Abstract
Objective
The aim of this study was to determine the association between the duration of systemic anticoagulation therapy (ACT) and the risk of further venous thromboembolism (VTE) in patients with superficial venous thrombosis (SVT).
Methods
A systematic review and meta-analysis were performed using searches of Medline and Cochrane Library databases in September 2023. Papers that provided VTE incidence within mid-term follow-up of ≥45 days in patients who received any ACT were included. Patients were categorized into subgroups according to the course of treatment: (1) no ACT (0 days); (2) ACT of ≤14 days; (3) ACT of 15 to 30 days; (4) ACT of 31 to 45 days; and (5) ACT of >45 days. Reported events were transformed to events per 100 patient-years, and a random-effects model was used to calculate pooled rates for proportions. The primary outcome (VTE) was a combination of SVT progression or recurrence with the occurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE). Secondary outcomes included major and clinically relevant non-major or minor bleeding.
Results
Twenty-four studies (10 randomized controlled trials and 14 cohort studies) combining outcomes in 12,341 patients were included in the quantitative synthesis. Minimum VTE and SVT recurrence or progression rates were observed with the ACT duration of 31 to 45 days of 16.2 (95% confidence interval [CI], 10.4-23.3) and 8.2 (95% CI, 3.1-15.8) events per 100 patient-years, respectively. Minimum DVT and PE rates observed with the treatment duration of 15 to 30 days were 5.5 (95% CI, 2.8-9.1) and 0.9 (95% CI, 0.5-1.3) events per 100 patient-years, respectively. Short-term treatment of ≤14 days was associated with the highest rates of VTE of 59.7 (95% CI, 37.7-86.4), DVT of 13.7 (95% CI, 9.6-18.4), and PE of 3.1 (95% CI, 1.4-5.6) events per 100 patient-years. Major bleeding rates were unrelated to the duration of ACT and did not exceed 0.5 events per 100 patient-years. The highest rate of clinically relevant non-major or minor bleeding was observed with ACT duration of 31 to 45 days of 14.2 (95% CI, 5.5-26.8) events per 100 patient-years. The most common risk factors for VTE included male sex, cancer, personal history of DVT, PE, or SVT, and thrombosis of non-varicose veins.
Conclusions
Prolonged systemic anticoagulation is associated with the tendency to decrease VTE rates in patients with lower limb SVT.
Keywords: Anticoagulation, Superficial vein thrombosis, Treatment, Venous thromboembolism
Superficial venous thrombosis (SVT) of lower limbs, also known as superficial thrombophlebitis, has been considered a self-limited benign disorder for a long time, with an incidence rate of 0.6 to 1.3 SVT events per 1000 patient-years.1, 2, 3 However, emerging evidence suggests a high prevalence of concurrent deep vein thrombosis (DVT) and pulmonary embolism (PE) in 18% and 7% of all patients with SVT, respectively.4 The cohort studies with a mid-term follow-up of 3 months showed an occurrence of further venous thromboembolism (VTE), including SVT progression or recurrence, new DVT, and/or PE in 3% to 10% of all patients.5, 6, 7 In the long-term follow-up, a history of SVT has shown an association with a VTE risk increase of 5.4 to 8.6 times, and the risk of VTE recurrence after SVT is similar to proximal DVT.8, 9, 10 All such findings suggest SVT should be combined with DVT and PE into the group of VTE and be treated with anticoagulation, as stated in the recent guidelines.11,12 The previous meta-analysis included different SVT treatment approaches (nonsteroidal anti-inflammatory drugs [NSAIDs], unfractionated heparin [UFH], low-molecular-weight heparin [LMWH], fondaparinux, rivaroxaban, warfarin, surgery, and no therapy) and showed that anticoagulation is associated with minimal events rate of further VTE.13 However, no standard anticoagulation type, dose, and duration were described. Although emerging evidence suggests a further increase of VTE rate by 50% to 70% after 45 days of anticoagulation in high-risk patients,14 a knowledge gap has been found on the association of prolonged anticoagulation with VTE risk in individuals with SVT. This study aims to address this gap.
Methods
The primary objective of this systematic review was to estimate the rate of VTE development during mid-term follow-up in patients with lower limb SVT without DVT and PE who received any systemic anticoagulation according to the treatment duration. Following the Patient, Intervention, Comparison, Outcome (PICO) rule, the main question was: “How does the duration of anticoagulation therapy impact the risk of venous thromboembolism in patients with superficial vein thrombosis of lower limbs within mid-term follow-up?” The systematic review protocol, including all planned analyses, was registered a priori through PROSPERO, an international database of prospectively registered systematic reviews in health and social care (CRD42021271486).
Search strategy
An electronic Medline and Cochrane Library search was done on September 1, 2023. It was performed according to the pre-specified strategy and keywords. Search terms for PubMed: ((("thrombophlebitis"[MeSH Terms]) OR (superficial vein thrombosis)) OR (superficial thrombophlebitis)) AND ("anticoagulants"[MeSH Terms]); search terms for Cochrane Library: ("superficial venous thrombosis"):ti,ab,kw OR ("thrombophlebitis"):ti,ab,kw AND ("anticoagulant therapy"):ti,ab,kw OR ("anticoagulation"):ti,ab,kw OR ("anticoagulant"):ti,ab,kw). Additionally, lists of references of the relevant publications and personal archives of the authors were screened for any complementary information. The search was restricted by language (English) but not publication date. No additional search was done for unpublished or preprint data, conference abstracts, and other papers not indexed.
References and titles were screened manually without any software. Two reviewers (I.S. and A.B.) independently screened the records for inclusion and exclusion criteria and selected eligible papers. Two reviewers (K.L. and E.D.) independently extracted data from the relevant articles, and the third (I.S.) checked accuracy. A collective discussion among all authors resolved any disagreements between the two reviewers. All relevant information was extracted according to the pre-specified plan through the Excel spreadsheet.
Full-text articles were chosen for the final analysis according to several criteria: (1) reports on unselected patients with symptomatic lower limb SVT without DVT and PE objectively confirmed by duplex ultrasound scan (DUS) in either a prospective cohort (PC), retrospective cohort (RC) study, or randomized controlled trial (RCT); (2) reported treatment with systemic anticoagulation of any type (UFH, LMWH, fondaparinux, vitamin K antagonist [VKA], direct oral anticoagulants), dose, and duration; (3) follow-ups of patients for no less than 45 days; and (4) objectively confirmed VTE event as an absolute number of patients or percentage during the follow-up period. Studies were excluded based on several criteria: (1) primary SVT was not confirmed; (2) known combination of patients with upper and lower limb SVT without providing separate outcomes for lower limb SVT; (3) did not identify the type, dose, and/or duration of anticoagulant therapy; (4) followed patients less than 45 days or did not report the duration of observation; (5) primary outcome was not confirmed; (6) primary outcome was not reported in relation with ACT duration; (7) not original research; (8) case report; and/or (9) not in English. The studies were also checked for data duplication and excluded if population overlap was identified. Among those, a more detailed paper was included.
The systematic review results were reported according to the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA).15
Outcome measures
The primary outcome was designed as any new VTE event that combined symptomatic or asymptomatic SVT progression or recurrence and development of new DVT or PE within a mid-term follow-up ≥45 days. The number of VTE events was calculated manually when individual outcomes were reported or used according to the definition of the original trial. The duration of follow-up was calculated from the diagnosis of SVT and the initiation of treatment, which usually does not match the time of symptom onset. Any of the appropriate imaging should have confirmed outcomes: (1) DUS for the symptomatic and asymptomatic SVT; (2) DUS or phlebography for symptomatic or asymptomatic DVT; and (3) pulmonary angiography or computed tomography pulmonary angiography or ventilation-perfusion scintigraphy for symptomatic PE. The diagnosis, codes, and/or prescription of anticoagulants were also suggested as a form of VTE confirmation in the individual retrospective cohort studies. Secondary outcomes included the components of the primary that were assessed separately: (1) SVT progression or recurrence; (2) new DVT; and (3) PE. The definition of every outcome was used according to the individual study. Also, (4) major bleeding and (5) clinically relevant non-major (CRNM) or minor bleeding, according to the criteria of original studies, were assessed as secondary outcomes.
As pre-specified, outcomes were measured in the different subgroups of patients according to the duration of anticoagulation: (1) ≤14 days; (2) 15 to 30 days; (3) 31 to 45 days; or (4) >45 days. These time intervals were suggested according to the most commonly used period of anticoagulation in the relevant papers. The duration was considered as an intention to treat in the randomized controlled trial and as treated in the cohort trials. If different courses of anticoagulation were reported separately, they were analyzed within the matched subgroups. Otherwise, the whole cohort was analyzed according to the mean or median duration. The use of a placebo in an RCT or absence of anticoagulation in most of the cohort (>90%) was considered as no treatment and was analyzed within a separate subgroup (0 days).
Assessment of study quality
The quality of the RCTs was evaluated using the Cochrane Risk of Bias assessment tool.16 The quality of observational studies was evaluated using the Newcastle-Ottawa Assessment scale for cohort studies.17 Two reviewers (K.L. and E.D.) assessed study quality independently, and a collective discussion of all authors resolved disagreements. A funnel plot analysis with Egger’s test was performed to assess for publication bias.
Strategy of data synthesis
Individual study events were converted to rates per patient-month by estimating the total observation period as the number of patients multiplied by the duration of follow-up. This calculation allowed for a better comparison of studies with different observation periods. The unit of 1 month was chosen due to a low number of participants with short follow-ups reported in some studies, so the number of events exceeded the patient-years and made the analysis of proportions impossible. Event rates were pooled using a pooled proportion meta-analysis with a random-effects model. The final results were translated to patient-years by multiplying patient-months by 12 and were represented with a weighted mean proportion with 95% confidence intervals (CIs) as per 100 patient-years. All analyses were performed using MedCalc statistical software (MedCalc Software Ltd; 2021). Data were analyzed as intention-to-treat, per-protocol, or as-treated according to the original publication. The χ2 test and I2 statistic were used to estimate heterogeneity. Heterogeneity was considered high if the χ2 value of P < .05 or I2 >50%.
Results
Search results
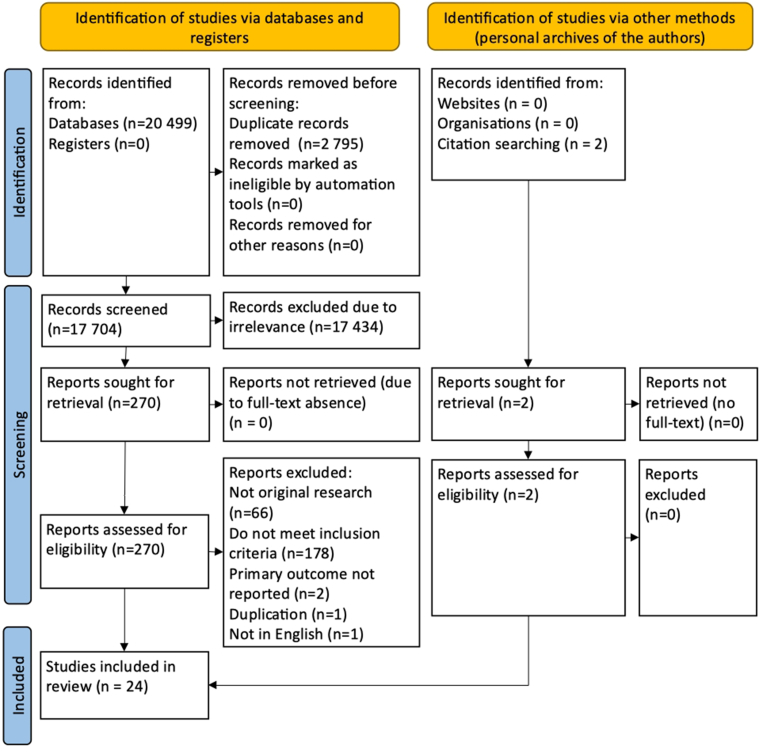
The initial search identified 20,499 records, and two additional papers were found by screening the reference lists of related articles. Only 24 were eligible and combined for the analysis (Supplementary Fig 1, online only). The quantitative synthesis included 10 randomized controlled trials,14,18, 19, 20, 21, 22, 23, 24, 25, 26 and 14 cohort studies3,5,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 containing data on 13,194 patients who received different treatments. The study by Karathanos et al was duplicated: the earlier paper represented a separate analysis of different outcomes (SVT progression or recurrence, DVT, and PE) according to the duration of anticoagulation, whereas the latest extended version allowed extraction of only combined results on VTE.34,35 So, both versions were included in the analysis of different outcomes. The results of the INSIGHTS-SVT trial were also published in two papers, but the latest one with extended follow-up was included.7,38
Supplementary Fig 1 (online only).
Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram.
The results of anticoagulation treatment were obtained from 12,341 patients who were followed for 63,015 patient-months or 5271 patient-years. The duration of anticoagulation varied from 3 to 200 days, and the follow-up time ranged between 74 and 1026 days, with a mean of 169 days. The mean age of patients was 59 years; varicose veins were reported in 66%, personal history of SVT in 19%, personal history of DVT and PE in 8%, family history of VTE in 6%, cancer in 4%, and thrombophilia in 2% of all participants. Detailed characteristics of the studies are presented in Tables I and II, and Supplementary Table I (online only).
Table I.
Summary of included studies
| Author | Design | Total number of patients | Comparison | Anticoagulation duration, days | Follow-up duration, days | VTE imaging | Reporting of asymptomatic VTE |
|---|---|---|---|---|---|---|---|
| Marchiori A et al, 200218 | RCT | 60 | UFH high/intermediate vs prophylactic dose | 28 | 180 | Serial DUS up to 90 days | SVT, DVT |
| STENOX, 200319 | RCT | 427 | Enoxaparin therapeutic or prophylactic dose vs tenoxicam vs placebo | 8-12 | 97 | Serial DUS and CTPA, V/Q, PA for symptomatic PE | SVT, DVT |
| Lozano FS et al, 200320 | RCT | 60 | Enoxaparin high/intermediate dose vs high ligation | 28 | 180 | DUS | N/A |
| Prandoni P et al (VESALIO), 200521 | RCT | 164 | Nadroparin high/intermediate vs prophylactic dose | 30 | 90 | Serial DUS | SVT, DVT |
| Decousus H et al (CALISTO), 201022 | RCT | 3002 | Fondaparinux prophylactic dose vs placebo | 45 | 77 | DUS, V/Q, CTPA, PA, autopsy if symptomatic patients | No |
| Rathbun SW et al, 201223 | RCT | 72 | Dalteparin high/intermediate dose vs ibuprofen | 7-14 | 90 | DUS or V/Q or CTPA if symptomatic | No |
| Cosmi B et al (STEFLUX), 201224 | RCT | 664 | Parnaparin intermediate or prophylactic dose for different duration | 10-30 | 93 | Serial DUS at 30 days; at 90 days on physician's decision | SVT, DVT |
| Spirkoska A et al, 201525 | RCT | 68 | Dalteparin intermediate vs prophylactic dose | 42 | 180 | Serial DUS at 6 weeks, 3 and 6 months | SVT, DVT |
| Beyer-Westendorf J et al (SURPRISE), 201714 | RCT | 472 | Rivaroxaban prophylactic vs fondaparinux prophylactic doses | 45 | 90 | DUS, phlebography, V/Q, CTPA, PA if symptomatic | No |
| Kearon C et al, 202026 | RCT | 85 | Rivaroxaban prophylactic vs placebo | 45 | 90 | DUS at 7 das as recommended | SVT, DVT |
| Ascer E et al, 199527 | PC | 20 | IV UFH followed by warfarin | 42 | 150 | Serial DUS within 2-4 days, 2 weeks, and 8 months | SVT, DVT |
| Gorty S et al, 200428 | PC | 60 | LMWH switched to OAC | 62 | 74 | Serial DUS | SVT, DVT |
| Decousus H et al (POST), 201022 | PC | 844 | Anticoagulation in 91% of patients by predominantly therapeutic (63%) or prophylactic (37%) doses of LWMH | 11 | 90 | Serial DUS at 8-14 days | SVT, DVT |
| Sartori M et al, 201629 | PC | 678 | LMWH therapeutic followed by an intermediate dose | 30 | 90 | Objectively confirmed if symptomatic | No |
| Samuelson B et al, 201630 | RC | 329 | LMWH or warfarin was provided in the minority of patients (4.3%) | 0 | 365 | Reported in the medical records | No |
| Blin P et al, 201731 | RC | 978 | Fondaparinux or LMWH in different doses and duration | 34 ± 18 or 19 ± 27 | 90 | DUS if symptomatic | No |
| Barco S et al (ICARO), 201732 | RC | 411 | LMWH predominantly in prophylactic or intermediate dose | 30 | 1026 | Objectively confirmed if symptomatic | No |
| Gouveia S et al, 201833 | RC | 60 | Enoxaparin prophylactic or intermediate dose in obese | 42 | 84 | Objectively confirmed if symptomatic | No |
| Geersing GJ et al, 20183 | RC | 2008 | LMWH was provided in the minority of patients (7.3%) | 0 | 90 | Reported in the medical records | No |
| Karathanos C et al (SeVEN), 202134 | PC | 660 | Tinzaparin intermediate dose | 14-120 (30) | 90 | Serial DUS at 1 month | SVT, DVT |
| Karathanos C et al (SeVEN), 202335 | PC | 956 | Tinzaparin intermediate doses of different duration | 3-200 (30) | 90 | Serial DUS at 1 month and phone call at 3 months | SVT, DVT |
| Clapham R et al, 202236 | RC | 54 | Rivaroxaban prophylactic dose | 42 | 90 | Symptomatic reported in the medical records, no information on imaging | No |
| Casian D et al, 202237 | RC | 190 | Open or endovascular surgery or anticoagulation of different types, doses, and duration | 28 | 180 | Serial DUS at 2 weeks and 1 month; DUS afterward if symptomatic | SVT and DVT |
| Rabe E et al (INSIGHTS-SVT), 202338 | PC | 872 | Fondaparinux, LMWH, and other anticoagulants in different doses and duration or no anticoagulation | 34.9 ± 15.7 or 26.2 ± 23.2 or 35-43 | 374 | DUS on physicians' decision | SVT |
CTPA, Computed tomography pulmonary angiography; DUS, duplex ultrasound scan; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; N/A, non-available; OAC, oral anticoagulant; PA, pulmonary angiography; PC, prospective cohort; PE, pulmonary embolism; RC, retrospective cohort; RCT, randomized controlled trial; SVT, superficial vein thrombosis; UFH, unfractionated heparin; V/Q, ventilation-perfusion scintigraphy; VTE, venous thromboembolism.
Table II.
Risk profile of the included patients
| Author | Mean age, years | Varicose veins, no. (%) | History of SVT, no. (%) | History of DVT/PE, no. (%) | Family history of VTE, no. (%) | Cancer, no. (%) | Thrombophilia, no. (%) |
|---|---|---|---|---|---|---|---|
| Marchiori et al, 200218 | 62 | 37 (61.7%) | 9 (15.0%) | 7 (11.7%) | N/A | 5 (8.3%) | N/A |
| STENOX, 200319 | 62 | N/A | N/A | 62 (14.5%) | N/A | 6 (1.4%) | N/A |
| Lozano et al, 200320 | 59 | 49 (81.7%) | 29 (48.3%) | N/A | N/A | N/A | N/A |
| Prandoni et al (VESALIO), 200521 | 63 | 100 (61.0%) | N/A | N/A | N/A | N/A | N/A |
| Decousus et al (CALISTO), 201022 | 57 | 2660 (88.6%) | 356 (11.9%) | 209 (7.0%) | N/A | 61 (2.0%) | 38 (1.3%) |
| Rathbun et al, 201223 | 51 | 32 (44.4%) | 7 (9.7%) | 5 (6.9%) | N/A | 7 (9.7%) | N/A |
| Cosmi et al (STEFLUX), 201224 | 63 | 498 (75.0%) | 191 (28.8%) | 58 (8.7%) | 78 (11.7%) | N/A | 31 (4.7%) |
| Spirkoska et al, 201525 | 60 | 0 (0%) | N/A | N/A | N/A | 3 (5.0%) | N/A |
| Beyer-Westendorf et al (SURPRISE), 201739 | 61 | 330 (69.9%) | 229 (48.5%) | N/A | 55 (11.7%) | N/A | |
| Kearon C et al, 202026 | 59 | 58 (68.2%) | 25 (19.4%) | 8 (9.4%) | N/A | 2 (2.4%) | N/A |
| Ascer et al, 199527 | 63 | 10 (50.0%) | 4 (20.0%) | 0 (0%) | N/A | N/A | N/A |
| Gorty et al, 200428 | 52 | 48 (80.0%) | 24 (40.0%) | 10 (16.7%) | N/A | 4 (6.7%) | 12 (20.0%) |
| Decousus et al (POST), 20105 | 65 | 690 (81.8%) | 285 (33.8%) | 120 (14.2%) | 206 (24.4%) | 93 (11.0%) | 48 (5.7%) |
| Sartori et al, 201629 | 65 | 430 (63.4%) | N/A | N/A | N/A | 36 (5.3%) | N/A |
| Samuelson et al, 201630 | 59 | 85 (23.7%) | N/A | 12 (3.3%) | N/A | 29 (8.1%) | N/A |
| Blin et al, 201731 | 65 | 843 (86.2%) | 400 (40.9%) | 196 (20.0%) | N/A | 95 (9.7%) | 23 (2.4%) |
| Barco et al (ICARO), 201732 | 54 | 140 (34.1%) | 153 (37.2%) | 68 (16.5%) | 129 (31.4%) | 29 (7.1%) | N/A |
| Gouveia et al, 201833 | 51 | 43 (71.7%) | 7 (11.7%) | 21 (35.0%) | 4 (6.7%) | 0 (0%) | 2 (3.3%) |
| Geersing et al, 20183 | 56 | 782 (38.9%) | N/A | N/A | N/A | 81 (94.0%) | N/A |
| Karathanos et al (SeVEN), 202134 | 59 | 436 (66.1%) | 187 (28.3%) | 34 (5.2%) | 91 (13.8%) | 10 (1.5%) | N/A |
| Karathanos C et al (SeVEN), 202335 | 59 | 601 (62.9%) | 256 (26.8%) | 48 (5.0%) | 93 (9.7%) | 3 (0,3%) | N/A |
| Clapham R et al, 202236 | 61 | 41 (75.9%) | 9 (16.7%) | 16 (29.6%) | 8 (14.8%) | 1 (1.9%) | 1 (1.9%) |
| Casian D et al, 202237 | 60 | 104 (54.7%) | N/A | N/A | N/A | N/A | N/A |
| Rabe E et al (INSIGHTS-SVT), 202338 | 61 | 698 (80.0%) | 278 (31.9%) | 148 (17.0%) | 145 (16.6%) | 63 (7.2%) | 50 (5.7%) |
DVT, Deep vein thrombosis; N/A, not available; PE, pulmonary embolism; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
Six studies included patients who did not use anticoagulants. They were placebo groups in the STENOX19 and CALISTO trials22 and an RCT by Kearon et al26 that compared rivaroxaban (10 mg) with placebo. Two retrospective cohort studies with the assessment of medical records for diagnosis codes, disease-related free text, and anticoagulants that were prescribed in <10% of patients with established SVT without DVT and PE were also included. Geersing et al analyzed a database of general practitioners to identify patients seeking care for SVT.3 Still, they could not extract any information on the thrombus localization, so the combined data was used for the synthesis. In contrast, Samuelson et al reported separate results for SVT of lower extremities, accounting for 61% of the cohort.30 However, they presented only the total number of VTE events without clarification of DVT and PE. In the prospective cohort study INSIGHTS-SVT, 6.7% of patients did not receive anticoagulation, whereas separate outcomes of VTE were available for analysis.38
In four studies, patients received anticoagulation for ≤14 days. In two RCTs, treatment with LMWH was designed for 7 to 14 days and compared with NSAIDs and placebo.19,23 In the STEFLUX randomized controlled trial, one of three groups received an intermediate dose of LMWH for only 10 days, followed by a placebo for additional 20 days.24 In the prospective observational trial (POST), almost all patients received treatment with therapeutic (63%) or prophylactic (37%) doses of LWMH for a median of 11 days.5 Therapy with VKA was continued for a mean of 81 days in a limited number of patients (17%). Thus, these participants were considered as having a short-term treatment of ≤14 days. The authors did not present the localization of asymptomatic VTE detected by DUS at 8 to 14 days follow-up, so the total number of VTE did not match their localization.
Eleven studies reported the use of anticoagulants for 15 to 30 days. Three RCTs were designed to use LMWH or UFH for 28 to 30 days while comparing different regimens or in comparison with surgical treatment.18,20,21 In the STEFLUX study, two of three groups received either prophylactic or intermediate doses of parnaparin for 30 days, and these two groups were combined for the analysis.24 One prospective and one retrospective cohort trial assessed outcomes of SVT treatment with different doses of LMWH for 30 days.29,32 In the prospective cohort study by Casian et al, open or endovascular surgery was compared with administration of different anticoagulants in prophylactic or therapeutic doses for a median duration of 28 days.37 The last four cohort studies reported using anticoagulants separately according to the duration. In the study of Blin et al, fondaparinux was used for 34 ± 18 days and LMWH for 19 ± 27 days.31 Therefore, patients who received LMWH were considered to be treated for 15 to 30 days. According to Karathanos et al, tinzaparin was used for 30 (14-120 or 3-200) days and analyzed separately in two subgroups: (1) ≤1 month and (2) >1 month.34,35 The first subgroup was considered to have a duration of 15 to 30 days. In the INSIGHTS-SVT study, 25% of patients received LMWH for 26.2 ± 23.2 days (median, 21 days) and were considered to have treatment duration of 15 to 30 days.38
Ten studies assessed outcomes of anticoagulant therapy for 31 to 45 days. The CALISTO trial compared subcutaneous fondaparinux (2.5 mg) with placebo, and the SURPRISE trial compared oral rivaroxaban (10 mg) with the same dose of fondaparinux.14,22 The duration of treatment was 45 days in both studies. Kearon et al preliminarily halted an RCT aimed to compare rivaroxaban (10 mg) and placebo with a treatment duration of 45 days.26 A randomized controlled trial by Spirkoska et al compared intermediate and prophylactic doses of dalteparin in terms of thrombus regression.25 The duration of treatment was designed to be 42 days. Two cohort studies assessed intravenous UFH followed by warfarin and prophylactic or intermediate doses of LMWH for 42 days.27,33 The corresponding subgroups of patients from the above-mentioned cohort trials by Blin et al (fondaparinux with a mean duration of 34 ± 18 days) and Karathanos et al (a subgroup of >1 month) were all considered as having a treatment duration of 31 to 45 days.31,34,35 In the INSIGHTS-SVT trial, patients received fondaparinux for 34.9 ± 15.7 days (median, 39 days) and other anticoagulants for 35 to 43 days (figures are extracted from the table).38 Clapham et al analyzed treatment results with a prophylactic dose of rivaroxaban (10 mg) for 42 days in the retrospective cohort trial.36
Only one prospective cohort study assessed the use of LMWH followed by non-specified oral anticoagulant (OAC) for a mean of 62 days in comparison with the absence of anticoagulation and surgical treatment.28 These patients were matched with a subgroup of treatment duration of >45 days.
Assessment of quality
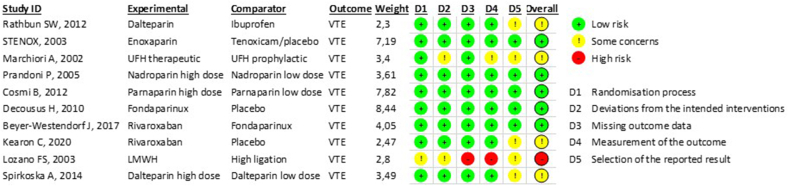
The quality assessment results are presented in Supplementary Fig 2 (online only) and Supplementary Table II (online only). In general, moderate-to-high quality was judged in the RCTs, and poor quality in the cohort trials due to the lack of comparability of cohorts based on the design and analysis controlled for confounders.
Supplementary Fig 2 (online only).
The results of bias (RoB) risk assessment of randomized clinical trials with RoB 2 Tool. VTE, Venous thromboembolism.
Assessment of publication bias
Funnel plots were generated by plotting the individual study-reported VTE event rate (proportion, x-axis) against the standard error (y-axis) for all pooled estimates obtained from studies. Publication bias was suggested by the asymmetry of the plot and confirmed by Egger’s test (P < .001).
Primary and secondary outcomes
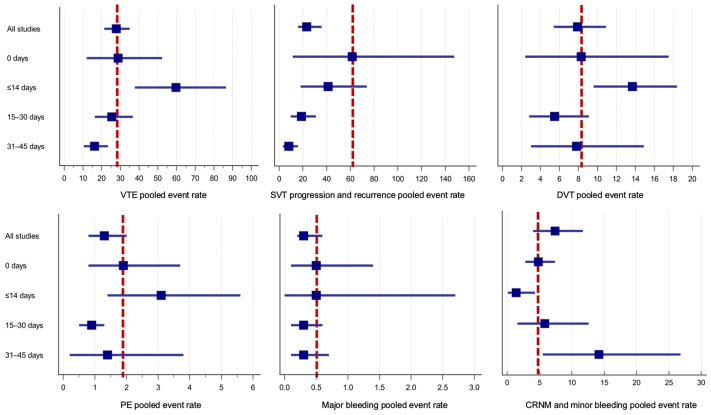
The VTE and bleeding rates according to the ACT duration, as extracted from the original trials, are represented in Supplementary Table III (online only). Table III and the Fig illustrate the pooled analysis results.
Table III.
The pooled event rate per 100 patient-years of venous thromboembolism (VTE) and bleeding according to the duration of anticoagulation treatment
| Duration of anticoagulation | VTE | SVT progression or recurrence | DVT | PE | Major bleeding | CRNM and minor bleeding |
|---|---|---|---|---|---|---|
| All studies | 27.8 (21.4-34.9) | 23.3 (15.8-35.8) | 7.9 (5.4-10.9) | 1.3 (0.8-2.0) | 0.3 (0.2-0.6)a | 7.4 (4.0-11.7) |
| 0 days | 28.8 (12.0-52.4) | 61.5 (11.5-147.6) | 8.3 (2.4-17.5) | 1.9 (0.8-3.7)a | 0.5 (0.1-1.4)a | 4.8 (2.8-7.4)a |
| ≤14 days | 59.7 (37.7-86.4) | 41.3 (17.9-73.9) | 13.7 (9.6-18.4)a | 3.1 (1.4-5.6)a | 0.5 (0.0-2.7)a | 1.4 (0.1-4.3)a |
| 15-30 days | 25.4 (16.3-36.6) | 18.8 (9.7-30.9) | 5.5 (2.8-9.1) | 0.9 (0.5-1.3)a | 0.3 (0.1-0.6)a | 5.8 (1.6-12.6) |
| 31-45 days | 16.2 (10.4-23.3) | 8.2 (3.1-15.8) | 7.8 (3.0-14.9) | 1.4 (0.2-3.8) | 0.3 (0.1-0.7)a | 14.2 (5.5-26.8) |
CRNM, Clinically relevant non-major; DVT, deep vein thrombosis; PE, pulmonary embolism; SVT, superficial vein thrombosis.
Indicates low heterogeneity (P > .05 and I2< 50%).
Fig.
The pooled event rates of primary and secondary outcomes. Data are presented as per 100 patient-years. Boxes indicate polled event rates and whisker–lower and upper 95% confidence interval (CI). The vertical dashed line corresponds to the polled event rate in the group of no anticoagulation (0 days). CRNM, Clinically relevant non-major; DVT, deep vein thrombosis; PE, pulmonary embolism; SVT, superficial venous thrombosis; VTE, venous thromboembolism.
The analysis of all studies for the primary outcome showed a high heterogeneity of results (P < .0001; I2= 94.14%). The pooled rate of VTE was estimated as 27.8 (95% CI, 21.4-34.9) per 100 patient-years with the incidence of SVT progression or recurrence, DVT, and PE as 23.3 (95% CI, 15.8-35.8), 7.9 (95% CI, 5.4-10.9), and 1.3 (95% CI, 0.8-2.0) per 100 patient-years, respectively. The rate of major bleeding was reported as 0.3 (95% CI, 0.2-0.6), and CRNM bleeding as 7.4 (95% CI, 4.0-11.7) per 100 patient-years.
In patients who did not receive anticoagulation (0 days), the event rate of VTE, SVT progression or recurrence, DVT, and PE was higher when compared with the pooled data from all studies. However, the rate of major bleeding did not differ, and the rate of CRNM bleeding was lower. The maximum incidence of VTE, DVT, and PE was observed in the studies with short-term anticoagulation of ≤14 days as 59.7 (95% CI, 37.7-86.4), 13.7 (95% CI, 9.6-18.4), and 3.1 (95% CI, 1.4-5.6) per 100 patient-years, respectively. These event rates were higher than the pooled data from all studies and compared with the absence of anticoagulation. However, short-term anticoagulation caused a reduction in the incidence of SVT progression or recurrence from 61.5 (95% CI, 11.5-147.6) to 41.3 (95% CI, 17.9-73.9) compared with no treatment. The rate of major bleeding was comparable to the absence of anticoagulation, whereas the rate of CRNM bleeding was the lowest (1.4; 95% CI, 0.1-4.3 per 100 patient-years).
The minimal VTE incidence was observed in prolonged anticoagulation of 31 to 45 days (16.2; 95% CI, 10.4-23.3 per 100 patient-years) followed by treatment of 15 to 30 days (25.4; 95% CI, 16.3-36.6 per 100 patient-years). The lowest rates of secondary outcomes were also observed in these groups with a minimum incidence of SVT progression and recurrence for the treatment of 31 to 45 days (8.2; 95% CI, 3.1-15.8 per 100 patient-years) and the lowest rate of DVT (5.5; 95% CI, 2.8-9.1 per 100 patient-years) and PE (0.9; 95% CI, 0.5-1.3 per 100 patient-years) for the treatment of 15 to 30 days. The rates of major bleeding were similar in both groups and did not differ from the pooled estimates of all studies, whereas the rate of CRNM or minor bleeding was the highest with anticoagulation of 31 to 45 days (14.2; 95% CI, 5.5-26.8 per 100 patient-years).
Only one study assessed treatment duration of more than 45 days with event rates of VTE, SVT, DVT, and PE of 141, 118, 24, and 0 per 100 patient-years, respectively, without any information on bleeding.28 These figures appeared to be much higher compared with others due to a limited number of patients and a short follow-up period.
The risk factors for VTE development within the follow-up period were also extracted from the relevant studies and presented in Table IV. One additional trial from the full-text analysis that did not fulfill the inclusion criteria (did not clarify the duration of anticoagulation) reported the risk factors, and the data from the STEFLUX trial were published separately.6,40 The most common (represented in ≥3 studies) factors that increased the VTE risk by 2 to 3 times were male sex, cancer, personal history of DVT, PE, and/or SVT, and thrombosis of non-varicose veins.
Table IV.
Risk factors for venous thromboembolism (VTE) development during the follow-up period
| STENOX, 200319 | Decousus et al (POST), 20105 | Galanaud et al (Optimev), 20116 | Cosmi et al (STEFLUX), 201224, 40 | Barco S et al (ICARO), 201732 | Blin et al, 201731 | Geersing et al, 20183 | Casian D et al, 202237 | Karathanos C et al (SeVEN), 202335 | Rabe E et al (INSIGHTS-SVT), 202338 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male sex | 2.2 (1.3-3.7) | 2.6 (1.4-4.9) | 3.5 (1.1-11.3) | 2.0 (1.2-3.5) | ||||||
| Cancer | 3.1 (1.2-8.5) | 3.1 (1.1-8.9) | 2.2 (0.9-4.9) | |||||||
| Inpatient treatment | 4.5 (1.3-15.3) | |||||||||
| History of VTE | 2.1 (1.1-4.0) | 2.2 (1.2-4.1) | 2.1 (1.3-3.2) | 2.5 (1.1-6.1) | 2.9 (1.5-5.6) | |||||
| Non-varicose veins | 2.1 (1.1-4.3) | 2.6 (1.3-5.0) | 1.8 (1.0-2.9) | |||||||
| Symptoms <7 days | 3.0 (1.4-6.3) | |||||||||
| Progressive stage of CVD | 2.8 (1.1-6.9) | |||||||||
| Increased BMI | 2.2 (1.3-3.2) | 1.06 (1.02-1.11) | ||||||||
| Thrombus <3 cm of junction | 2.5 (1.1-6.1) | |||||||||
| Thrombus length | 1.02 (1.0-1.05) | |||||||||
| Chronic- reduced mobility | 4.6 (1.5-14.2) | |||||||||
| Severe systemic infection | 7.6 (1.8-32.5) |
BMI, Body mass index; CVD, chronic venous disease (progressive CVD means stage 3 by Porter);
BMI increased means more than 25 kg/m2.
Cancer combines active and historical.
History of VTE combines personal and family history of superficial vein thrombosis, deep vein thrombosis, and pulmonary embolism.
Thrombus length reported as hazard ratio for every 1 cm.
Data are presented as the odds ratios or hazard ratios (with 95% confidence interval).
Discussion
Different approaches have been suggested to treat lower limb SVT. Topical agents, NSAIDs, anticoagulants, heparinoids, elastic compression, and surgical intervention aim to reduce the symptoms of inflammation and prevent further VTE due to SVT extension into deep veins and to prevent the occurrence of new DVT and PE. Systemic anticoagulation appeared to be the most protective for VTE in thrombosis length of >5 cm.11, 12, 13,41 In the case of smaller thrombus, local cold, topical agents, NSAIDs, and elastic compression are indicated without systemic anticoagulation.11,12,39, 42, 43, 44 VTE threat increases if thrombus length is more than 5 cm, predominantly if it is localized above the knee or near the connection with deep veins. Studies on systemic anticoagulation included primarily patients with extensive SVT not reaching the junction. However, those with thrombus located within a 3 to 5 cm near junction were generally excluded, so the best treatment approach for them is not established (Supplementary Table I, online only). The current guidelines empirically equate such SVT with proximal DVT and suggest full-dose therapeutic anticoagulation for 6 to 12 weeks.11,12 However, a recent analysis of the RIETE registry by Prandoni et al did not find any differences between full therapeutic and prophylactic anticoagulation that was administrated predominantly for 12 weeks in patients with SVT within 3 cm near a junction (the study was considered ineligible due to the absence of separate outcomes for different treatment durations).45
Thereby, systemic anticoagulation, as evaluated in the current systematic review, corresponds to extensive SVT with a length of >5 cm, not reaching the junction by 3 to 5 cm (Supplementary Table I, online only). The previous meta-analyses focused on the type and doses of anticoagulation but not on duration. The authors did not find any significant differences between low (prophylactic) and high (intermediate or therapeutic) doses of LMWH while judging subcutaneous fondaparinux at 2.5 mg for 45 days as the best treatment associated with the lowest rate of VTE.13,41 This conclusion was based on the results of the largest randomized controlled trial CALISTO and insights from a few cohort trials.7,22,31,38 However, the comparison of fondaparinux with LMWH or other anticoagulants does not consider treatment duration. In most trials, LMWH was used for a short period of ≤14 or 15 to 30 days.
In the current meta-analysis, the treatment duration of ≤14 days was associated with the highest rate of VTE, which was even higher than that with no anticoagulation. These findings may be biased by large cohort trials, where patients without estimated risk did not develop VTE despite the absence of systemic anticoagulation.3,30,38 It can be assumed that most of them had a limited SVT without additional VTE risk factors in contrast with other trials where individuals with higher VTE risk were treated with short-term anticoagulation. Like in DVT treatment, limited anticoagulation for SVT may be insufficient to control hypercoagulation and prevent VTE recurrence.46
Combining the results of the previous analyses based on type or dose indicates that the actual efficacy of the treatment with LMWH may be underestimated. Notably, in cohort trials by Rabe et al and Blin et al, fondaparinux showed superiority over LMWH.31,38 However, the mean duration of therapy with fondaparinux was 35 ± 16 and 34 ± 8 days, respectively, compared with 26 ± 23 and 19 ± 27 days for LMWH, respectively.
In this study, we chose an alternative approach and combined different types and doses of anticoagulation according to the duration of treatment. An apparent tendency toward VTE risk reduction with prolonged therapy was observed. Treatment for 15 to 30 and 31 to 45 days was associated with the lowest rates of VTE. However, this rate was relatively high, particularly in terms of SVT progression or recurrence and DVT. Our findings are similar to the results of the previous meta-analysis by Duffet et al, who found that prolonged use of LMWH may reduce the event rate of DVT and PE from 18.3 (95% CI, 8.3-31.1) events per 100 patient-years with the treatment duration less than 30 days to 10.0 (95% CI, 5.3-16.1) events per 100 patient-years with the course of 30 to 42 days.13 However, the results of the SURPRISE trial suggest that 45 days of anticoagulation may not be enough for patients at high risk of VTE.14 Within 45 days after treatment cessation, the incidence of VTE increased from 3% to 7% and 2% to 7% in groups of rivaroxaban and fondaparinux, respectively. Unfortunately, we could not find any strong evidence of prolonged treatment lasting more than 45 days to be used in quantitative synthesis. Karathanos et al reported the use of LMWH up to 200 days with a mean duration of 30 days.35 Actually, less than one-half of patients continued treatment after 30 days, and only 1.2% continued after 90 days. The authors did not find any difference in the outcomes between subgroups of patients treated for less and more than 30 days. However, this study of real clinical practice was biased, and prolonged treatment was prescribed for patients at a higher risk, particularly those with a history of DVT and thrombus location above the knee.
Individual VTE risk factors may play a pivotal role in the development of new thrombosis after cessation of anticoagulation and may be used for treatment adjustment. Unfortunately, not all studies correctly reported the most common risk factors, which may lead to inadequate treatment and an increase in new thrombotic events. Male sex, cancer, personal history of DVT, PE, and/or SVT, and thrombosis of non-varicose veins were identified as the most common risk factors that could be considered for individual treatment adjustment. Thus, treatment for more than 45 days may be beneficial in selected patients at high risk of VTE. However, such suggestions must be tested in randomized controlled trials to estimate the risk/benefit ratio.
Our review has limitations. The primary outcome was designed as any new VTE, combined SVT progression or recurrence, DVT, and PE. It was chosen due to strong evidence of SVT association with the development of clinically significant DVT and PE, and its progression or recurrence may indicate treatment failure. However, not all analyzed papers reported individual outcomes, and VTE could be defined as DVT and PE only according to the design of original trials. This fact introduced additional heterogeneity in the primary outcome analysis. The data from RCTs and cohort trials of varying quality and consistency were combined, and the statistical analysis of the difference between groups was unavailable. The reported pooled proportions are based on study-level data rather than individual patient data. Most studies did not present the follow-up as patient-years, so patient-months and patient-years were calculated based on the intended or median follow-up rather than on actual individual observation periods. The proportions estimated for relatively short observation periods may be valid for the assumption of an event rate that is consistent over time. Events that occurred within the follow-up period included both on- and off-anticoagulation. Mortality rates were not analyzed due to a low number of events in the previous reviews. Information on the further treatment after cessation of anticoagulation, particularly the surgical removal of varicose veins that may affect the risk of SVT recurrence and DVT or PE occurrence, was not available for analysis. Finally, most calculations were associated with high heterogeneity.
The systematic review and meta-analysis results suggest prolonged systemic anticoagulation is associated with decreased VTE rates in patients with lower limb SVT. The optimal duration of anticoagulant treatment needs to be established in future trials.
Author Contributions
Conception and design: KL
Analysis and interpretation: KL, ED, IS, AB
Data collection: KL, ED, IS, AB
Writing the article: KL, ED
Critical revision of the article: KL, ED, IS, AB
Final approval of the article: KL, ED, IS, AB
Statistical analysis: KL
Obtained funding: Not applicable
Overall responsibility: KL
Disclosures
None.
Footnotes
Additional material for this article may be found online at www.jvsvenous.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
PROSPERO registry number: CRD42021271486.
Appendix
Additional material for this article may be found online at www.jvsvenous.org
Appendix (online only).
Supplementary Table I (online only).
Inclusion and exclusion criteria in the analyzed trials
| Author | Inclusion criteria | Exclusion criteria | Patient selection according to thrombus location close to the junction |
|---|---|---|---|
| Marchiori A et al, 200218 |
|
|
SVT within 1 cm of the SFJ excluded |
| STENOX, 200319 |
|
|
Those who required ligation or full therapeutic anticoagulation excluded |
| Lozano FS et al, 200320 |
|
|
SVT with thrombus close to the junction included |
| Prandoni P et al (VESALIO), 200521 |
|
|
SVT within 3 cm of the SFJ excluded |
| Decousus H et al (CALISTO), 201022 |
|
|
SVT within 3 cm of the SFJ excluded |
| Rathbun SW et al, 201223 |
|
|
Not reported |
| Cosmi B et al (STEFLUX), 201224 |
|
|
SVT within 3 cm of SFJ or SPJ excluded |
| Spirkoska A et al, 201525 |
|
|
SVT within 5 cm of the SFJ or 3 cm of SPJ excluded |
| Beyer-Westendorf J et al (SURPRISE), 201714 |
|
|
SVT within 3 cm of SFJ excluded |
| Kearon C et al, 202026 |
|
|
Those who required surgical management or full therapeutic anticoagulation excluded |
| Ascer E et al, 199527 |
|
|
SVT with thrombus close to the junction included |
| Gorty S et al, 200428 |
|
|
Not reported |
| Decousus H et al (POST), 20105 |
|
|
Not reported |
| Sartori M et al, 201629 |
|
|
SVT within 3 cm of the SFJ excluded |
| Samuelson B et al, 201630 |
|
Not indicated | Not reported |
| Blin P et al, 201731 |
|
|
Not reported |
| Barco S et al (ICARO), 201732 |
|
|
Not reported |
| Gouveia S et al, 201833 |
|
|
SVT within 3 cm of the SFJ excluded |
| Geersing GJ et al, 20183 |
|
|
Not reported |
| Karathanos C et al (SeVEN), 202134 |
|
|
SVT within 3 cm of the SFJ or SPJ excluded |
| Karathanos C et al (SeVEN), 202335 |
|
|
SVT within 3 cm of the SFJ or SPJ excluded |
| Clapham R et al, 202236 |
|
|
SVT within 3 cm of the SFJ excluded |
| Casian D et al., 202237 |
|
|
Not reported |
| Rabe E et al (INSIGHTS-SVT), 202338 |
|
|
SVT within 3 cm of the SFJ excluded |
DUS, Duplex ultrasound scan; DVT, deep vein thrombosis; GP, general practitioner; GSV, great saphenous vein; ICD-CM, International Classification of Diseases, Clinical Modification; ICPC, International Classification of Primary Care; LMWH, low-molecular weight heparin; NSAID, non-steroidal anti-inflammatory drug; PE, pulmonary embolism; SFJ, sapheno-femoral junction; SPJ, sapheno-popliteal junction; SSV, small saphenous vein; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
Supplementary Table II (online only).
Risk of bias Newcastle-Ottawa for cohort studies
| Author | Summary: Selection (max. four stars) |
Summary: Comparability (max. two stars) |
Summary: Outcome (max. three stars) |
Quality |
|---|---|---|---|---|
| Ascer E et al, 199527 | ☆☆☆ | – | ☆☆ | Poor |
| Gorty S et al, 200428 | ☆☆☆☆ | – | ☆☆☆ | Poor |
| Decousus H et al (POST), 20105 | ☆☆☆ | – | ☆☆☆ | Poor |
| Sartori M et al, 201629 | ☆☆☆ | – | ☆☆ | Poor |
| Samuelson B et al, 201630 | ☆☆ | – | ☆☆ | Poor |
| Blin P et al, 201731 | ☆☆☆☆ | – | ☆☆ | Poor |
| Barco S et al (ICARO), 201732 | ☆☆☆ | – | ☆☆☆ | Poor |
| Gouveia S et al, 201833 | ☆☆☆ | – | ☆☆ | Poor |
| Geersing GJ et al, 20183 | ☆☆ | – | ☆☆ | Poor |
| Karathanos C et al, 202134 | ☆☆☆☆ | – | ☆☆☆ | Poor |
| Karathanos C et al, 202335 | ☆☆☆☆ | ☆☆ | ☆☆☆ | Good |
| Clapham R,et al, 202236 | ☆☆☆ | – | ☆☆☆ | Poor |
| Rabe E et al, 202338 | ☆☆☆☆ | – | ☆☆ | Poor |
| Casian D et al, 202237 | ☆☆☆☆ | ☆ | ☆☆☆ | Good |
Thresholds for converting the Newcastle-Ottawa scales to the Agency for Healthcare Research and Quality standards (good, fair, and poor):
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain;
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain;
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
Supplementary Table III (online only).
The main results of the trials used for quantitative synthesis
| Author | Anticoagulation duration, days | No. patients in the group | Total VTE, n (%) | SVT progression or recurrence, n (%) | DVT, n (%) | PE, n (%) | Major bleeding, n (%) | CRNM and minor bleeding, n (%) |
|---|---|---|---|---|---|---|---|---|
| Marchiori A et al, 200218 | 28 | 60 | 26 (43.3) | 19 (31.7) | 6 (10.0) | 1 (1.7) | 0 (0) | N/A |
| STENOX, 200319 | 18-21 | 216 | 36 (16.7) | 32 (14.8) | 9 (4.2) | 2 (0.9) | 0 (0) | 1 (0.5) |
| 0 | 112 | 38 (33.9) | 37 (33.0) | 5 (4.5) | 0 (0) | 0 (0) | 1 (0.9) | |
| Lozano FS et al, 200320 | 28 | 30 | 3 (10.0) | 3 (10.0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.7) |
| Prandoni P et al (VESALIO), 200521 | 30 | 164 | 13 (7.9) | 7 (4.3) | 5 (3.0) | 1 (0.6) | 0 (0) | N/A |
| Decousus H et al (CALISTO), 201022 | 45 | 1502 | 17 (1.1) | 13 (0.9) | 4 (0.3) | 0 (0) | 1 (0.1) | 16 (1.1) |
| 0 | 1500 | 102 (6.8) | 80 (5.3) | 19 (1.3) | 6 (0.4) | 1 (0.1) | 15 (1.0) | |
| Rathbun SW et al, 201223 | 7-14 | 37 | 4 (10.8) | 2 (5.4) | 1 (2.7) | 1 (2.7) | 0 (0) | 0 (0) |
| Cosmi B et al (STEFLUX), 201224 | 10 | 217 | 48 (22.1) | 37 (17.1) | 10 (4.6) | 1 (0.5) | 0 (0) | 0 (0) |
| 30 | 446 | 50 (11.2) | 39 (8.7) | 10 (2.2) | 1 (0.2) | 0 (0) | 2 (0.4) | |
| Spirkoska A et al, 201525 | 42 | 68 | 3 (4.4) | 0 (0) | 2 (2.9) | 1 (1.5) | 0 (0) | 4 (5.9) |
| Beyer-Westendorf J et al (SURPRISE), 201714 | 45 | 472 | 30 (6.4) | 23 (4.9) | 8 (1.7) | 0 (0) | 0 (0) | 40 (8.5) |
| Kearon C et al, 202026 | 45 | 43 | 1 (2.3) | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) | 1 (2.3) |
| 0 | 42 | 5 (11.9) | 5 (11.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Ascer E et al, 199527 | 42 | 12 | 2 (16.7) | 0 (0) | 2 (16.7) | 0 (0) | N/A | N/A |
| Gorty S et al, 200428 | 62 | 21 | 6 (28.6) | 5 (23.8) | 1 (4.8) | 0 (0) | N/A | N/A |
| Decousus H et al (POST), 20105 | 11 | 586 | 58 (9.9) | 28 (4.8) | 15 (2.6) | 3 (0.5) | N/A | N/A |
| Sartori M et al, 201629 | 30 | 678 | 36 (5.3) | 29 (4.3) | 6 (0.9) | 1 (0.1) | 0 (0) | N/A |
| Samuelson B et al, 201630 | 0 | 200 | 15 (7.5) | N/A | N/A | N/A | N/A | N/A |
| Blin P et al, 201731 | 34±18 | 735 | 24 (3.3) | N/A | N/A | N/A | 0 (0) | N/A |
| 19±27 | 127 | 7 (5.5) | N/A | N/A | N/A | 0 (0) | N/A | |
| Barco S et al (ICARO), 201732 | 30 | 411 | 152 (37.0) | 100 (24.3) | 37 (9.0) | 12 (2.9) | 3 (0.7) | N/A |
| Gouveia S et al, 201833 | 42 | 60 | 11 (18.3) | 4 (6.7) | 5 (8.3) | 2 (3.3) | 0 (0) | 3 (5.0) |
| Geersing GJ et al, 20183 | 0 | 1957 | 32 (1.6) | N/A | N/A | N/A | N/A | N/A |
| Karathanos C et al (SeVEN), 202134 | ≤30 | 347 | 6 (1.7) | 4 (1.2) | 2 (0.6) | 0(0) | 1 (0.3) | 2 (0.6) |
| >30 | 313 | 9 (2.9) | 5 (1.6) | 3 (1.0) | 1 (0.3) | 0 (0) | 2 (0.6) | |
| Karathanos C et al (SeVEN), 202335 | ≤30 | 552 | 17 (3.1%) | N/A | N/A | N/A | N/A | N/A |
| >30 | 404 | 16 (4.0%) | N/A | N/A | N/A | N/A | N/A | |
| Clapham R et al, 202236 | 42 | 52 | 1 (1.9) | 0 (0) | 1 (1.9) | 0 (0) | 0 (0) | 1 (1.9) |
| Casian D et al, 202237 | 28 | 105 | 6 (5.7) | 5 (4.8) | 1 (1.0) | 0 (0) | 0 (0) | 6 (5.7) |
| Rabe E et al (INSIGHTS-SVT), 202338 | 35-43 | 596 | 65 (10.9) | N/A | N/A | N/A | 1 (0.2) | N/A |
| 21 | 218 | 49 (22.5) | N/A | N/A | N/A | 2 (0.9) | N/A | |
| 0 | 58 | 7 (12.1) | N/A | N/A | N/A | 0 (0) | N/A |
CRNM, Clinically relevant non-major; DVT, deep vein thrombosis; N/A, non-available; PE, pulmonary embolism; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
VTE is represented as a combination of SVT progression or recurrence, DVT, and PE when available or according to the definitions of the original trials; does not always match the sum of individual events due to reporting issues.
References
- 1.Coon W.W., Willis P.W., Keller J.B. Venous thromboembolism and other venous disease in the Tecumseh community health study. Circulation. 1973;48:839–846. doi: 10.1161/01.cir.48.4.839. [DOI] [PubMed] [Google Scholar]
- 2.Frappé P., Buchmuller-Cordier A., Bertoletti L., et al. Annual diagnosis rate of superficial vein thrombosis of the lower limbs: the STEPH community-based study. J Thromb Haemostasis. 2014;12:831–838. doi: 10.1111/jth.12575. [DOI] [PubMed] [Google Scholar]
- 3.Geersing G.J., Cazemier S., Rutten F., Fitzmaurice D.A., Hoes A.W. Incidence of superficial venous thrombosis in primary care and risk of subsequent venous thromboembolic sequelae: a retrospective cohort study performed with routine healthcare data from The Netherlands. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Minno M., Ambrosino P., Ambrosini F., Tremoli E., Di Minno G., Dentali F. Prevalence of deep vein thrombosis and pulmonary embolism in patients with superficial vein thrombosis: a systematic review and meta-analysis. J Thromb Haemostasis. 2016;14:964–972. doi: 10.1111/jth.13279. [DOI] [PubMed] [Google Scholar]
- 5.Decousus H., Quéré I., Presles E., et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–224. doi: 10.7326/0003-4819-152-4-201002160-00006. [DOI] [PubMed] [Google Scholar]
- 6.Galanaud J.P., Genty C., Sevestre M.A., et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost. 2011;105:31–39. doi: 10.1160/TH10-06-0406. [DOI] [PubMed] [Google Scholar]
- 7.Bauersachs R., Gerlach H.E., Heinken A., et al. Management and outcomes of patients with isolated superficial vein thrombosis under real life conditions (INSIGHTS-SVT) Eur J Vasc Endovasc Surg. 2021;62:241–249. doi: 10.1016/j.ejvs.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 8.van Langevelde K., Lijfering W.M., Rosendaal F.R., Cannegieter S.C. Increased risk of venous thrombosis in persons with clinically diagnosed superficial vein thrombosis: results from the MEGA study. Blood. 2011;118:4239–4241. doi: 10.1182/blood-2011-05-356071. [DOI] [PubMed] [Google Scholar]
- 9.Cannegieter S.C., Horváth-Puhó E., Schmidt M., et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood. 2015;125:229–235. doi: 10.1182/blood-2014-06-577783. [DOI] [PubMed] [Google Scholar]
- 10.Galanaud J.P., Sevestre M.A., Pernod G., et al. Long-term risk of venous thromboembolism recurrence after isolated superficial vein thrombosis. J Thromb Haemost. 2017;15:1123–1131. doi: 10.1111/jth.13679. [DOI] [PubMed] [Google Scholar]
- 11.Kakkos S.K., Gohel M., Baekgaard N., et al. Editor's choice - European society for vascular surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61:9–82. doi: 10.1016/j.ejvs.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Gloviczki P., Lawrence P.F., Wasan S.M., et al. The 2023 Society for Vascular Surgery, American venous forum, and American vein and lymphatic society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II. J Vasc Surg Venous Lymphat Disord. 2023;11:101670. doi: 10.1016/j.jvsv.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Duffett L., Kearon C., Rodger M., Carrier M. Treatment of superficial vein thrombosis: a systematic review and meta-analysis. Thromb Haemost. 2019;119:479–489. doi: 10.1055/s-0039-1677793. [DOI] [PubMed] [Google Scholar]
- 14.Beyer-Westendorf J., Schellong S.M., Gerlach H., et al. Prevention of thromboembolic complications in patients with superficial-vein thrombosis given rivaroxaban or fondaparinux: the open-label, randomised, non-inferiority SURPRISE phase 3b trial. Lancet Haematol. 2017;4:e105–e113. doi: 10.1016/S2352-3026(17)30014-5. [DOI] [PubMed] [Google Scholar]
- 15.Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 16.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Peterson J., Welch V., Losos M., Tugwell P. Ottawa Hospital Research Institute; 2011. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; pp. 1–12. [Google Scholar]
- 18.Marchiori A., Verlato F., Sabbion P., et al. High versus low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica. 2002;87:523–527. [PubMed] [Google Scholar]
- 19.Superficial Thrombophlebitis Treated By Enoxaparin Study Group A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med. 2003;163:1657–1663. doi: 10.1001/archinte.163.14.1657. [DOI] [PubMed] [Google Scholar]
- 20.Lozano F.S., Almazan A. Low-molecular-weight heparin versus saphenofemoral disconnection for the treatment of above-knee greater saphenous thrombophlebitis: a prospective study. Vasc Endovascular Surg. 2003;37:415–420. doi: 10.1177/153857440303700605. [DOI] [PubMed] [Google Scholar]
- 21.Prandoni P., Tormene D., Pesavento R., Vesalio Investigators Group High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind, randomized trial. J Thromb Haemost. 2005;3:1152–1157. doi: 10.1111/j.1538-7836.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- 22.Decousus H., Prandoni P., Mismetti P., et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232. doi: 10.1056/NEJMoa0912072. [DOI] [PubMed] [Google Scholar]
- 23.Rathbun S.W., Aston C.E., Whitsett T.L. A randomized trial of dalteparin compared with ibuprofen for the treatment of superficial thrombophlebitis. J Thromb Haemost. 2012;10:833–839. doi: 10.1111/j.1538-7836.2012.04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosmi B., Filippini M., Tonti D., et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum) J Thromb Haemostasis. 2012;10:1026–1035. doi: 10.1111/j.1538-7836.2012.04727.x. [DOI] [PubMed] [Google Scholar]
- 25.Spirkoska A., Jezovnik M.K., Poredos P. Time course and the recanalization rate of superficial vein thrombosis treated with low-molecular-weight heparin. Angiology. 2015;66:381–386. doi: 10.1177/0003319714533183. [DOI] [PubMed] [Google Scholar]
- 26.Kearon C., Carrier M., Gu C.S., et al. Rivaroxaban compared to placebo for the treatment of leg superficial vein thrombosis: a randomized trial. Semin Thromb Hemost. 2020;46:977–985. doi: 10.1055/s-0040-1718891. [DOI] [PubMed] [Google Scholar]
- 27.Ascer E., Lorensen E., Pollina R.M., Gennaro M. Preliminary results of a nonoperative approach to saphenofemoral junction thrombophlebitis. J Vasc Surg. 1995;22:616–621. doi: 10.1016/s0741-5214(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 28.Gorty S., Patton-Adkins J., DaLanno M., Starr J., Dean S., Satiani B. Superficial venous thrombosis of the lower extremities: analysis of risk factors, and recurrence and role of anticoagulation. Vasc Med. 2004;9:1–6. doi: 10.1191/1358863x04vm516oa. [DOI] [PubMed] [Google Scholar]
- 29.Sartori M., Favaretto E., Migliaccio L., et al. The incidence of heparin-induced thrombocytopenia in patients treated with low molecular weight heparin for superficial vein thrombosis. Thromb Res. 2016;139:154–157. doi: 10.1016/j.thromres.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Samuelson B., Go A.S., Sung S.H., Fan D., Fang M.C. Initial management and outcomes after superficial thrombophlebitis: the cardiovascular research network venous thromboembolism study. J Hosp Med. 2016;11:432–434. doi: 10.1002/jhm.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blin P., Sevestre M.A., Pouchain D., Gillet J.L. Management and 3-month outcomes of isolated superficial vein thrombosis of the lower limb: a real-world cohort study. Thromb Res. 2017;157:117–119. doi: 10.1016/j.thromres.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Barco S., Pomero F., Di Minno M.N.D., et al. Clinical course of patients with symptomatic isolated superficial vein thrombosis: the ICARO follow-up study. J Thromb Haemost. 2017;15:2176–2183. doi: 10.1111/jth.13840. [DOI] [PubMed] [Google Scholar]
- 33.Gouveia S., Roberts L.N., Czuprynska J., Patel R.K., Arya R. Single centre experience of the management of superficial vein thrombosis with prophylactic low-molecular-weight heparin. Br J Haematol. 2018;181:682–684. doi: 10.1111/bjh.14673. [DOI] [PubMed] [Google Scholar]
- 34.Karathanos C., Chatzis D., Latzios P., Papakostas I., Goumas K., Giannoukas A.D. Treatment of superficial vein thrombosis with intermediate dose of tinzaparin: a real word cohort study - the seVEN EXTension study. Phlebology. 2021;36:423–431. doi: 10.1177/0268355520947300. [DOI] [PubMed] [Google Scholar]
- 35.Karathanos C., Kakkos S.K., Georgiadis G., et al. Risk of recurrent thromboembolic events according to treatment duration in patients with superficial vein thrombosis treated with intermediate dose of tinzaparin. Phlebology. 2023;38:141–149. doi: 10.1177/02683555221143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clapham R.E., Speed V., Czuprynska J., et al. Rivaroxaban for the treatment of superficial vein thrombosis, experience at King's College Hospital. Br J Haematol. 2022;196:e3–e6. doi: 10.1111/bjh.17757. [DOI] [PubMed] [Google Scholar]
- 37.Casian D., Bzovii F., Culiuc V., Gutu E. Urgent surgery versus anticoagulation for treatment of superficial vein thrombosis in patients with varicose veins. Vasa. 2022;51:174–181. doi: 10.1024/0301-1526/a001000. [DOI] [PubMed] [Google Scholar]
- 38.Rabe E., Hoffmann U., Schimke A., et al. Determinants of late venous thromboembolic events after acute isolated superficial vein thrombosis in daily practice: 12-month results of the INSIGHTS-SVT study. Eur J Vasc Endovasc Surg. 2023;66:697–704. doi: 10.1016/j.ejvs.2023.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Beyer-Westendorf J. Controversies in venous thromboembolism: to treat or not to treat superficial vein thrombosis. Hematology Am Soc Hematol Educ Program. 2017;2017:223–230. doi: 10.1182/asheducation-2017.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosmi B., Filippini M., Campana F., et al. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum) Thromb Res. 2014;133:196–202. doi: 10.1016/j.thromres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Di Nisio M., Wichers I.M., Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2013;14 doi: 10.1002/14651858.CD004982.pub5. [DOI] [PubMed] [Google Scholar]
- 42.Nicolaides A.N., Fareed J., Kakkar A.K., et al. Prevention and treatment of venous thromboembolism--International Consensus Statement. Int Angiol. 2013;32:111–260. [PubMed] [Google Scholar]
- 43.Gloviczki P. CRC Press; 2017. Handbook of venous and lymphatic disorders : guidelines of the American Venous Forum. [Google Scholar]
- 44.Guyatt G.H., Akl E.A., Crowther M., Gutterman D.D., Schuunemann H.J. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prandoni P., Pesavento R., Bilora F., et al. No difference in outcome between therapeutic and preventive anticoagulation in patients with superficial vein thrombosis involving the saphenous-femoral junction. Vasc Med. 2022;27:290–292. doi: 10.1177/1358863X211066962. [DOI] [PubMed] [Google Scholar]
- 46.Boutitie F., Pinede L., Schulman S., et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. Bmj. 2011;342:d3036. doi: 10.1136/bmj.d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]