [DOI] [PubMed] [Google Scholar]
- Marz R., Wohlhueter R. M., Plagemann P. G. Relationship between thymidine transport and phosphorylation in Novikoff rat hepatoma cells as analyzed by a rapid sampling technique. J Supramol Struct. 1977;6(3):433–440. doi: 10.1002/jss.400060316. [DOI] [PubMed] [Google Scholar]
- Matarese G. P., Rossi G. B. Effect of interferon on growth and division cycle of Friend erythroleukemic murine cells in vitro. J Cell Biol. 1977 Nov;75(2 Pt 1):344–354. doi: 10.1083/jcb.75.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen B., Tyrsted G. Induction of thymidine kinases in phytohaemagglutinin-stimulated human lymphocytes. Biochim Biophys Acta. 1977 Oct 4;478(3):364–375. doi: 10.1016/0005-2787(77)90152-6. [DOI] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Dynamics of pyrimidine deoxynucleoside triphosphate pools in relationship to DNA synthesis in 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1347–1351. doi: 10.1073/pnas.80.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panniers L. R., Clemens M. J. Inhibition of cell division by interferon: changes in cell cycle characteristics and in morphology of Ehrlich ascites tumour cells in culture. J Cell Sci. 1981 Apr;48:259–279. doi: 10.1242/jcs.48.1.259. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Uridine transport in Novikoff rat hepatoma cells and other cell lines and its relationship to uridine phosphorylation and phosphorolysis. J Cell Physiol. 1978 Oct;97(1):49–72. doi: 10.1002/jcp.1040970107. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Synthesis of new proteins associated with the induction of interferon in human fibroblast cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4918–4922. doi: 10.1073/pnas.77.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Secher D. S., Burke D. C. A monoclonal antibody for large-scale purification of human leukocyte interferon. Nature. 1980 Jun 12;285(5765):446–450. doi: 10.1038/285446a0. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Lukey T. Kinetic considerations for the regulation of adenosine and deoxyadenosine metabolism in mouse and human tissues based on a thymocyte model. Biochim Biophys Acta. 1982 Mar 29;696(3):299–307. doi: 10.1016/0167-4781(82)90061-6. [DOI] [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Tovey M., Brouty-Boyé D., Gresser I. Early effect of interferon on mouse leukemia cells cultivated in a chemostat. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2265–2269. doi: 10.1073/pnas.72.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]



