Abstract
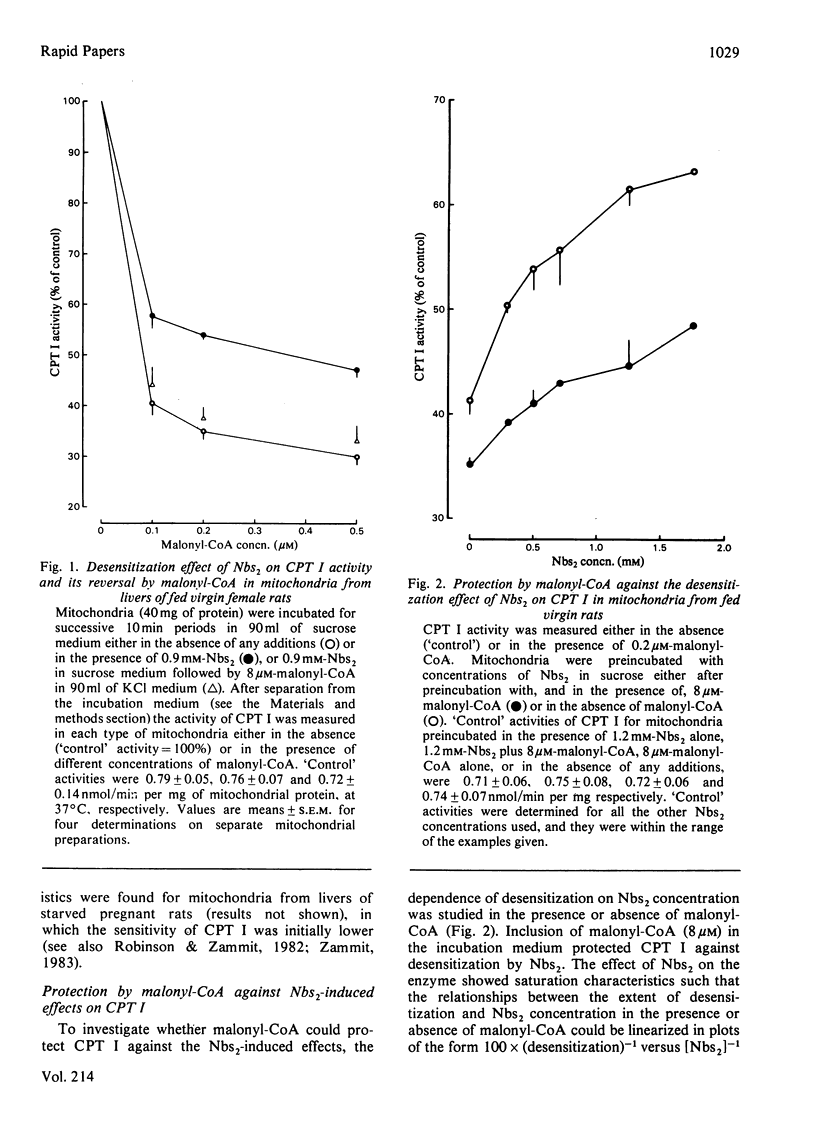
Preincubation of rat liver mitochondria with 5,5'-dithiobis-(2-nitrobenzoic acid) (Nbs2) followed by removal of excess reagent by washing the mitochondria with 0.5 mM-reduced glutathione resulted in a desensitization of carnitine palmitoyltransferase (CPT) I activity to malonyl-CoA inhibition. The effect was not observed if mitochondria were washed with 0.5 mM-dithiothreitol. The desensitization effect of Nbs2 could be reversed by a second incubation in the presence of 8 microM-malonyl-CoA. In addition, malonyl-CoA, when present simultaneously with Nbs2, protected CPT I activity against the desensitization effect of the thiol-group reagent. These results suggest that malonyl-CoA exerts an effect on one or more thiol groups of the enzyme, and that this effect is related to the ability of the metabolite to sensitize CPT I to malonyl-CoA inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Importance of experimental conditions in evaluating the malonyl-CoA sensitivity of liver carnitine acyltransferase. Studies with fed and starved rats. Biochem J. 1981 Nov 15;200(2):217–223. doi: 10.1042/bj2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Stakkestad J. A., Bremer J. The outer carnitine palmitoyltransferase and regulation of fatty acid metabolism in rat liver in different thyroid states. Biochim Biophys Acta. 1983 Feb 7;750(2):244–252. doi: 10.1016/0005-2760(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. Increased sensitivity of carnitine palmitoyltransferase I activity to malonyl-CoA inhibition after preincubation of intact rat liver mitochondria with micromolar concentrations of malonyl-CoA in vitro. Biochem J. 1983 Mar 15;210(3):953–956. doi: 10.1042/bj2100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]


