Abstract
Objective
A large vein diameter is associated with higher recanalization rates after endovenous thermal ablation procedures of the great saphenous vein (GSV) and small saphenous vein (SSV). However, relatively few studies have explored the relationship between vein diameter and recanalization rates after mechanochemical ablation (MOCA).
Methods
We conducted a retrospective review of patients with chronic venous insufficiency who underwent MOCA of the GSV or SSV from 2017 to 2021 at a single hospital. Patients with no follow-up ultrasound examination were excluded. Patients were classified as having a large (≥1 cm) or small (<1 cm) treated vein. The primary outcomes were 2-year recanalization and reintervention of the treated segment.
Results
A total of 186 MOCA procedures during the study period were analyzed. There was no differences in age, gender, history of venous thromboembolic events, use of anticoagulation, obesity, or length of treated segment between the cohorts. Patients with large veins were less likely to have stasis ulcers compared with those with small veins (3.2% vs 21.5%; P < .05 on Fisher exact test). Patients with large veins had a higher incidence of postoperative local complications (24.2% vs 7.2%, P < .05 on χ2 test). A survival analysis with Cox proportional hazards showed no significant difference in recanalization rates with larger vein diameters. However, obesity was found to correlate significantly with recanalization.
Conclusions
A large vein diameter was not associated with higher recanalization rates after MOCA of the GSVs and SSVs. However, obesity was found to correlate with recanalization rates.
Keywords: Venous insufficiency, Endovascular methods, Mechanochemical ablation, Treatment failure, Obesity
Article Highlights.
-
•
Type of Research: Single-center, retrospective, longitudinal study
-
•
Key Findings: We identified 186 mechanochemical ablation procedures. Survival analysis with Cox proportional hazards modeling showed no significant difference in recanalization rates between large and small diameter veins (hazard ratio, 0.47; P = .16). However, obesity was found to significantly correlate with recanalization (hazard ratio, 3.84; P = .03).
-
•
Take Home Message: Rates of recanalization after mechanochemical ablation were found to be higher in obese patients than in nonobese patients. Further work is needed to study whether morbidly obese patients might be better served by alternative ablation techniques.
Chronic venous insufficiency is a prevalent condition with significant impacts on quality of life,1,2 ranging from psychological distress to functional limitations.3,4 Current guidelines published jointly by the Society for Vascular Surgery and the American Venous Forum recommend that moderate and severe symptomatic axial reflux in the great saphenous vein (GSV) and small saphenous vein (SSV) should be treated operatively, with a preference for endovascular approaches whenever possible.5
Mechanochemical ablation (MOCA) is a nonthermal, endovascular vein ablation method in which a spinning catheter ablates the endothelium of the target vein mechanically while simultaneously spraying a sclerosant along the vessel wall.6 In contrast with endovenous laser ablation (EVLA) and radiofrequency ablation (RFA),7 MOCA is a nonthermal method and does not require tumescent anesthesia. In principle, MOCA might decrease the risk of damage to nerves and other surrounding structures from iatrogenic heating or tumescent anesthesia.
In the case of other endovascular modalities, numerous prior studies have explored whether patient-specific factors are correlated with outcomes. For instance, prior studies suggest that recanalization rates after foam sclerotherapy and thermal ablation are worse in large diameter veins.8, 9, 10 Unfortunately, in the case of MOCA, there is limited evidence as to whether patient-specific factors such as age, sex, obesity, or vein diameter correlate with rates of recanalization. At present, there is relatively little evidence on whether MOCA produces comparable outcomes to other endovascular treatments or open surgery, especially in large diameter veins.
Given the paucity of evidence on this question, we conducted a retrospective analysis of our institutional experience with MOCA to explore whether the rates of recanalization after MOCA were associated with vein diameter or other patient-specific factors.
Methods
Data collection
We conducted a retrospective cohort study of patients at a single safety-net hospital who underwent MOCA to the GSV or SSV between 2017 and 2021. Institutional review board approval was obtained. An initial database of patients was assembled from the electronic medical record by selecting all patients with a Current Procedural Terminology code of 36473 or 36474 within the specified time range. We excluded from the study all patients who underwent MOCA to veins other than the GSV or SSV, as well as patients without a follow-up ultrasound examination.
All procedures were outpatient, single-day procedures conducted in vascular suites at a single hospital and affiliated vascular clinic. Ultrasound examinations were conducted at a vascular laboratory accredited by the Intersocietal Accreditation Commission and interpreted by vascular surgeons certified as Registered Physicians in Vascular Interpretation by the Alliance for Physician Certification and Advancement. Ultrasound examinations were collected in our usual fashion and included imaging of the saphenofemoral junction; the proximal, mid, and distal thirds of the thigh; at the level of the knee; and the proximal, middle, and distal thirds of the calf.
The maximum vessel diameter on pre-operative ultrasound was identified for each patient. Patients were grouped into large (>1.0 cm) and small (≤1.0 cm) diameter vein cohorts by maximum vein diameter. We additionally collected data on patient demographics, comorbidities at the time of treatment, initial Clinical-Etiology-Anatomy-Pathology (CEAP) clinical class,11 symptoms, and ultrasound characteristics at initial presentation and follow-up. For the purposes of survival modeling, we grouped CEAP classes C4, C5, and C6 as high and C2 and C3 as moderate. Of note, our institution's electronic medical record lists Hispanic as a race. Patients marked as Hispanic race in our data generally did not have additional race data available in the electronic medical record.
Outcomes
The primary outcome was a 2-year complete recanalization of the treated segment. Recanalization is defined as complete patency of the treated vein segment with flow visualized on follow-up duplex ultrasound examination. At our institution, we typically schedule at least one short-term (<6 months) and one long-term (>6 months) follow-up visit with ultrasound imaging, followed by annual follow-up visits whenever possible.
Secondary outcomes were reintervention within 2 years, postoperative pain, and local complications including hematoma, hyperpigmentation, burning sensation, paresthesias, and saphenous neuralgia. We additionally tracked the incidence of local ablation-associated thrombosis, which presents similarly to endovenous heat-induced thrombosis after thermal ablation.
Data analysis
Statistical analysis was performed in R Studio software (R Core Team 2020; The R Foundation for Statistical Computing, Vienna, Austria). Categorical outcomes were compared with χ2 tests and Fisher exact tests, where a P value of <.05 was considered statistically significant.
Recanalization rates were then studied using Cox proportional hazards survival models. We treat continued occlusion of the treated segment as analogous to patient survival and occlusion as analogous to patient mortality in the setting of survival models.10 Because we were interested initially in the relationship between vein diameter and recanalization, we first fit an unadjusted model of recanalization rates vs vein diameter. After fitting this initial unadjusted model, we subsequently built adjusted models with patient-level covariates. We built an adjusted model with vein diameter, sex, age, and obesity (body mass index [BMI] of ≥30.0 kg/m2) as variables. Following standard practice, we restricted the adjusted model to no more than 1 variable per 10 observations. We proceeded by removing individual variables and comparing the reduced model against the full model using a log-likelihood ratio test and systematically eliminating variables that do not improve model fit.
Subsequently, we repeated the survival modeling with alternative model specifications to assess for robustness of the results. Because there is no established standard for determining whether a given vein diameter should be considered large or small, we conducted a sensitivity analysis with different choices of cutoffs between large and small vein cohorts and with vein diameter as a continuous variable. We also repeated modeling with patient BMI as a continuous variable.
Results
Patient cohort
We initially located the records of 280 venous ablation procedures performed on 232 patients during the study period. Of these 280 procedures, 186 were MOCA procedures performed on 161 patients. Of the 186 MOCA ablations identified, 153 treated veins had a diameter of <1 cm, including 13 SSVs. The remaining 33 MOCA procedures were ablations of GSVs with diameters of ≥1 cm. Preoperative characteristics of the large (≥1 cm) vein diameter and small (<1 cm) vein diameter cohorts are summarized in Table I.
Table I.
Demographics and preoperative characteristics of the large (≥1 cm) vein diameter and small (<1 cm) vein diameter cohorts
| >1.0 cm (n = 31) | ≤1.0 cm (n = 130) | P value | |
|---|---|---|---|
| Age, years | 50.6 ± 12.1 | 51.8 ± 11.5 | .869 |
| Sex | |||
| Female | 22 (71.0) | 84 (64.6) | .507 |
| Race/ethnicitya | |||
| African American | 13 (41.9) | 69 (53.1) | .380 |
| Hispanic | 16 (51.6) | 42 (32.3) | |
| White | 2 (6.5) | 14 (10.8) | |
| Asian | 0 (0.0) | 1 (0.8) | |
| Other | 0 (0.0) | 4 (3.1) | |
| CEAP clinical classa | |||
| 2 | 2 (6.5) | 18 (13.8) | .042∗ |
| 3 | 16 (51.6) | 49 (37.7) | |
| 4 | 10 (32.3) | 26 (20.0) | |
| 5 | 2 (6.5) | 9 (6.9) | |
| 6 | 1 (3.2) | 28 (21.5) | |
| Anticoagulation | |||
| Warfarina | 1 (3.2) | 5 (3.8) | 1 |
| DOACsa | 1 (3.2) | 4 (3.1) | 1 |
| Comorbidities | |||
| Obese | 19 (61.3) | 74 (56.9) | .683 |
| History of DVTa | 3 (9.7) | 13 (10.0) | 1 |
| Vein diameter | |||
| Median, mm | 12.0 ± 3.98 | 6.40 ± 1.85 | <.001b |
| Treated segment length | |||
| Median, mm | 44.0 ± 9.92 | 44.5 ± 11.7 | .157 |
| Treated veina | |||
| GSV | 31 (100.0) | 121 (93.1) | .205 |
| SSV | 0 (0.0) | 9 (6.9) |
CEAP, Clinical-Etiology-Anatomy-Pathology; DOAC, direct-acting anticogaulatn; DVT, deep vein thrmbosis; GSV, great saphenous vein; SSV, small saphenous vein.
Values are mean ± standard devition or number (%).
For patients who had multiple procedures, the demographics at earliest presentation are listed. Continuous variables were compared with t tests, and categorical variables were compared with χ2 tests and Fisher exact tests. CEAP classes varied significantly between groups (P < .05 on Fisher exact test). Patients in the small vein cohort were more likely to present with C6 disease (P < .05 on χ2 test). There were no significant differences in demographic characteristics or length of treated segment between the two groups.
Compared with Fisher exact test due to small cohort numbers (<5 patients per group).
P < .05.
In five procedures across four patients, the time to initial follow-up ultrasound examination was >1 year. Excluding these five procedures, the median time to the first follow-up ultrasound examination was 10 days. In the majority of cases (138 procedures [74.2%]), the first follow-up ultrasound examination was done within 30 days. The median time to last known follow-up ultrasound examination was 132.0 days. Follow-up ultrasound examinations beyond 1 year were available in 68 cases (36.6%) across 90 patients (48.4%), and of these, 38 procedures (20.4% of all procedures) had ultrasound follow-up of >2 years. The median time to last clinic follow-up up was 344 days.
Patient characteristics
The CEAP clinical classification varied significantly between the two vein cohorts as compared by a Fisher exact test across all CEAP classes (P < .05). Patients in the large vein cohort were less likely to have CEAP class C6 disease (venous stasis ulcers) compared with the small vein cohort (3.0% vs 22.2%; P < .05 on Fisher exact test). Most patients in either cohort presented with class C2 disease (varicose veins) or class C3 disease (edema) (58.1% in the large vein cohort vs 51.5% in the small vein cohort). The vast majority of patients in the large and small vein diameter cohorts were female (71.0% and 64.6%, respectively). There were no statistically significant differences in age, sex, history of venous thromboembolic events, or anticoagulation between the large and small diameter cohorts. Importantly, there was no difference in the length of the treated segment between the two groups.
Primary outcome
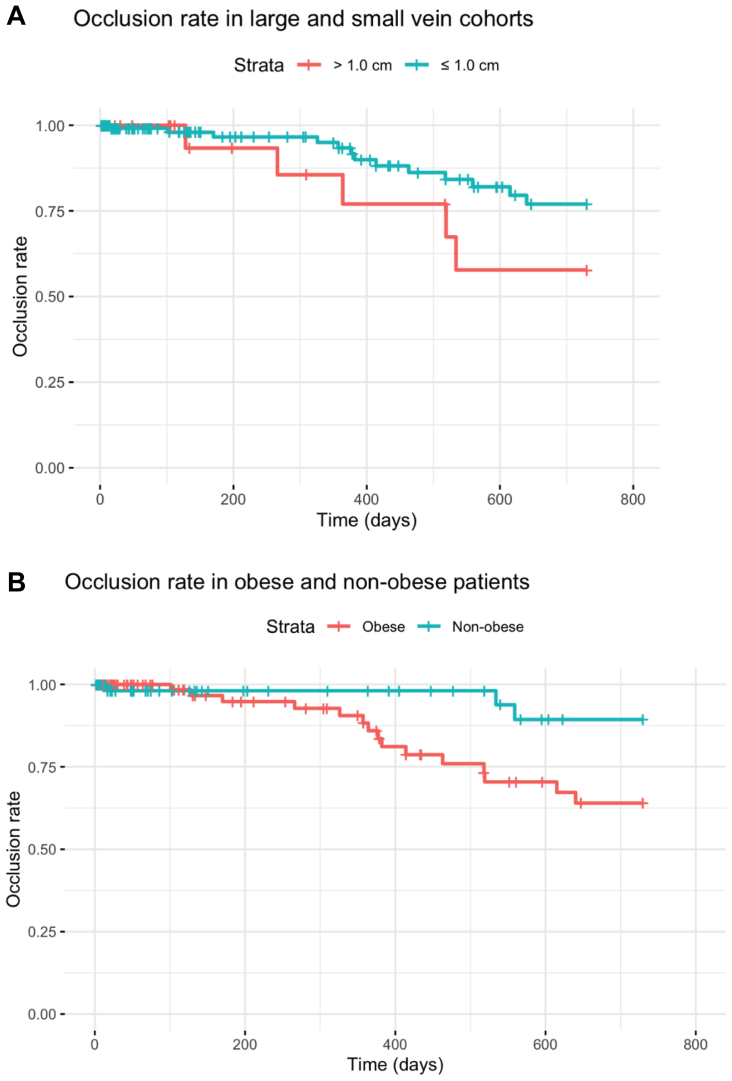
At 2 years after the procedure, there were no significant differences between large and small vein cohorts with regard to overall rate of recanalization (15.2% vs 8.5%; P = .326) or reintervention (6.1% vs 2.0%; P = .216) of the treated segment. Observed occlusion rates by vein cohort are plotted in the Fig, A.
Fig.
(A) Observed rate of occlusion over time in large vein and small vein cohorts. (B) Observed rate of occlusion over time in obese and nonobese patients.
An unadjusted Cox model of 2 -year recanalization rates vs vein diameter did not produce a statistically significant result at the predetermined significance level of P < .05 (hazard ratio [HR], 0.47 for large vein cohort; P = .16).
An initial adjusted Cox model of 2-year recanalization rates vs vein diameter with age, sex, obesity, and CEAP class as covariates found that large vein diameter was associated with worse recanalization rates (HR, 0.27; P = .03). However, the number of observations (18 instances of recanalization) is insufficient to support a model with this many variables. We, therefore, proceeded to eliminate covariates using the log likelihood test as described in the Methods. Via this method, we eliminated age, sex, and CEAP class as covariates that did not improve model fit, resulting in a reduced, adjusted model of recanalization vs vein diameter with obesity as the sole covariate. Log likelihood tests showed that no other variables collected could improve model fit. In this reduced, adjusted model, vein diameter was not associated with recanalization rate (HR, 0.46; P = .14). However, obesity was a significant predictor of recanalization (HR, 3.84; P = .03).
Because obesity was the sole significant predictor of recanalization, we then built an unadjusted Cox model of survival as a function of obesity without accounting for vein diameter, which showed a significant relationship between obesity and recanalization rates (HR, 3.85; P = .03). Based on this finding, we subsequently compared the observed recanalization rates among obese and nonobese patients. Observed occlusion rates over time by vein diameter cohort are plotted in the Fig, B. The overall rate of recanalization recorded among obese patients was 13.5% (15 of 111 cases) compared with 4% (3 of 75 cases) among nonobese patients.
Model robustness
Using alternative specifications for model variables did not affect the above results. In particular, specifying an unadjusted model with patient BMI as a continuous variable instead of using a categorical variable for obesity produced a comparable result (unadjusted model HR of 1.06; P = .04). Modeling recanalization with vein diameter as a continuous variable instead of a categorical variable produced no significant correlation between diameter and recanalization (HR, 1.05; P = .36). Similarly, using a large vein cutoff of 0.6 cm9 again produced no significant relationship between vein diameter and recanalization (HR, 0.48; P = .192).
Secondary outcomes
In the postoperative period, patients with large veins had a higher overall incidence of postoperative complications (24.2% vs 7.2%; P < .05) as shown in Table II.
Table II.
Recanalization, reintervention, and complication rates by vein diameter compared with χ2 tests
| >1.0 cm (n = 33) | ≤ 1.0 cm (n = 153) | P value | |
|---|---|---|---|
| Recanalization within 2 years | 5 (15.2) | 13 (8.5) | .326 |
| Reintervention within 2 years | 2 (6.1) | 3 (2.0) | .206 |
| Complications | |||
| Any complication | 8 (24.2) | 11 (7.2) | .011 |
| Ablation-associated thrombosis | 3 (9.1) | 2 (1.3) | – |
| Pain | 4 (12.1) | 4 (2.6) | – |
| Hematoma | 2 (6.1) | 4 (2.6) | – |
| Hyperpigmentation | 0 (0.0) | 3 (2.0) | – |
Values are number (%).
Patients in the large vein cohort were more likely to develop a complication (P < .05). Rates of ablation-associated thrombosis, postoperative pain, and hematoma were higher in the large vein cohort. Two patients in the small vein cohort developed postoperative hyperpigmentation. The χ2 tests are unreliable with such small patient numbers (<5 affected patients per complication per group) and are therefore omitted. There were no instances of postoperative burning sensation, paresthesias, saphenous neuralgia, puncture site complications, or induration in either group.
Recanalization and reintervention rates did not vary significantly between vein diameter cohorts. Patients in the large vein cohort were more likely to develop a complication (P < .05 on χ2 test). Rates of thrombosis and postoperative pain were higher in the large vein cohort (P < .05 on Fisher exact test). Rates of hematoma were not significantly different between cohorts. Two patients in the small vein cohort developed postoperative hyperpigmentation. There were no recorded instances of postoperative burning sensation, paresthesias, saphenous neuralgia, puncture site complications, or induration in either group.
Discussion
Joint guidelines from the Society for Vascular Surgery and American Venous Forum recommend that symptomatic axial reflux of the GSV and SSV be treated with endovascular ablation.5 However, few randomized controlled trials have compared the outcomes of surgical and endovascular vein treatments. Data are particularly deficient regarding MOCA. In a randomized, controlled, nonblinded trial, in Helsinki, Finland, Vähäaho et al9 found that post-MOCA recanalization rates were inferior to recanalization rates after thermal ablation. Prior studies have also suggested that recanalization rates after other endovascular modalities, such as foam sclerotherapy and thermal ablation, are worse in larger diameter veins.8, 9, 10 In thermal ablation, recanalization rates also correlate with the length of the treated vein segment. Further complicating matters, technical outcomes may not always predict clinical outcomes and patients' subjective experiences. A landmark, multicenter, randomized controlled trial by Brittenden et al12 comparing laser ablation, foam sclerotherapy, and open surgery found that overall clinical outcomes and patient satisfaction were comparable across treatments, even though the technical success rates varied significantly.
There are few data on whether vein diameter or other patient factors correlate with recanalization rates after MOCA. In the aforementioned unblinded trial, Vähäaho et al9 randomized patients with CEAP clinical class 2, 3, or 4 venous insufficiency to treatment with EVLA, RFA, or MOCA to the GSV. Their results suggested that MOCA had a slightly higher 3-year recanalization rate relative to EVLA and RFA and that post-MOCA recanalization occurred somewhat more frequently with vein diameters of >0.6 cm. However, the group sizes in this study were small, with only a total of 50 MOCA cases analyzed, and it seems13 that there were no patients with vein sizes of >1.2 cm in the MOCA group. In contrast with these conclusions, a retrospective cohort study by Chen et al14 found that occlusion rates after MOCA were comparable with occlusion rates after other endovascular modalities and found no higher rate of occlusion with vein diameters of >0.75 cm.
Given this paucity of gold standard data from randomized controlled trials, we conducted a retrospective study of MOCA outcomes at our institution, a safety net hospital in the southeastern United States. Because vascular surgeons at our institution frequently perform MOCA ablation and generally prefer this technique to thermal methods, a large cohort of 186 cases was available. Overall recanalization rates in our experience were similar to rates reported in other studies after other thermal and nonthermal endovenous ablation procedures.15,16 Using a survival analysis with Cox proportional hazards models, we found no statistically significant difference in recanalization rates between patients with large vein diameters and those with small vein diameters. Although we have used a cutoff of 1 cm, other authors have suggested smaller cutoffs. As there is no consensus on which diameter veins should be considered large, we conducted a sensitivity analysis using alternative cutoffs, which did not affect our conclusions. Only five cases of reintervention occurred in our cohort. In the setting of such small cohort numbers, there was no statistical significance to the difference in incidence by vein diameter.
We also found that rates of recanalization were higher in obese patients, a topic that has not been explored widely in the prior literature. Work by Ahmed et al17 found that obesity was correlated with greater rates of recanalization of perforator veins after thermal ablation. However, Ahmed et al17 found that axial vein recanalization was not greater in obese patients after thermal ablation. In the present study, obese patients did have a significantly higher rate of axial vein recanalization after MOCA. Further research is needed to understand whether MOCA to the GSV or SSV in obese patients presents a greater risk of technical failure compared with thermal methods.
The incidence of postoperative pain and local complications was significantly higher in the large vein cohort (24.2% vs 7.2%; P < .05). The broader literature shows MOCA is generally well-tolerated by patients,14,18 but further work is needed to explore whether subsets of patients, such as those with larger vein diameters, have greater local anesthesia needs or increased risk of local complications.
This study is limited by its retrospective, single-institution design. Although we have collected data on 186 separate MOCA procedures, we have documented only 18 cases of recanalization. This factor limits survival modeling to one or two variables at best.19 Moreover, the length of follow-up is limited in our present study. Joint guidelines from the Society for Vascular Surgery and American Venous Forum recommend that patients undergoing an initial ablation for symptomatic axial reflux in the superficial venous trunks be followed for ≥3 months.5 Owing to the paucity of evidence on appropriate follow-up, the level of this recommendation is an ungraded clinical practice only, and no guidelines are available at present for long-term follow-up. At our institution, we attempt to schedule one short-term (<6 months) and one long-term (>1 year) follow-up with an ultrasound examination for all patients, followed by biannual follow-up visits whenever possible. Unfortunately, only one-half of the patients in this study had a follow-up ultrasound examination >1 year after their procedure. This factor limits our ability to compare longer term outcomes after MOCA. As is standard practice, we use a BMI cutoff of 30.0 kg/m2 to define obesity. However, BMI does not account for differences in weight distribution. It is possible that differences in adiposity between, for instance, the abdomen and the treated leg, could affect recanalization rates. As suggested by Ahmed et al,17 future prospective studies should consider additional measures of body weight distribution, such as waist circumference, leg circumference, or waist-to-hip ratios.
Conclusions
In this retrospective, single-institution study of MOCA of the GSV and SSV, we found no statistically significant difference in recanalization rates between small and large diameter veins. However, pain and local complications occurred more frequently in patients with large diameter veins. Rates of recanalization were found to be higher in obese patients than in nonobese patients. Further work is needed to study whether morbidly obese patients or patients with large veins might be better served by thermal ablation or saphenectomy compared with MOCA and to assess the generalizability of these results to other institutions.
Author Contributions
Conception and design: VP, AW, JB-G
Analysis and interpretation: VP, AW, RR, CR, MG-T, JB-G
Data collection: AW, JB-G
Writing the article: VP, AW, JB-G
Critical revision of the article: VP, AW, RR, CR, MG-T, JB-G
Final approval of the article: VP, AW, RR, CR, MG-T, JB-G
Statistical analysis: VP, AW, JB-G
Obtained funding: Not applicable
Overall responsibility: VP, JB-G
Disclosures
None.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Beebe-Dimmer J.L., Pfeifer J.R., Engle J.S., Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Criqui M.H. Chronic venous disease in an ethnically diverse population: the San Diego population study. Am J Epidemiol. 2003;158:448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhardt R.T., Raffetto J.D. Chronic venous insufficiency. Circulation. 2014;130:333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 4.Mallick R., Lal B.K., Daugherty C. Relationship between patient-reported symptoms, limitations in daily activities, and psychological impact in varicose veins. J Vasc Surg Venous and Lymphatic Disorder. 2017;5:224–237. doi: 10.1016/j.jvsv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Gloviczki P., Lawrence P.F., Wasan S.M., et al. The 2022 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part I. Duplex scanning and treatment of superficial truncal reflux. J Vasc Surg Venous and Lymphatic Disorder. 2023;11:231–261.e6. doi: 10.1016/j.jvsv.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Mueller R.L., Raines J.K. ClariVein mechanochemical ablation: background and procedural details. Vasc Endovascular Surg. 2013;47:195–206. doi: 10.1177/1538574413477216. [DOI] [PubMed] [Google Scholar]
- 7.Aurshina A., Alsheekh A., Kibrik P., Hingorani A., Marks N., Ascher E. Recanalization after endovenous thermal ablation. Ann Vasc Surg. 2018;52:158–162. doi: 10.1016/j.avsg.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Toniolo J., Chiang N., Munteanu D., Russell A., Hao H., Chuen J. Vein diameter is a predictive factor for recanalization in treatment with ultrasound-guided foam sclerotherapy. J Vasc Surg Venous and Lymphatic Disorder. 2018;6:707–716. doi: 10.1016/j.jvsv.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Vähäaho S., Halmesmäki K., Mahmoud O., Albäck A., Noronen K., Venermo M. Three-year results of a randomized controlled trial comparing mechanochemical and thermal ablation in the treatment of insufficient great saphenous veins. J Vasc Surg Venous and Lymphatic Disorder. 2021;9:652–659. doi: 10.1016/j.jvsv.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Van der Velden S.K., Lawaetz M., De Maeseneer M.G.R., et al. Predictors of recanalization of the great saphenous vein in randomized controlled trials 1 Year after endovenous thermal ablation. Eur J Vasc Endovasc Surg. 2016;52:234–241. doi: 10.1016/j.ejvs.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Lurie F., Passman M., Meisner M., et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8:342–352. doi: 10.1016/j.jvsv.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 12.Brittenden J., Cotton S.C., Elders A., et al. A randomized trial comparing treatments for varicose veins. N Engl J Med. 2014;371:1218–1227. doi: 10.1056/NEJMoa1400781. [DOI] [PubMed] [Google Scholar]
- 13.Cairols M. Commentary on ‘predictors for recanalization of the great saphenous vein in RCTs 1 Year after endovenous thermal ablation’: the dark side of systematic reviews. Eur J Vasc Endovasc Surg. 2016;52:242. doi: 10.1016/j.ejvs.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Chen A.J., Ulloa J.G., Torrez T., et al. Mechanochemical endovenous ablation of the saphenous vein: a look at contemporary outcomes. Ann Vasc Surg. 2022;82:7–12. doi: 10.1016/j.avsg.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Nayman A., Yildiz I., Koca N., Deniz S., Koplay M., Oguzkurt L. Risk factors associated with recanalization of incompetent saphenous veins treated with radiofrequency ablation catheter. Diagn Interv Imaging. 2017;98:29–36. doi: 10.1016/j.diii.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen L.H., Lawaetz M., Bjoern L., Vennits B., Blemings A., Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079–1087. doi: 10.1002/bjs.7555. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed T., Portnoy R., Chachati G., et al. Correlation of body mass index with recanalization risk after endovenous thermal ablation. J Vasc Surg Venous and Lymphatic Disorder. 2022;10:82–86. doi: 10.1016/j.jvsv.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed A.H., Leung C., Wallace T., Smith G., Carradice D., Chetter I. A randomized controlled trial of endovenous laser ablation versus mechanochemical ablation with ClariVein in the management of superficial venous Incompetence (LAMA trial) Ann Surg. 2021;273:e188–e195. doi: 10.1097/SLA.0000000000003749. [DOI] [PubMed] [Google Scholar]
- 19.Ogundimu E.O., Altman D.G., Collins G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. 2016;76:175–182. doi: 10.1016/j.jclinepi.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]