Abstract
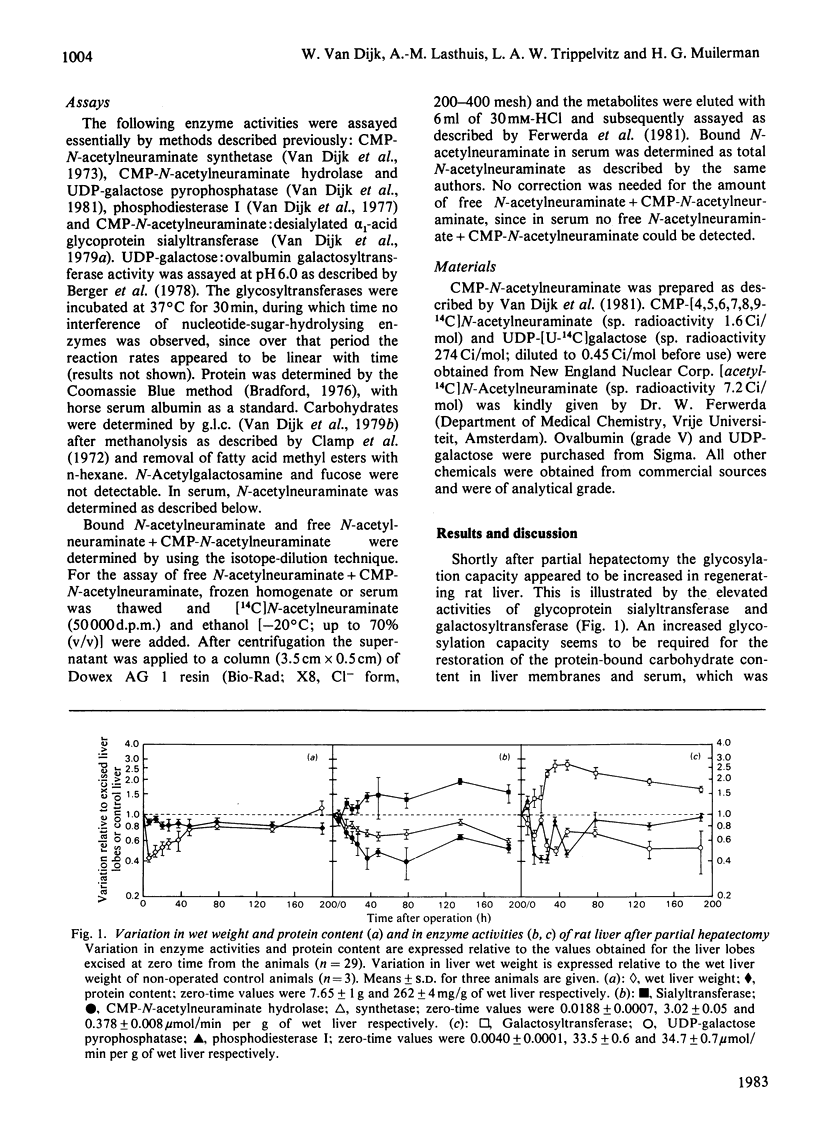
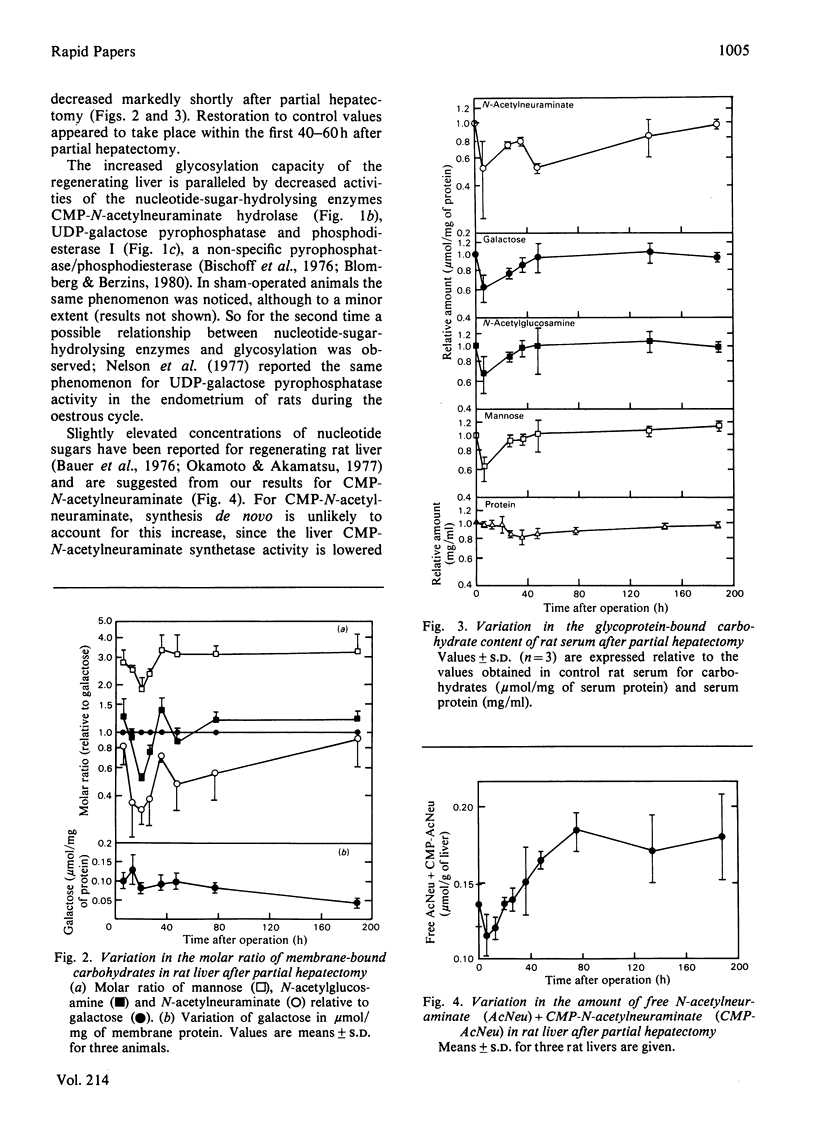
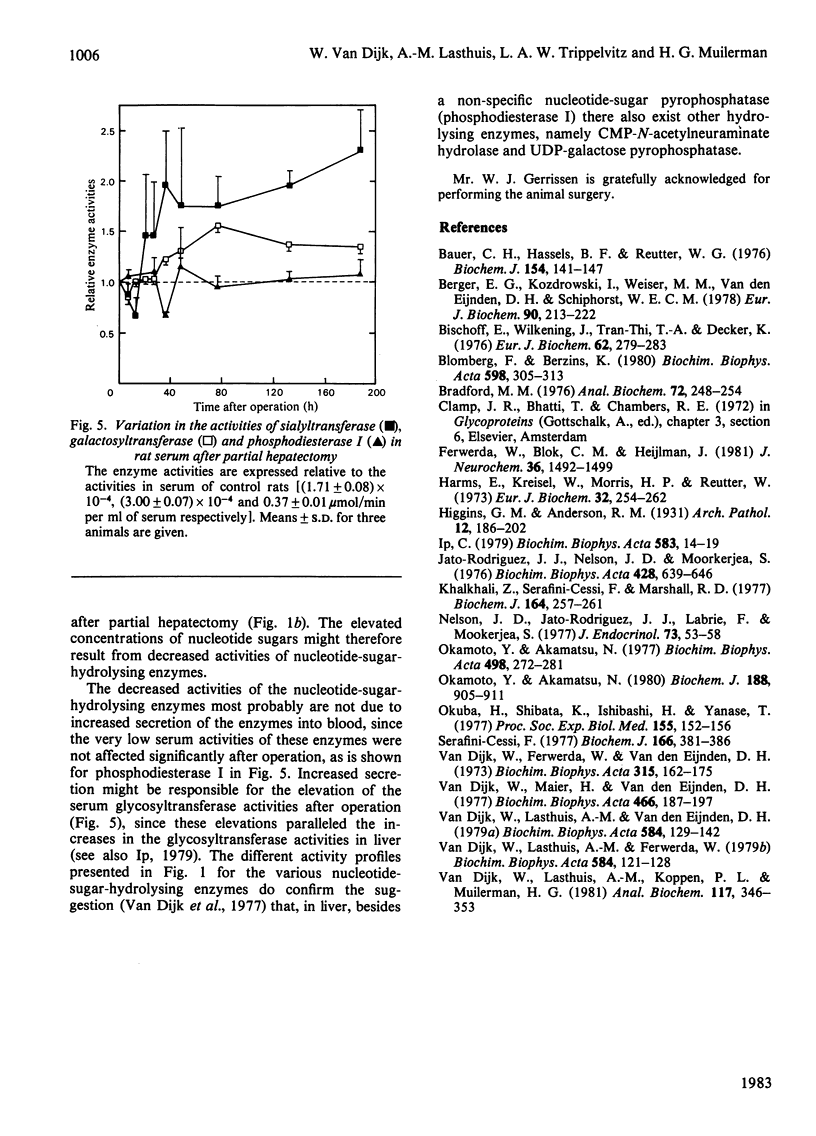
In regenerating rat liver the activities of CMP-N-acetylneuraminate hydrolase and UDP-galactose pyrophosphatase were decreased to 40-50% of control values within 35 h after partial hepatectomy. In the same time period the activities of sialyltransferase and galactosyltransferase were increased, and the initial sharp decrease in the carbohydrate content of liver and serum glycoproteins was largely restored. The antiparallel nature of these events is suggestive of an involvement of nucleotide-sugar-hydrolysis enzymes in rat liver glycoprotein biosynthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. H., Hassels B. F., Reutter W. G. Galactose metabolism in regenerating rat liver. Biochem J. 1976 Jan 15;154(1):141–147. doi: 10.1042/bj1540141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. G., Kozdrowski I., Weiser M. M., van den Eijnden D. H., Schiphorst W. E. Human serum galactosyltransferase: distinction, separation and product identification of two galactosyltransferase activities. Eur J Biochem. 1978 Oct;90(2):213–222. doi: 10.1111/j.1432-1033.1978.tb12593.x. [DOI] [PubMed] [Google Scholar]
- Bischoff E., Wilkening J., Tran-Thi T. A., Decker K. Differentiation of the nucleotide pyrophosphatases of rat-liver plasma membranes and endoplasmic reticulum by enzymic iodination. Eur J Biochem. 1976 Feb 16;62(2):279–283. doi: 10.1111/j.1432-1033.1976.tb10158.x. [DOI] [PubMed] [Google Scholar]
- Blomberg F., Berzins K. Alkaline phosphodiesterase-active antigens in plasma membranes of rat liver. Biochim Biophys Acta. 1980 May 23;598(2):305–313. doi: 10.1016/0005-2736(80)90008-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ferwerda W., Blok C. M., Heijlman J. Turnover of free sialic acid, CMP-sialic acid, and bound sialic acid in rat brain. J Neurochem. 1981 Apr;36(4):1492–1499. doi: 10.1111/j.1471-4159.1981.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Harms E., Kreisel W., Morris H. P., Reutter W. Biosynthesis of N-acetylneuraminic acid in Morris hepatomas. Eur J Biochem. 1973 Jan 15;32(2):254–262. doi: 10.1111/j.1432-1033.1973.tb02605.x. [DOI] [PubMed] [Google Scholar]
- Ip C. Effect of partial hepatectomy and hydrocortisone administration on liver and serum sialyltransferase activities. Biochim Biophys Acta. 1979 Feb 19;583(1):14–19. doi: 10.1016/0304-4165(79)90304-0. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Nelson J. D., Mookerjea S. Effect of estradiol and progesterone on UDPgalactose pyrophosphatase activity in the endometrium of ovariectomized rats. Biochim Biophys Acta. 1976 May 28;428(3):639–646. doi: 10.1016/0304-4165(76)90193-8. [DOI] [PubMed] [Google Scholar]
- Khalkhali Z., Serafini-Cessi F., Marshall R. D. The UDP-N-acetylglucosamine-asparagine-sequon N-acetyl-beta-D-glucosaminyltransferase activity in preparations of rough endoplasmic reticulum from regenerating rat liver. Biochem J. 1977 Apr 15;164(1):257–261. doi: 10.1042/bj1640257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. D., Jato-Rodriguez J. J., Labrie F., Mookerjea S. Glycosyltransferase and UDP-galactose pyrophosphatase activities in the endometrium during oestrous cycle of the rat. J Endocrinol. 1977 Apr;73(1):53–58. doi: 10.1677/joe.0.0730053. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Akamatsu N. Metabolism of sialic acid in regenerating rat liver. Biochem J. 1980 Jun 15;188(3):905–911. doi: 10.1042/bj1880905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Akamatsu N. Synthesis in vitro of glycoprotein in regenerating rat liver. Biochim Biophys Acta. 1977 Jul 21;498(1):272–281. doi: 10.1016/0304-4165(77)90265-3. [DOI] [PubMed] [Google Scholar]
- Okubo H., Shibata K., Ishibashi H., Yanase T. Regulation of N-acetylneuraminic acid synthesis following injury and partial hepatectomy. Proc Soc Exp Biol Med. 1977 Jun;155(2):152–156. doi: 10.3181/00379727-155-39763. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F. Sialyltransferase activity in regenerating rat liver. Biochem J. 1977 Sep 15;166(3):381–386. doi: 10.1042/bj1660381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W., Lasthuis A. M., Koppen P. L., Muilerman H. G. A universal and rapid spectrophotometric assay of CMP-sialic acid hydrolase and nucleoside-diphosphosugar pyrophosphatase activities and detection in polyacrylamide gels. Anal Biochem. 1981 Nov 1;117(2):346–353. doi: 10.1016/0003-2697(81)90791-0. [DOI] [PubMed] [Google Scholar]
- Van Dijk W., Maier H., Van den Eijnden D. H. CMP-N-acetylneuraminic acid hydrolase, an ectoenzyme distributed unevenly over the hepatocyte surface. Biochim Biophys Acta. 1977 Apr 1;466(1):187–197. doi: 10.1016/0005-2736(77)90218-8. [DOI] [PubMed] [Google Scholar]
- van Dijk W., Ferwerda W., van den Eijnden D. H. Subcellular and regional distribution of CMP-N-acetylneuraminic acid synthetase in the calf kidney. Biochim Biophys Acta. 1973 Jul 5;315(1):162–175. doi: 10.1016/0005-2744(73)90139-3. [DOI] [PubMed] [Google Scholar]
- van Dijk W., Lasthuis A. M., Ferwerda W. Preparation and chemical characterisation of calf Tamm-Horsfall glycoprotein. Biochim Biophys Acta. 1979 Apr 18;584(1):121–128. doi: 10.1016/0304-4165(79)90242-3. [DOI] [PubMed] [Google Scholar]
- van Dijk W., Lasthuis A. M., van den Eijnden D. H. Glycoprotein biosynthesis in calf kidney. Glycoprotein sialyltransferase activities towards serum glycoproteins and calf Tamm-Horsfall glycoprotein. Biochim Biophys Acta. 1979 Apr 18;584(1):129–142. doi: 10.1016/0304-4165(79)90243-5. [DOI] [PubMed] [Google Scholar]



