Abstract
Background and Aims
Leaf traits are known to be strong predictors of plant performance and can be expected to (co)vary along environmental gradients. We investigated the variation, integration, environmental relationships and evolutionary history of leaf functional traits in the genus Coffea, typically a rainforest understorey shrub, across Africa. A better understanding of the adaptive processes involved in leaf trait evolution can inform the use and conservation of coffee genetic resources in a changing climate.
Methods
We used phylogenetic comparative methods to investigate the evolution of six leaf traits measured from herbarium specimens of 58 African Coffea species. We added environmental data and data on maximum plant height for each species to test trait–environment correlations in various (sub)clades, and we compared continuous trait evolution models to identify variables driving trait diversification.
Key Results
Substantial leaf trait variation was detected across the genus Coffea in Africa, which was mostly interspecific. Of these traits, stomatal size and stomatal density exhibited a clear trade-off. We observed low densities of large stomata in early-branching lineages and higher densities of smaller stomata in more recent taxa, which we hypothesize to be related to declining CO2 levels since the mid-Miocene. Brownian motion evolution was rejected in favor of white noise or Ornstein–Uhlenbeck models for all traits, implying these traits are adaptively significant rather than driven by pure drift. The evolution of leaf area was likely driven by precipitation, with smaller leaves in drier climates across the genus.
Conclusions
Generally, Coffea leaf traits appear to be evolutionarily labile and governed by stabilizing selection, though evolutionary patterns and correlations differ depending on the traits and clades considered. Our study highlights the importance of a phylogenetic perspective when studying trait relationships across related taxa, as well as the consideration of various taxonomic ranges.
Keywords: Adaptation, Africa, climate, Coffea, coffee, leaf traits, stabilizing selection, stomata, tropics
INTRODUCTION
Since the emergence of the first vascular plants over 400 million years ago, evolution has seen the unfolding of a remarkable diversity of leaf traits (Hao and Xue, 2013). Morphologically, the appearance of a leaf is composed of various traits expressed to different degrees. Since specific leaf trait combinations are known to have evolved in synchrony with a plant’s survival strategy, leaf traits strongly predict a plant’s performance in the environment it grows in (Poorter and Bongers, 2006; Violle et al., 2007). Since traits are often phenotypically integrated, several leaf functional traits have been compiled into the leaf economics spectrum (LES) (Wright et al., 2004). This framework represents a continuum of survival strategies, ranging from fast to slow return on investment in leaf biomass (Wright et al., 2004; Jones et al., 2013; Reich, 2014). Furthermore, because functional traits form the interface between the plant and its surroundings, evolutionary change in these traits facilitates the adaptation of plants to a changing environment (Ackerly et al., 2000; Ackerly, 2004; Jones et al., 2013).
Leaf functional traits, by definition, have the capacity to influence the performance or fitness of an organism in its environment (Reich et al., 2003; McGill et al., 2006). They have been related to performance measures such as water use efficiency (e.g. Xu and Zhou, 2008; Drake et al., 2013) and tend to covary with environmental variables, such as temperature, precipitation and nutrient availability (Givnish, 1987; Wright et al., 2017). Leaf size, for instance, is often larger in hot and wet tropical climates, while smaller leaves predominate in cooler or more arid regions (Wright et al., 2017). Plants have also shown a reduced stomatal size and increased stomatal density when exposed to drought stress (Xu and Zhou, 2008), though this response is likely species-specific (Zhang et al., 2012). Further, specific leaf area (SLA, i.e. leaf area per unit of dry mass) has been found to increase with increasing soil nutrient levels (Andersen et al., 2012) and soil moisture content (Chaturvedi and Raghubanshi, 2018) in tropical forest species.
Despite the wealth of research focusing on leaf trait evolution at different taxonomic levels (e.g. Flores et al., 2014; Glade-Vargas et al., 2018), sources of trait variation often remain poorly documented, particularly in tropical forest understories. The lack of data leads to gaps in our understanding of the adaptive potential of tropical taxa. A closer look at particularly understudied floras, such as the Afrotropical understorey, is required to gain insights into the current state and evolutionary history of these ecosystems (Verbeeck et al., 2011). Broad studies of leaf trait evolution across Africa are lacking, though some studies have been performed at smaller geographic scales (e.g. Lauterbach et al., 2016; Wigley et al., 2016). As the understorey of African tropical forests harbors important crop wild relatives, including numerous wild coffee species, an enhanced focus on these species is also crucial for the long-term conservation of their genetic resources. Although some research has been done on leaf trait diversity in the Congo Basin canopy layer (Verbeeck et al., 2014; Kafuti et al., 2020), knowledge on leaf traits of understorey species remains very limited (but see Hatangi et al., 2023).
Coffee is a valuable commodity worldwide, with global consumption exceeding ten million tons annually (International Coffee Organization, 2023). The popular beverage is brewed from seeds of a few Coffea species; C. arabica and C. canephora make up the vast majority of the global coffee market, which exceeds a total value of 88 billion US dollars (International Coffee Organization, 2023; Statista, 2023). Despite this duopoly in the global market, other Coffea species, such as C. liberica and C. stenophylla, have shown promise as alternative beverage species (Davis et al., 2021, 2022). The genus Coffea encompasses 131 species known to date, after the inclusion of 20 species formerly classified as a sister genus, Psilanthus (Robbrecht and Manen, 2006; Davis et al., 2007, 2011; Hamon et al., 2017) and the recent description of seven new species (Davis and Rakotonasolo, 2021; Stoffelen et al., 2021). Most Coffea species are native to continental Africa or Madagascar, with a few species occurring in Comoros and Mayotte, the Mascarene islands, in parts of Asia, or in Northern Australia (Davis, 2003; Hamon et al., 2017). Most of these species have relatively narrow geographic ranges (Davis et al., 2006). Nonetheless, some species, such as C. canephora and C. liberica, occur across large areas of the African continent (Herrera and Lambot, 2017). These widespread species tend to inhabit moist, tropical habitats, or areas along rivers or wetlands. In contrast, other Coffea species can be found in drier shrublands, or in deciduous forests with a distinct dry season (Maurin et al., 2007; Herrera and Lambot, 2017).
Two major clades have been identified within the genus: a relatively small xeno-coffee (XC) clade, and a more diverse eu-coffee (EC) clade (Hamon et al., 2017). The sparsity of the XC clade is assumed to be related to haploidy and selfing within the clade, whereas nearly all EC species are diploid and self-incompatible (Davis et al., 2005; Hamon et al., 2017). The XC clade is dispersed over tropical West, Central and East Africa as well as Asia and Northern Australia, while the EC clade encompasses two subclades occurring across continental tropical Africa (AFR subclade) and the West Indian Ocean Islands (WIOI subclade) (Davis, 2003; Hamon et al., 2017). Extant taxonomic diversity has been influenced by several environmental and geographic factors. For example, the Dahomey Gap, an arid savannah region separating West and Central African forests, is known to have influenced the genetic diversity and speciation within Coffea by acting as a barrier to gene flow (Berthaud, 1986; Maurin et al., 2007; Gomez et al., 2009; Cubry et al., 2013). Further, speciation on the West Indian Ocean Islands has been shown to be rapid and radial, suggesting a possible adaptive radiation in the WIOI subclade (Davis et al., 2006; Anthony et al., 2010). Learning more about the macroevolution of leaf traits in these species, specifically in response to environmental variables such as climate, can inform their conservation to safeguard the genetic resources within these species.
Previous studies investigating leaf trait variation in Coffea have focused on only one or a few species (e.g. Buchanan et al., 2019; Dutra Giles et al., 2019; Dubberstein et al., 2021). Moreover, although several studies have assessed the macroevolution of leaf functional traits across a given taxonomic group (e.g. Flores et al., 2014; Onstein et al., 2016; Glade-Vargas et al., 2018), the drivers and mode of leaf trait evolution across a principally rainforest understorey genus such as Coffea remain unexplored. For example, a different pattern of leaf trait evolution can be expected between continental African (AFR) and Malagasy (WIOI) species, due to their independent evolutionary histories and different range extents (Hamon et al., 2017). Furthermore, species that have adapted to survive in drier habitats could be expected to reflect these adaptations in their leaf traits, for example via lower SLA values (Chaturvedi and Raghubanshi, 2018). Also, functional trait variation at the population level is a prerequisite for natural selection and adaptive trait change.
Due to most Coffea species’ fairly narrow distribution range (Davis et al., 2006), we expect interspecific trait variation to exceed intraspecific variation for all traits. We also expect some traits to show a certain degree of integration. For example, stomatal density and stomatal size are generally inversely correlated across taxa (Brodribb et al., 2013). Overall, we predict that trait–environment relationships will generally be weaker than trait–trait correlations, due to the large amount of trait variation found between coexisting species (Leishman et al., 2000; Wright et al., 2004; Moles et al., 2005; Vandelook et al., 2012; Jones et al., 2013). Seed mass, for example, can vary across several orders of magnitude between co-occurring species, reflecting different survival strategies (Leishman et al., 2000; Moles et al., 2005). Finally, by evaluating whether trait evolution occurred independently from the phylogenetic relationships between taxa, it is possible to shed light on the importance of adaptation and constraints in trait evolution. While some species or traits may exhibit rapid responses to environmental change, others may not possess the phenotypic variation or heritability required for rapid adaptation.
At present, these hypotheses have remained untested in Coffea. Current knowledge on evolutionary drivers in Coffea is incomplete, and a better understanding of the processes involved in trait evolution could shed light on the adaptive value of the studied leaf traits in wild coffee plants. Additionally, knowledge on trait–trait and trait–climate relationships is essential to foster more resilient and sustainable coffee cultivation. Via the use of phylogenetic comparative methods, we aim to (1) estimate the relative degree of intra- and interspecific leaf functional trait variation across 58 African Coffea species that are broadly distributed across the genus, across Africa and across habitats; (2) analyze potential correlated evolution between traits; and (3) explore which environmental factors are the most likely drivers of leaf trait evolution across the genus. We also attempt to discern different life history strategies among Coffea clades or species and to identify the evolutionary trajectories that led to the current trait diversity in the genus.
MATERIALS AND METHODS
Data collection
A data set of trait measurements was compiled from 780 leaves across 167 herbarium accessions of 62 species. Most of the included species (n = 58) belonged to the genus Coffea, except for four outgroup species from related genera (Belonophora coriacea, Calycosiphonia spathicalyx, Tricalysia congesta and Bertiera iturensis). Of the 58 Coffea species, two were representatives of the former Psilanthus genus. The samples included in this study were well distributed throughout the genus, across habitats and across Africa. Sample coordinates ranged from 25.10°S to 8.53°N and from 10.00°W to 57.43°E. The leaf functional traits included in the analyses were leaf area (square meters), SLA (square meters per kilogram), stomatal density (stomata per square millimeter), stomatal length (micrometers), stomatal width (micrometers) and pore width (i.e. aperture width, micrometers). Data on leaf dry mass and pore length were also compiled, but these traits were not further considered due to strong correlations with leaf area (rs = 0.898, P < 0.001) and stomatal length (rs = 0.817, P < 0.001), respectively. Leaf area was measured by scanning leaves using a standard A4 flatbed scanner and estimating area using ImageJ (Schneider et al., 2012). Leaf dry mass was determined by weighing individual leaves, after a week of calibration to laboratory moisture conditions (50–60 % RH). To correct for potential bias in SLA estimates due to shrinkage during the drying of herbarium specimens (Blonder et al., 2012; Perez et al., 2020), we calculated average shrinkage factors for species of which live specimens were available in Meise Botanic Garden greenhouses. The methodology of this correction is presented in the Appendix.
For the same leaves, stomatal prints were taken from the abaxial side using the nail varnish method as explained in Meeus et al. (2020). Two prints per leaf were taken in the middle of the leaf at opposite sides of the main vein, from which three photomicrographs of 1600 × 1200 pixels were taken per leaf print (dimensions = 344 × 258 µm; area view field = 0.09 mm2) using a digital microscope (VH-5000 Ver 1.5.1.1, Keyence). A single photomicrograph was created by stacking several digital images taken at different focal planes to increase the depth of the resulting image. All stomata that fell entirely within the view field were counted and converted to stomata per square millimeter to obtain stomatal density. Stomatal measurements were performed on the same digital photographs using ImageJ version 1.53v (Schneider et al., 2012) on three stomata per leaf across five leaves (i.e. 15 stomata) per accession. Stomatal dimensions were not measured for three species (C. homollei, C. arenesiana and C. coursiana) due to a lack of source material. One leaf was removed from the data set due to an exceptionally low leaf weight at around five times the area of the other leaves from this accession, which was attributed to a potential data entry error (specimen Vermoesen 2182; Supplementary Data Table S1). This yielded stomatal measurements of 742 leaves representing 159 accessions across 59 species (55 Coffea + 4 outgroup species). Trait values for all leaves were averaged per species to obtain a comprehensive data set including a single mean trait value per species (Supplementary Data Table S2). Standard errors were added to the averaged trait data, and leaf area was log-transformed before analysing the data to avoid violating normality of residuals.
Additionally, data on maximum plant height for each species were compiled from various literature sources (Leroy, 1961; Bridson, 1994; Stoffelen, 1998; Davis and Rakotonasolo, 2001, 2008; Ruffo et al., 2002; Davis and Mvungi, 2004; Davis et al., 2006). These values were added to the data set, along with geographic coordinates and altitude of all accessions. Geographic coordinates were obtained by georeferencing based on the locality information provided on the specimens, which was detailed up to at least the nearby village level.
Climate data for each accession were retrieved from WorldClim (Fick and Hijmans, 2017) for each of the given coordinates in the form of 19 bioclimatic variables, which were subsequently averaged at species level. To reduce the dimensionality of these variables, a phylogenetically corrected PCA was performed on the correlation matrix to decompose the climate data into phylogenetically structured principal components (PCs) using R package phytools (Revell, 2012). Three interpretable PCs were retained, jointly explaining 79 % of the variance in the climate data. These three PCs were added to the dataset and used later as predictors in the phylogenetic regression analyses and evolutionary models.
Phylogenetic tree construction
Phylogenetic information included in the analyses was based on the phylogeny of Hamon et al. (2017). We pruned their phylogeny to retain only those species for which data were readily available to us, using the drop.tip function in R package ape (Paradis and Schliep, 2019). The full tree including outgroups was ensured to be ultrametric (using the chronos command in ape with a smoothing parameter of zero) and dichotomous (using multi2di in ape) before rescaling its length to 1. Subtrees were then extracted from the full phylogeny to include (1) only Coffea species, (2) only the EC clade, (3) only the AFR subclade, and (4) only the WIOI subclade. The XC clade was not extracted to a separate subtree due to its small size. These subtrees allowed for the isolation of different clades, to then run independent regression analyses for each of them (see section Phylogenetic regressions). All analyses were performed in R version 4.3.0 (R Core Team, 2023).
Inter- and intraspecific variation
The proportions of inter- and intraspecific variation in the trait values were estimated using one-way ANOVA. Although the assumption of independence was violated, the results are presented heuristically. We then applied a phylogenetically corrected PCA to the trait data to identify axes of multivariate trait evolution using phytools (Revell, 2012). The results were visualized in biplots, as well as in phylomorphospace plots as implemented in phytools to visualize phylogenetic patterns.
Phylogenetic signal
We estimated Pagel’s lambda (λ) (Pagel, 1999), a measure of phylogenetic signal in the trait data, with the phylosig command in phytools while accounting for standard error in the data. Pagel’s λ varies between 0 and 1, with 0 indicating phylogenetically independent trait evolution and 1 indicating that the trait followed a perfect Brownian model of evolution. Signal was estimated in all considered clades and subclades.
Models of continuous trait evolution
To test whether trait evolution had been driven by environmental variables, we fitted different models reflecting different evolutionary processes. These models allow interpretations of the evolutionary processes involved in the diversification of Coffea leaf functional traits. The first and simplest model used was a non-phylogenetic white noise (WN) model, which assumes phylogenetic independence (λ = 0). This model is equivalent to drawing traits randomly from a normal distribution, independently of the phylogeny (Pagel, 1999; Münkemüller et al., 2015). The second model was simple Brownian motion (BM), which is a constant-variance random-walk model that assumes that λ = 1 and that traits change randomly over time at a given rate (Freckleton et al., 2002; Revell et al., 2008; Meireles et al., 2020). This model implies that the evolution of the trait has been driven by pure drift, though the same pattern could be found under natural selection fluctuating in direction and intensity through time (O’Meara et al., 2006; Losos, 2008). Third, we fitted an Ornstein–Uhlenbeck (OU) model (Hansen, 1997; Butler and King, 2004; Hansen et al., 2008), which can be used to model evolution towards an optimal trait value θ. OU models contain a stochasticity parameter, σ2, which expresses the intensity of random fluctuations in trait values, and an adaptive parameter, α, which measures the rate of trait change towards the optimum. An OU model with a single optimum (OU1) can represent constrained evolution or inertia. WN models can also be described as variations on OU models, but with α = ∞ (Münkemüller et al., 2015). If leaf trait evolution is adaptive, a WN or OU model of evolution should be the best fit to the data (Hansen, 1997; Butler and King, 2004; Beaulieu et al., 2012; Münkemüller et al., 2015).
Since optima are expected to vary with evolutionary drivers such as climate, we also fitted four OU models in which the evolutionary optimum varies continuously as a function of a given putative driver of trait evolution (OUA = altitude; OUT = temperature, i.e. climate PC1; OUD = drought, i.e. climate PC2; OUS = seasonality, i.e. climate PC3). In all OU models, we also estimated phylogenetic half-life (t1/2 = ln(2)/α), another measure of phylogenetic signal (Hansen, 1997). This metric represents the time required for the average trait value to move halfway towards the optimum θ.
The WN models were fitted using the fitContinuous function in R package geiger (Pennell et al., 2014). We used the brown.fit and slouch.fit commands in R package slouch (Kopperud et al., 2020) to fit the BM and OU1 models, as well as the more complex OUT, OUD, OUS and OUA models. Model fit was assessed using AICc, and measurement error was always incorporated into the models.
Phylogenetic regressions
To test for correlated evolution between traits and for any effects of environmental variables on trait evolution, we fitted regression models for each leaf trait across four different phylogenies (i.e. the genus Coffea, the EC clade, the AFR subclade and the WIOI subclade), each time with all other traits and environmental variables (i.e. climate PC1, PC2, PC3, altitude, latitude and longitude) as predictors. Since leaf traits are known to vary with plant size in some taxa (Price et al., 2014), maximum plant height was also included as a predictor in the regression models to account for possible allometric relationships between leaf traits and plant size.
To account for any non-independence in the species data due to common ancestry, we used phylogenetic generalized least squares (PGLS) regressions with the pgls command from R package caper (Orme et al., 2018). Applying a phylogenetic correction to a linear regression based only on univariate estimates of phylogenetic signal can lead to erroneous inferences (Hansen and Orzack, 2005; Revell, 2010). Therefore, pgls estimates phylogenetic signal simultaneously with the regression parameters. A backward model selection approach was used to remove the least significant predictors one by one until model AICc was minimal.
RESULTS
Distribution of variance
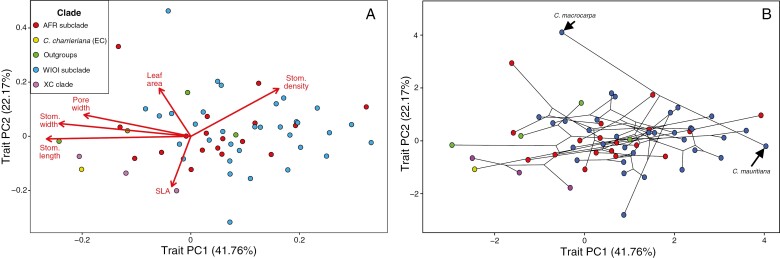
Interspecific trait differences were significant for all traits and explained most of the variance for all traits except pore width (Table 1, Supplementary Data Fig. S1). Leaf trait variation between species is illustrated in Fig. 1. The phylogenetic PCA of the trait data resulted in two meaningful PC axes, jointly explaining 64 % of the variance in the trait data (Fig. 2A, B). Trait PC1 showed strong negative loadings for stomatal dimensions (stomatal length, −0.94; stomatal width, −0.86; pore width, −0.70) and a substantial positive loading for stomatal density (−0.57) (Supplementary Data Table S3). PC axis 1 can therefore be regarded as a continuum from species with large stomata (generally at lower densities) to species with small stomata (generally at higher densities), explaining 42 % of the trait variance. The second trait PC axis had a negative loading for SLA (−0.66) and positive loadings for stomatal density and leaf area (both +0.63), explaining another 22 % of the trait variance. This PC axis therefore represents a spectrum from low leaf investment (small leaves, lower stomatal density, high SLA) to high leaf investment (larger leaves with higher stomatal density and low SLA).
Table 1.
Variance explained by between-species differences for each studied leaf trait across Coffea, estimated by one-way ANOVA. For stomatal density, leaf area and SLA, n = 58; for stomatal length, stomata width and pore width, n = 55.
| Stomatal density | Leaf area | SLA | Stomatal length | Stomatal width | Pore width | |
|---|---|---|---|---|---|---|
| Adjusted R2 | 0.60 | 0.70 | 0.74 | 0.61 | 0.53 | 0.36 |
| F-value | F 57,631 = 18.91 | F 57,631 = 29.6 | F 57,631 = 35.63 | F 54,601 = 19.61 | F 54,601 = 14.87 | F 54,601 = 7.79 |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
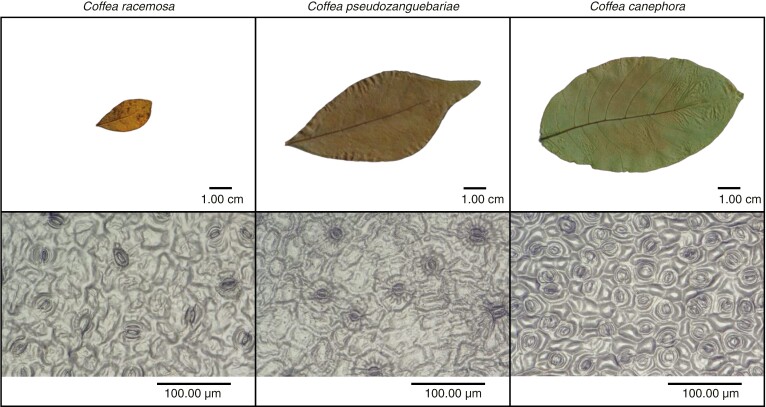
Fig. 1.
Examples of interspecific variation in leaf size (top), stomatal density (bottom), and stomatal dimensions (bottom) in three Coffea species.
Fig. 2.
PCA showing the relationships between leaf traits, showing the first two PC axes. Data points indicate species (n = 62) and are color-coded according to their clade. (A) Biplot. (B) Phylomorphospace plot with lines representing phylogenetic relationships. AFR, African; EC, eu-coffee; WIOI, West Indian Ocean Islands; XC, xeno-coffee. Clade nomenclature is based on Hamon et al. (2017).
Univariate phylogenetic signal
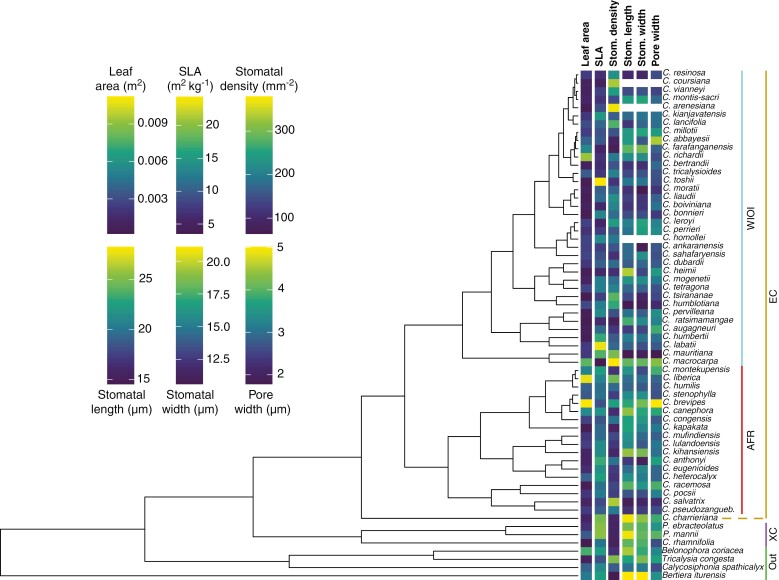
Within the subclades of the genus Coffea, none of the tested traits showed significant phylogenetic signal except stomatal density in the WIOI subclade (λ = 0.69, P = 0.015) (Fig. 3). Phylogenetic signal in leaf area was marginally significant across the EC clade (λ = 0.55, P = 0.087). Across the genus, significant signal was detected for SLA (λ = 0.52, P = 0.008) and stomatal length (λ = 0.73, P = 0.006), while leaf area remained marginally significant (λ = 0.69, P = 0.075). When including outgroups, phylogenetic signal in stomatal width became significant (λ = 0.57, P = 0.006), along with SLA (λ = 0.54, P = 0.018) and stomatal length (λ = 0.75, P = 0.001). All other traits were randomly distributed across the considered clade, with more related species not being significantly more similar to each other than less related lineages.
Fig. 3.
Phylogenetic tree of all studied species (n = 62) with values for the six considered traits shown at the tips of the tree. For stomatal length, stomatal width and pore width, trait values were missing for C. arenesiana, C. coursiana and C. homollei. Clades are delimited by the bars at the right-hand side. WIOI, West Indian Ocean Islands; AFR, African; EC, eu-coffee; XC, xeno-coffee. Clade nomenclature is based on Hamon et al. (2017).
Multivariate phylogenetic signal
The PGLS regressions did not detect phylogenetic signal (λ) significantly different from zero in any of the considered leaf traits across Coffea or across the EC clade, despite yielding non-zero maximum likelihood (ML) estimates for some traits (Table 2, Supplementary Data Table S4). However, ML estimates for λ became significantly different from 0 for some traits across the subclades. For stomatal density and stomatal length in the AFR subclade, λ = 1. In the WIOI subclade, signal for stomatal density was strong, but significantly below 1 (λ = 0.945; 95 % CI = 0.801, 0.992).
Table 2.
Multiple PGLS regressions fitted by maximum likelihood across Coffea without outgroups for each trait. Significant predictors are marked in bold. For stomatal density, leaf area and SLA, n = 58; for stomatal length, stomata width and pore width, n = 55.
| Trait | Variable | Standardized regression coefficient | Standard error | t-value | P-value |
|---|---|---|---|---|---|
|
Stomatal density
λ = 0.652 (NA; 0.891) Adjusted R2 = 0.311 |
Intercept | 0.11 | 0.53 | 5.49 | <0.001 |
| SLA | −0.25 | 0.11 | −2.15 | 0.036 | |
| Stomatal length | −0.79 | 0.18 | −4.41 | <0.001 | |
| Stomatal width | 0.31 | 0.17 | 1.81 | 0.076 | |
|
Leaf area
λ = 0.742 (NA; 0.963) Adjusted R2 = 0.3258 |
Intercept | −0.57 | 0.61 | −10.70 | <0.001 |
| Maximum height | 0.31 | 0.10 | 3.17 | 0.003 | |
| Pore width | 0.18 | 0.10 | 1.86 | 0.069 | |
| Latitude | 0.59 | 0.24 | 2.45 | 0.018 | |
| Climate PC2 | −0.41 | 0.11 | −3.84 | <0.001 | |
| Climate PC3 | 0.35 | 0.16 | 2.22 | 0.031 | |
|
SLA
λ = 0 (NA; 0.635) Adjusted R2 = 0.1796 |
Intercept | 0.00 | 0.12 | 0.67 | 0.509 |
| Leaf area | −0.30 | 0.14 | −2.05 | 0.046 | |
| Stomatal length | −0.11 | 0.14 | −0.79 | 0.432 | |
| Longitude | −0.41 | 0.18 | −2.34 | 0.024 | |
| Altitude | 0.87 | 0.47 | 1.84 | 0.072 | |
| Climate PC1 | 0.75 | 0.43 | 1.74 | 0.088 | |
| Climate PC2 | −0.19 | 0.14 | −1.39 | 0.170 | |
| Climate PC3 | 0.44 | 0.32 | 1.37 | 0.177 | |
|
Stomatal length
λ = 0 (NA; 0.524) Adjusted R2 = 0.7764 |
Intercept | 0.00 | 0.06 | 3.42 | 0.001 |
| Stomatal density | −0.29 | 0.07 | −4.16 | <0.001 | |
| Stomatal width | 0.61 | 0.08 | 8.06 | <0.001 | |
| Pore width | 0.23 | 0.07 | 3.16 | 0.003 | |
|
Stomatal width
λ = 0 (NA; 0.169) Adjusted R2 = 0.6893 |
Intercept | 0.00 | 0.08 | 3.01 | 0.004 |
| Leaf area | 0.16 | 0.08 | 2.08 | 0.043 | |
| Stomatal length | 0.82 | 0.08 | 10.71 | <0.001 | |
| Climate PC3 | 0.13 | 0.08 | 1.67 | 0.100 | |
|
Pore width
λ = 0 (NA; 0.172) Adjusted R2 = 0.3416 |
Intercept | 0.00 | 0.11 | 2.40 | 0.020 |
| Leaf area | 0.17 | 0.11 | 1.49 | 0.141 | |
| Stomatal length | 0.57 | 0.11 | 5.15 | <0.001 |
Relationships among leaf traits
Across all examined Coffea species, PGLS regressions showed that stomatal density was significantly negatively related to SLA (t51 = −2.15, P = 0.036) and stomatal length (t51 = −4.41, P < 0.001), with a marginally significant positive relation to stomatal width (t51 = 1.81, P = 0.076). Leaf area was significantly positively related to maximum plant height (t49 = 3.17, P = 0.003), and a marginally significant positive effect of pore width on leaf area was also detected (t49 = 1.86, P = 0.069). For SLA, the best regression model revealed a negative relationship with leaf area (t47 = −2.05, P = 0.046). Stomatal length was negatively predicted by stomatal density (t51 = −4.16, P < 0.001). Also, stomatal width (t51 = 8.06, P < 0.001) and pore width (t51 = 3.16, P = 0.003) both had significantly positive effects on stomatal length. Leaf area (t51 = 2.08, P = 0.043) and stomatal length (t51 = 10.71, P < 0.001) were the only significant predictors of stomatal width, both having a positive effect. Finally, pore width was significantly related to only one independent variable: stomatal length (t52 = 5.15, P < 0.001), of which the effect was positive.
Regressions restricted to the EC clade showed qualitatively similar results to the regressions across the genus for trait–trait relationships. Significant predictors differed only for SLA, where a marginally significant negative relationship with maximum plant height (t46 = −1.86, P = 0.069) was observed. Differences became more apparent when further narrowing the analyses to the AFR and WIOI subclades. Leaf area was positively related to stomatal density in the AFR subclade (t14 = 3.51, P = 0.003), which was not the case in any other clade. Leaf area was also positively related to maximum plant height in the WIOI subclade (t28 = 2.59, P = 0.015). Further, the relationship between SLA and leaf area, which was negative across the genus, was significantly positive across the AFR subclade (t15 = 2.60, P = 0.020), and SLA was inversely related to maximum plant height (t15 = −5.44, P < 0.001) in the AFR subclade. Finally, stomatal width was significantly positively related to maximum plant height in the AFR subclade (t15 = 3.83, P = 0.002).
Relationships between leaf traits and environment
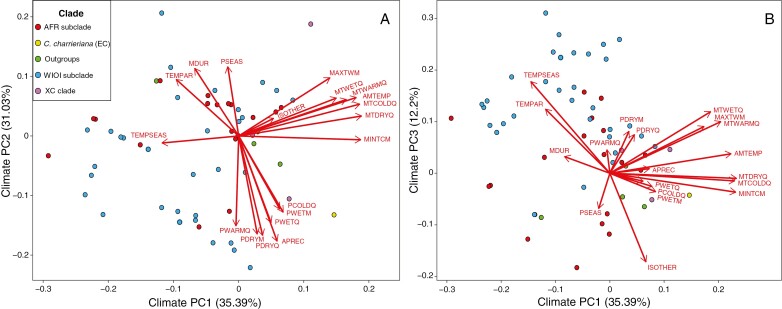
From the phylogenetic PCA applied to the 19 bioclimatic variables, three PCs were retained and added to the data set. The three retained PCs jointly explained 79 % of the variance in bioclimatic variables. PC1 and PC2 explained 35 and 31 % of the variance, respectively (Fig. 4, Supplementary Data Table S5). PC1 represented an axis of temperature, with higher values indicating hotter climates. The strongest loadings for PC2 were all negatively related to precipitation. This axis thus generally represented an axis of drought, with higher PC2 values indicating less rainfall. The third PC was interpreted as an axis of temperature seasonality, where higher PC3 values indicate stronger seasonal temperature fluctuations.
Fig. 4.
PCA of the 19 bioclimatic variables drawn from WorldClim (Fick and Hijmans, 2017). Data points indicate species (n = 62) and are color-coded according to their clade. (A) PC1 versus PC2. (B) PC1 versus PC3. AFR, African; EC, eu-coffee; WIOI, West Indian Ocean Islands; XC, xeno-coffee. Clade nomenclature is based on Hamon et al. (2017). AMTEMP, annual mean temperature; MDUR, mean diurnal temperature range (mean of monthly difference between maximum and minimum temperature); ISOTHER, isothermality (MDUR/TEMPAR × 100); TEMPSEAS, temperature seasonality (standard deviation × 100); MAXTWM, maximum temperature of the warmest month; MINTCM, minimum temperature of the coldest month; TEMPAR, temperature annual range (MAXTWM − MINTCM); MTWETQ, mean temperature of the wettest quarter; MTDRYQ, mean temperature of the driest quarter; MTWARMQ, mean temperature of the warmest quarter; MTCOLDQ, mean temperature of the coldest quarter; APREC, annual precipitation; PWETM, precipitation of the wettest month; PDRYM, precipitation of the driest month; PSEAS, precipitation seasonality (coefficient of variation); PWETQ, precipitation of the wettest quarter; PDRYQ, precipitation of the driest quarter; PWARMQ, precipitation of the warmest quarter; PCOLDQ, precipitation of the coldest quarter.
Significant relationships between environmental variables and leaf traits were uncommon across the genus (Table 2). For stomatal density and the three stomatal dimensions, no significant relations with environmental variables were detected (though climate PC3 was retained as a non-significant predictor in the best-fitting model for stomatal width). For SLA, all three climate PCs as well as altitude and longitude were retained in the final model, but only the negative relation to longitude was significant (t47 = −2.34, P = 0.024). The positive effects of altitude (t47 = 1.84, P = 0.072) and climate PC1 (t47 = 1.74, P = 0.088) on SLA were marginally significant. The only leaf trait strongly affected by our environmental variables was leaf area, with significant positive relations to latitude (t49 = 2.45, P = 0.018) and climate PC3 (t49 = 2.22, P = 0.031), and a strong negative relation to climate PC2 (t49 = −3.84, P < 0.001).
Trait–environment associations across the EC clade were similar, with only predictors of SLA showing qualitative differences. The association between longitude and SLA was no longer significant in the EC clade, while the positive relation between SLA and altitude (t46 = 2.31, P = 0.026) and climate PC1 (t46 = 2.28, P = 0.027) became significant. As was the case for trait–trait relationships, patterns differed substantially when looking only at the AFR or WIOI subclades. Whereas stomatal density was not related to climate at a larger phylogenetic scale, it showed significant negative associations with altitude (t11 = −7.16, P < 0.001), climate PC1 (t11 = −7.87, P < 0.001) and climate PC3 (t11 = −2.81, P = 0.017) in the AFR subclade, and positive associations with altitude (t26 = 2.62, P = 0.014) and climate PC3 (t26 = 4.75, P < 0.001) in the WIOI subclade. Leaf area showed significant positive relations with altitude (t14 = 3.12, P = 0.008) and climate PC1 (t14 = 4.02, P = 0.001) in the AFR subclade and a negative association with climate PC2 (t28 = −2.63, P = 0.014) across the WIOI subclade. SLA was not related to climate or geography in the AFR subclade. In contrast, analysis of SLA in the WIOI subclade uncovered a significant negative relationship with latitude (t28 = −3.15, P = 0.004) and positive associations with altitude (t28 = 3.17, P = 0.004) and climate PC1 (t28 = 2.85, P = 0.008). For stomatal length, significant environmental associations were only detected in the AFR subclade: negative associations with longitude (t11 = −3.97, P = 0.002) and climate PC2 (t11 = −2.67, P = 0.022), and a positive association with climate PC3 (t11 = 3.08, P = 0.011). Stomatal width and pore width showed no environmental association in any of the tested clades.
Evolutionary model comparisons
Model comparisons revealed that OU models outperformed the BM model for all traits, but the WN model was a significantly better fit for stomatal density, stomatal width and pore width (ΔAICc > 2, Table 3, Supplementary Data Table S6). Leaf area was best explained by the OUD model (AICc = 139.8), significantly outperforming all other models (ΔAICc > 2). For SLA, the OUS model (AICc = 317.2) was the best fit, slightly outperforming the OU1 model (AICc = 318.6). Stomatal length showed the lowest AICc value for the OU1 model (AICc = 281.2). Finally, PGLS regression models displayed significantly lower AICc values than the evolutionary models for all traits except SLA (Table 3).
Table 3.
Model AICc values across Coffea without outgroups for each trait. The lowest AICc value for each trait (not considering the PGLS models) is marked in bold. For stomatal density, leaf area and SLA, n = 58; for stomatal length, stomata width and pore width, n = 55.
| Model | Stomatal density | Leaf area | SLA | Stomatal length | Stomatal width | Pore width |
|---|---|---|---|---|---|---|
| WN | 624.1 | 150.9 | 321.7 | 288.8 | 248.9 | 104.5 |
| BM | 677.2 | 173.1 | 343.7 | 289.4 | 288.0 | 196.3 |
| OU1 | 626.3 | 143.6 | 318.6 | 281.2 | 251.0 | 106.1 |
| OUT | 627.0 | 145.4 | 320.4 | 283.4 | 251.1 | 106.3 |
| OUD | 627.1 | 139.8 | 320.9 | 282.9 | 252.9 | 108.3 |
| OUS | 626.3 | 145.9 | 317.2 | 283.4 | 253.4 | 108.0 |
| OUA | 628.5 | 145.9 | 320.3 | 283.5 | 253.0 | 108.0 |
| PGLS | 603.9 | 130.1 | 321.7 | 207.8 | 186.3 | 81.9 |
DISCUSSION
Leaf functional traits play a key role in environmental adaptability. Their variation and evolution across a genus can shed light on the factors driving evolutionary change, and on the adaptive value of specific traits. This information can be of use in strategies for conservation and crop improvement. Our results confirm that Coffea species have diverged substantially in the measured leaf functional traits over evolutionary time, contributing to the mapping of trait variation in the genus. Intraspecific trait differences were less substantial compared with interspecific differences for five of the six measured traits, and traits evolved in a correlated fashion at different taxonomic levels. It was also clear that climate played an important role in driving the evolution of some leaf functional traits in Coffea and that phylogenetic patterns in these traits differed between subclades.
Genus-wide leaf trait patterns
Our results suggest that leaf traits in Coffea have evolved in a rather continuous mode: earlier-branching taxa, such as the outgroups, the XC clade and C. charrieriana, tend to have fewer, larger stomata than more recently derived species such as e.g. C. mauritiana. This clear trade-off between stomatal size and density has been shown in other taxa (e.g. Doheny-Adams et al., 2012; Brodribb et al., 2013), and was evident in our trait PCA and PGLS regressions. This observation may suggest a shift in trait evolution throughout the diversification of the genus. However, we detected no clear climatic drivers of this shift, indicating that it may have been driven by variables not considered in this study. As higher densities of smaller stomata have been associated with increased stomatal conductance (Franks and Beerling, 2009; Drake et al., 2013), we hypothesize that this shift may have been a response to steadily decreasing atmospheric [CO2] throughout the diversification of Coffea over the past 12 million years (Hamon et al., 2017; Rae et al., 2021; Haworth et al., 2023). Lower [CO2] could have led species to increase their stomatal conductance to maintain the same growth rates, explaining the trend observed here.
Unlike stomatal size and density, SLA and leaf area were poorly related, both across the genus and within the subclades. Contrasting relationships across clades and the relatively unrelated environmental associations of these traits support the conclusions of Ackerly et al. (2002), who found that leaf area and SLA were not correlated across 22 shrub species in a 51-ha contiguous chaparral patch including elevation and insolation gradients. Their results indicated that these traits are related to different aspects of plant performance in an environmental context. Our study suggests that this conclusion is also valid across macroclimate gradients in African Coffea species. Further, leaf area increases with maximum plant height across the genus Coffea, aligning with our expectations based on literature (Jensen and Zwieniecki, 2013; Price et al., 2014; Peel et al., 2017). The work of Price et al. (2014) showed that traits in the LES do not tend to vary with maximum plant height across species, except for leaf area.
When considering all included Coffea species or the EC clade, leaf area was strongly negatively related to drought, and positively to latitude. Species with larger leaves thus occur in environments with more rainfall and in more equatorial regions, as has been observed in other taxa (e.g. Givnish, 1984; Peppe et al., 2011). We hypothesized that higher aridity would select for smaller leaf sizes to limit water loss via transpiration, reflecting a functional trade-off between drought tolerance and photosynthetic productivity (Poole and Miller, 1981; Thuiller et al., 2004; Wright et al., 2017; Chaturvedi and Raghubanshi, 2018). For example, in a study on the genus Leucadendron in the Cape Floristic Region, Thuiller et al. (2004) observed significantly smaller leaves in species in arid environments than in species in more moist habitats.
Overall, trait–climate relationships across the genus were relatively weak, as we had hypothesized. It is likely that trait evolution is influenced by multiple factors that differ strongly across the broad geographic range of the genus, making it difficult to discern direct drivers across Coffea as a whole. Patterns within subclades may thus be easier to detect and interpret, highlighting the importance of clade-specific analyses, especially when clades are also geographically isolated.
Contrasting patterns between subclades
Within the AFR subclade, stomatal density exhibited strong positive relationships with leaf area and with maximum plant height, relationships that were not present (or not as prominent) in the WIOI subclade. Taller AFR subclade species and species with larger leaves thus tend to have more stomata per square millimeter, a result that is contrary to observations in other taxa (Kouwenberg et al., 2007; Carins Murphy et al., 2012; Peel et al., 2017; Conesa et al., 2020). For example, Carins Murphy et al. (2012) studied the subtropical tree species Toona ciliata and found that more shaded (i.e. shorter) plants have larger leaves due to the expansion of epidermal cells, leading to lower stomatal densities. This supports our observation that shorter species have lower stomatal densities, but the positive association between leaf size and plant height (in contrast with Carins Murphy et al., 2012) excludes the possibility of a leaf size-mediated relationship between plant height and stomatal density. The cause of our contrarian findings requires further study.
The negative relationships between stomatal density and both temperature and altitude in the AFR subclade indicate that species in cooler environments have higher stomatal densities. This observation is again in direct contrast to patterns detected in other temperate (Kouwenberg et al., 2007) and subtropical (Liu et al., 2020) species, though some studies have found ambiguous or no patterns in this relationship in both (sub)tropical (Hill et al., 2014; Zhao et al., 2016) and temperate (Wang et al., 2014) climates. For example, Liu et al. (2020) observed increasing stomatal densities with elevation in three subtropical mountain species, whereas Zhao et al. (2016) observed no relationship between stomatal density and altitude across 105 angiosperm species in (sub)tropical montane forests in Yunnan (Southwestern China). The relation between stomatal density and the environment may thus differ across taxa and across habitats. On the African continent, species in warmer environments and/or at lower altitudes show a higher density of smaller stomata, thereby accelerating the rate of gas exchange and providing more potential for evaporative cooling (Drake et al., 2013; Hill et al., 2014). The same functional explanations could apply to the WIOI subclade, where the positive relationship between stomatal density and temperature seasonality aligns with the observations of Drake et al. (2013): higher stomatal densities (and thus smaller stomata) have more dynamic stomatal characteristics and may therefore be better adapted to respond to temperature fluctuations throughout the year (Drake et al., 2013).
Specific leaf area was also negatively related to maximum plant height in the AFR subclade. A core trait of the LES, high SLA values indicate proportionally lower levels of leaf investment (Wright et al., 2004; Reich, 2014). The more shaded leaves of shorter plants are known to have higher SLA values than sun leaves to enhance light capture (e.g. Niinemets, 2010; Legner et al., 2014; Paź-Dyderska et al., 2020), a pattern that appears to hold across the AFR subclade of Coffea. The fact that we did not observe this relationship in the WIOI subclade is likely due to the more homogeneous distribution of plant height in this subclade (results not shown) or due to the wider variety of vegetation structures inhabited by Malagasy species (Davis et al., 2006).
Leaf area was positively related to altitude and temperature in the AFR subclade, but not in the WIOI subclade. Leaf size has often been related to temperature (Ackerly et al., 2002; Royer et al., 2005; Wright et al., 2017; Glade-Vargas et al., 2018), though patterns are not consistent across taxa or taxonomic levels and depend on other factors such as rainfall or solar irradiance. Larger leaves have a higher potential for evaporative cooling, though this cooling requires sufficient water availability and should thus be conditional on sufficiently high moisture levels.
Evolutionary processes shaping leaf trait evolution
The non-uniform distribution of leaf trait values across the genus emphasizes the importance of a phylogenetic perspective when evaluating trait differences. Our results show that a pure drift BM model of evolution poorly predicted leaf functional trait evolution across Coffea.
Though univariate estimates showed significant phylogenetic signal for SLA and stomatal length across Coffea, multivariate phylogenetic signal was not significantly different from zero for any trait across the genus. An intrinsic property of OU models (and thus also of WN models; Münkemüller et al., 2015) is that evolutionary history becomes gradually less important over time, reducing phylogenetic signal (Felsenstein, 1988; Blomberg et al., 2003; Ackerly, 2009). However, our results showed that modes of trait evolution differed among subclades. In the AFR subclade, multivariate phylogenetic signal λ was high and was not significantly different from 1 for stomatal density and stomatal length. In the WIOI subclade, λ was also high for stomatal density, but stomatal length showed no phylogenetic signal. These differences may be partially explained by the different evolutionary trajectory of these clades, such as a rapid radiation of the WIOI clade across Madagascar and the Mascarenes (Anthony et al., 2010).
The evolution of stomatal density, stomatal width and pore width was better explained by a phylogeny-independent WN model than by any of the phylogenetic models fitted in this study. This could be due to rapid evolution along the phylogeny, or due to strong environmental influences on these traits (Glade-Vargas et al., 2018; Capunitan et al., 2020). The latter may be the case for stomatal density, which showed strong but differing associations with climate in each subclade.
The evolution of stomatal length was best approximated by the OU1 model, suggesting stabilizing selection towards a single optimum value for the entire genus. The best-fitting model for leaf area was the OUD model, whereas the OUS model provided the best fit for SLA. Evolutionary divergence in these three traits was thus constrained compared with a pure drift BM model, which is supported by the absence of multivariate phylogenetic signal and the high observed rates of adaptation. Moreover, leaf area and SLA appeared to be driven by precipitation and seasonality, respectively. In the case of leaf area, this interpretation is also supported by the PGLS regressions: leaf area was negatively related to climate PC2 across all clades except the AFR subclade. We can thus state with confidence that the evolution of leaf area across Coffea is driven by variation in precipitation experienced by the different species. Conversely, for SLA, relationships observed in the PGLS regressions do not appear to corroborate the trait’s evolution in response to temperature seasonality. Climate PC3 was not a significant predictor of SLA in any of the tested clades. Moreover, the difference in fit between the OUS and OU1 models for SLA was minimal and non-significant (ΔAICc = 1.4). We are therefore more hesitant to state that the evolution of SLA has been driven by temperature seasonality in Coffea, but we did observe constrained evolution and thus stabilizing selection on SLA within the genus.
For all traits except SLA, the AICc value of the PGLS model was substantially lower than the values of the evolutionary WN, BM and OU models, indicating that the inclusion of trait–trait and trait–environment associations explained a meaningful proportion of trait variation that was not considered in the other models. The benefit of these evolutionary models over PGLS is that they are explicitly evolutionarily interpretable, whereas PGLS models are simply regression models correcting for trait similarity resulting from common ancestry (Hansen, 1997).
Our results suggest that trait diversification across Coffea is substantially constrained compared with the expectation under pure drift (BM) evolution. This ecological similarity among related species can result from multiple processes, however, including insufficient variation, gene flow, genetic constraints or stabilizing selection (Wiens and Graham, 2005; Losos, 2008; Mitchell et al., 2018). Our analyses show that two highly derived sister species exhibit markedly different leaf traits: C. mauritiana and C. macrocarpa (Fig. 2B). The former was sampled on the island of Réunion, whereas the latter is endemic to Mauritius (Nowak et al., 2014). Their strong trait divergence despite their phylogenetic proximity suggests that isolation and novel niche availability following dispersal led to rapid trait evolution and divergence. Thus, rapid evolution is possible within the genus, and we deem strong genetic constraints unlikely to inhibit trait divergence across Coffea. Gene flow may occur at small scales between species but is unlikely to constrain evolutionary divergence at the genus level, or at the wide spatial scale considered here. We therefore consider stabilizing selection to be the driving force behind the constrained evolution of the considered leaf traits across the genus. Leaf traits are known to be more labile and adapt rapidly relative to other plant traits, such as seed or floral traits, which are generally more evolutionarily conserved (Ackerly, 2009; Vandelook et al., 2018). Strong stabilizing selection would thus act efficiently on the considered leaf traits, exemplified by the absence of phylogenetic signal and the high rates of adaptation. In contrast to our observation that leaf traits are evolutionarily labile, Glade-Vargas et al. (2018) detected significant phylogenetic signal for 14 out of 20 leaf traits in the Nothofagaceae family, indicating that these leaf traits were evolutionarily conserved. However, they considered only univariate estimates of phylogenetic signal and did not account for environmental effects.
Methodological limitations
Because of statistical limitations in fitting OU models on small phylogenies (Beaulieu et al., 2012; Cooper et al., 2016), we did not consider evolutionary model comparisons in the AFR and WIOI subclades. We also chose not to consider more complex models of continuous trait evolution (e.g. mvSLOUCH; Bartoszek et al., 2012) for the sake of interpretability. These models may nonetheless provide useful insights into the mechanisms and drivers of Coffea leaf trait evolution and should be considered in future studies.
Due to our exclusive use of herbarium specimens, the trait variation detected in this study is likely an underestimation of the variation present in wild populations and may suffer from unstandardized sampling, particularly in WIOI species, which occur in a broad range of vegetation types (Davis et al., 2006). Contrarily, since most central African Coffea species grow in the rainforest understorey, phenotypic variation due to variation in light exposure can be considered minimal in central African species (Hatangi et al., 2023). Despite these limitations, the use of herbarium accessions allows studies at a large geographical scale including almost half of all known Coffea species, which would not be feasible otherwise. Nonetheless, more sampling and improved data on habitat, vegetation structure and local climate remains necessary.
Conclusions
Our study seems to indicate that the evolution of leaf functional traits has been a complex process in Coffea. Interrelations with other traits and with various environmental variables, differing across clades, make it difficult to disentangle individual drivers of trait evolution. We show that the genus Coffea exhibits substantial leaf functional trait variation, and we observed a directional shift from low densities of large stomata towards higher densities of smaller stomata, potentially driven by a decline in atmospheric [CO2] during the divergence of Coffea. Also, by incorporating both ecological variables and explicit evolutionary models in our analyses, we found that the evolution of leaf area was most likely driven by differences in precipitation across the distribution of Coffea. We could confirm that leaf traits tend to adapt rapidly, with an important role for stabilizing selection, and that trait evolution across Coffea was not governed by pure drift. Contrasting correlations and differing evolutionary processes across different clades also highlight the relevance of a phylogenetic perspective in comparative studies, showing that the strength and direction of trait relationships depends on the considered taxonomic scope and geographic range. Our study can fundamentally be of relevance to crop improvement programmes, as our results suggest that smaller-leaved species are adapted to drier climates. Therefore, breeding programmes may consider leveraging leaf area in climate adaptation strategies. Our findings contribute more broadly to our understanding of the variation and adaptive significance of leaf functional traits in Coffea.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: individual leaf data set. Table S2: species-level data set. Table S3: loadings of the trait PCA. Table S4: regression tables and other output of PGLS across all considered clades. Table S5: loadings of the climate PCA. Table S6: parameter output of the evolutionary models. Figure S1: boxplots of trait values across the genus.
ACKNOWLEDGEMENTS
We would like to thank Mieke Rotteveel, Sofie Meeus and Martine Borremans for help with data collection and Ann Bogaerts for facilitating access to the BR herbarium.
Appendix
Shrinkage correction
To correct for potential bias introduced in SLA values by leaf shrinkage of herbarium specimens, 145 fresh leaves were sampled without petioles across 29 live accessions of 11 Coffea species. These leaves were weighed using a precision scale (1 mg accuracy) and scanned with a flatbed scanner (Canon CanoScan 9000F Mark II) before and after fully drying in a herbarium oven. Fresh and dry leaf areas were measured with ImageJ open access software version 1.53v (Schneider et al., 2012), after which leaf shrinkage factors were calculated as the ratio of dry area to fresh area. We then used dry area, which has been found to be a good predictor of shrinkage (Blonder et al., 2012), to predict shrinkage factors for all other leaves in the data set using a simple linear regression: (shrinkage factor = 0.008851 × loge(dry area) + 0.895520, P < 0.001, though residuals deviated from normality: Shapiro–Wilk normality test, W = 0.87496, P < 0.001). Data on SLA (calculated using dry area) were divided by the obtained shrinkage factors, resulting in SLA data with a correction for leaf shrinkage.
Contributor Information
Aiden Hendrickx, Meise Botanic Garden, 1860 Meise, Belgium; Division of Ecology, Evolution, and Biodiversity Conservation, KU Leuven, 3000 Leuven, Belgium; KU Leuven Plant Institute, 3001 Leuven, Belgium.
Yves Hatangi, Meise Botanic Garden, 1860 Meise, Belgium; Université de Kisangani, 2012 Kisangani, DR Congo; Liège University, Gembloux Agro-Bio Tech, 5030 Gembloux, Belgium.
Olivier Honnay, Division of Ecology, Evolution, and Biodiversity Conservation, KU Leuven, 3000 Leuven, Belgium; KU Leuven Plant Institute, 3001 Leuven, Belgium.
Steven B Janssens, Meise Botanic Garden, 1860 Meise, Belgium; Division of Molecular Biotechnology of Plants and Micro-organisms, KU Leuven, 3001 Leuven, Belgium.
Piet Stoffelen, Meise Botanic Garden, 1860 Meise, Belgium.
Filip Vandelook, Meise Botanic Garden, 1860 Meise, Belgium; Division of Ecology, Evolution, and Biodiversity Conservation, KU Leuven, 3000 Leuven, Belgium; KU Leuven Plant Institute, 3001 Leuven, Belgium.
Jonas Depecker, Meise Botanic Garden, 1860 Meise, Belgium; Division of Ecology, Evolution, and Biodiversity Conservation, KU Leuven, 3000 Leuven, Belgium; KU Leuven Plant Institute, 3001 Leuven, Belgium.
FUNDING
This work was financially supported by an FWO research project grant (G090719N) and by the Belgian Science Policy Office (Belspo)-funded COBECORE project (R/175/A3/COBECORE).
CONFLICT OF INTEREST
We report no conflicts of interest.
LITERATURE CITED
- Ackerly DD. 2004. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecological Monographs 74: 25–44. [Google Scholar]
- Ackerly DD. 2009. Conservatism and diversification of plant functional traits: evolutionary rates versus phylogenetic signal. Proceedings of the National Academy of Sciences of the USA 106: 19699–19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerly DD, Dudley SA, Sultan SE, et al. 2000. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50: 979–995. [Google Scholar]
- Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP.. 2002. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130: 449–457. [DOI] [PubMed] [Google Scholar]
- Andersen KM, Endara MJ, Turner BL, Dalling JW.. 2012. Trait-based community assembly of understory palms along a soil nutrient gradient in a lower montane tropical forest. Oecologia 168: 519–531. [DOI] [PubMed] [Google Scholar]
- Anthony F, Diniz LEC, Combes MC, Lashermes P.. 2010. Adaptive radiation in Coffea subgenus Coffea L. (Rubiaceae) in Africa and Madagascar. Plant Systematics and Evolution 285: 51–64. [Google Scholar]
- Bartoszek K, Pienaar J, Mostad P, Andersson S, Hansen TF.. 2012. A phylogenetic comparative method for studying multivariate adaptation. Journal of Theoretical Biology 314: 204–215. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Jhwueng DC, Boettiger C, O’Meara BC.. 2012. Modeling stabilizing selection: expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution 66: 2369–2383. [DOI] [PubMed] [Google Scholar]
- Berthaud J. 1986. Les ressources génétiques pour l’amélioration des caféiers Africains diploïdes. PhD thesis, Université de Paris-Sud, France. [Google Scholar]
- Blomberg SP, Garland T, Ives AR.. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Blonder B, Buzzard V, Simova I, et al. 2012. The leaf-area shrinkage effect can bias paleoclimate and ecology research. American Journal of Botany 99: 1756–1763. [DOI] [PubMed] [Google Scholar]
- Bridson DM. 1994. Additional notes on Coffea (Rubiaceae) from tropical East Africa. Kew Bulletin 49: 331–342. [Google Scholar]
- Brodribb TJ, Jordan GJ, Carpenter RJ.. 2013. Unified changes in cell size permit coordinated leaf evolution. New Phytologist 199: 559–570. [DOI] [PubMed] [Google Scholar]
- Buchanan S, Isaac ME, Van den Meersche K, Martin AR.. 2019. Functional traits of coffee along a shade and fertility gradient in coffee agroforestry systems. Agroforestry Systems 93: 1261–1273. [Google Scholar]
- Butler MA, King AA.. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. American Naturalist 164: 683–695. [DOI] [PubMed] [Google Scholar]
- Capunitan DC, Johnson O, Terrill RS, Hird SM.. 2020. Evolutionary signal in the gut microbiomes of 74 bird species from Equatorial Guinea. Molecular Ecology 29: 829–847. [DOI] [PubMed] [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ.. 2012. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant, Cell and Environment 35: 1407–1418. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Raghubanshi A.. 2018. Leaf size and specific leaf area of tropical deciduous trees increase with elevation in soil moisture content. International Journal of Hydrology 2: 466–469. [Google Scholar]
- Conesa MA, Muir CD, Molins A, Galmés J.. 2020. Stomatal anatomy coordinates leaf size with Rubisco kinetics in the Balearic Limonium. AoB Plants 12: plz050. [Google Scholar]
- Cooper N, Thomas GH, Venditti C, Meade A, Freckleton RP.. 2016. A cautionary note on the use of Ornstein Uhlenbeck models in macroevolutionary studies. Biological Journal of the Linnean Society of London 118: 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubry P, de Bellis F, Pot D, Musoli P, Leroy T.. 2013. Global analysis of Coffea canephora Pierre ex Froehner (Rubiaceae) from the Guineo-Congolese region reveals impacts from climatic refuges and migration effects. Genetic Resources and Crop Evolution 60: 483–501. [Google Scholar]
- Davis AP. 2003. A new combination in Psilanthus (Rubiaceae) for Australasia, and nomenclatural notes on Paracoffea. Novon 13: 182–184. [Google Scholar]
- Davis AP, Mvungi EF.. 2004. Two new and endangered species of Coffea (Rubiaceae) from the Eastern Arc Mountains (Tanzania) and notes on associated conservation issues. Botanical Journal of the Linnean Society 146: 237–245. [Google Scholar]
- Davis AP, Rakotonasolo F.. 2001. Three new species of Coffea L. (Rubiaceae) from Madagascar. Adansonia, Sér. 3 23: 137–146. [Google Scholar]
- Davis AP, Rakotonasolo F.. 2008. A taxonomic revision of the baracoffea alliance: nine remarkable Coffea species from western Madagascar. Botanical Journal of the Linnean Society 158: 355–390. [Google Scholar]
- Davis AP, Rakotonasolo F.. 2021. Six new species of coffee (Coffea) from northern Madagascar. Kew Bulletin 76: 497–511. [Google Scholar]
- Davis AP, Bridson DM, Rakotonasolo F.. 2005. A reexamination of Coffea subgenus Baracoffea and comments on the morphology and classification of Coffea and Psilanthus (Rubiaceae-Coffeeae). In: Keating RC, Hollowell VC, Croat TB. eds. A Festschrift for William G. D’Arcy: the legacy of a taxonomist. St Louis: Missouri Botanical Garden Press, 399–420. https://www.biodiversitylibrary.org/item/281930#page/1/mode/1up. [Google Scholar]
- Davis AP, Govaerts R, Bridson DM, Stoffelen P.. 2006. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Botanical Journal of the Linnean Society 152: 465–512. [Google Scholar]
- Davis AP, Chester M, Maurin O, Fay MF.. 2007. Searching for the relatives of Coffea (Rubiaceae, Ixoroideae): the circumscription and phylogeny of Coffeeae based on plastid sequence data and morphology. American Journal of Botany 94: 313–329. [DOI] [PubMed] [Google Scholar]
- Davis AP, Tosh J, Ruch N, Fay MF.. 2011. Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of molecular and morphological data; implications for the size, morphology, distribution and evolutionary history of Coffea. Botanical Journal of the Linnean Society 167: 357–377. [Google Scholar]
- Davis AP, Mieulet D, Moat J, Sarmu D, Haggar J.. 2021. Arabica-like flavour in a heat-tolerant wild coffee species. Nature Plants 7: 413–418. [DOI] [PubMed] [Google Scholar]
- Davis AP, Kiwuka C, Faruk A, Walubiri MJ, Kalema J.. 2022. The re-emergence of Liberica coffee as a major crop plant. Nature Plants 8: 1322–1328. [DOI] [PubMed] [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE.. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society of London, Series B 367: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ.. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberstein D, Oliveira MG, Aoyama EM, et al. 2021. Diversity of leaf stomatal traits among Coffea canephora Pierre ex A. Froehner genotypes. Agronomy 11: 1126. [Google Scholar]
- Dutra Giles JA, Ferreira AD, Partelli FL, et al. 2019. Divergence and genetic parameters between Coffea sp. genotypes based in foliar morpho-anatomical traits. Scientia Horticulturae 245: 231–236. [Google Scholar]
- Felsenstein J. 1988. Phylogenies and quantitative characters. Annual Review of Ecology and Systematics 19: 445–471. [Google Scholar]
- Fick SE, Hijmans RJ.. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Flores O, Garnier E, Wright IJ, et al. 2014. An evolutionary perspective on leaf economics: phylogenetics of leaf mass per area in vascular plants. Ecology and Evolution 4: 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ.. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences of the USA 106: 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M.. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. American Naturalist 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. 1987. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytologist 106: 131–160. [Google Scholar]
- Givnish TJ. 1984. Leaf and canopy adaptations in tropical forests. In: Medina E, Mooney HA, Vázquez-Yánes C. eds. Physiological ecology of plants of the wet tropics. The Hague: Dr W. Junk Publishers, 12: 51–84. doi: 10.1007/978-94-009-7299-5_6 [Google Scholar]
- Glade-Vargas N, Hinojosa LF, Leppe M.. 2018. Evolution of climatic related leaf traits in the family Nothofagaceae. Frontiers in Plant Science 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Dussert S, Hamon P, Hamon S, De Kochko A, Poncet V.. 2009. Current genetic differentiation of Coffea canephora Pierre ex A. Froehn in the Guineo-Congolian African zone: cumulative impact of ancient climatic changes and recent human activities. BMC Evolutionary Biology 9: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon P, Grover CE, Davis AP, et al. 2017. Genotyping-by-sequencing provides the first well-resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species: GBS coffee phylogeny and the evolution of caffeine content. Molecular Phylogenetics and Evolution 109: 351–361. [DOI] [PubMed] [Google Scholar]
- Hansen TF. 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51: 1341–1351. [DOI] [PubMed] [Google Scholar]
- Hansen TF, Orzack SH.. 2005. Assessing current adaptation and phylogenetic inertia as explanations of trait evolution: the need for controlled comparisons. Evolution 59: 2063–2072. [PubMed] [Google Scholar]
- Hansen TF, Pienaar J, Orzack SH.. 2008. A comparative method for studying adaptation to a randomly evolving environment. Evolution 62: 1965–1977. [DOI] [PubMed] [Google Scholar]
- Hao S, Xue J.. 2013. The early Devonian posongchong flora of Yunnan – a contribution to an understanding of the evolution and early diversification of vascular plants. Beijing, China: Science Press. [Google Scholar]
- Hatangi Y, Nshimba H, Stoffelen P, et al. 2023. Leaf traits of understory woody species in the Congo Basin forests changed over a 60-year period. Plant Ecology and Evolution 156: 339–351. [Google Scholar]
- Haworth M, Marino G, Materassi A, Raschi A, Scutt CP, Centritto M.. 2023. The functional significance of the stomatal size to density relationship: interaction with atmospheric [CO2] and role in plant physiological behaviour. Science of the Total Environment 863: 160908. [DOI] [PubMed] [Google Scholar]
- Herrera JC, Lambot C.. 2017. The coffee tree – genetic diversity and origin. In: Folmer B. ed. The craft and science of coffee. London, United Kingdom: Academic Press, 1–16. doi: 10.1016/B978-0-12-803520-7.00001-3. [DOI] [Google Scholar]
- Hill KE, Guerin GR, Hill RS, Watling JR.. 2014. Temperature influences stomatal density and maximum potential water loss through stomata of Dodonaea viscosa subsp. angustissima along a latitude gradient in southern Australia. Australian Journal of Botany 62: 657–665. [Google Scholar]
- International Coffee Organization. 2023. Coffee market report, December 2023. https://www.icocoffee.org/documents/cy2023-24/cmr-1223-e.pdf.
- Jensen KH, Zwieniecki MA.. 2013. Physical limits to leaf size in tall trees. Physical Review Letters 110: 018104. [DOI] [PubMed] [Google Scholar]
- Jones CS, Martínez-Cabrera HI, Nicotra AB, Mocko K, Marais EM, Schlichting CD.. 2013. Phylogenetic influences on leaf trait integration in Pelargonium (Geraniaceae): convergence, divergence, and historical adaptation to a rapidly changing climate. American Journal of Botany 100: 1306–1321. [DOI] [PubMed] [Google Scholar]
- Kafuti C, Bourland N, De Mil T, et al. 2020. Foliar and wood traits covary along a vertical gradient within the crown of long-lived light-demanding species of the Congo Basin semi-deciduous forest. Forests 11: 35. [Google Scholar]
- Kopperud B, Pienaar J, Voje K, Orzack SH, Hansen TF.. 2020. slouch: Stochastic Linear Ornstein-Uhlenbeck Comparative Hypotheses (2.1.4). Retrieved November 10, 2023 from 10.1086/426002. [DOI] [Google Scholar]
- Kouwenberg LLR, Kürschner WM, McElwain JC.. 2007. Stomatal frequency change over altitudinal gradients: prospects for paleoaltimetry. Reviews in Mineralogy and Geochemistry 66: 215–241. [Google Scholar]
- Lauterbach M, van der Merwe P de W, Keßler L, Pirie MD, Bellstedt DU, Kadereit G.. 2016. Evolution of leaf anatomy in arid environments – a case study in southern African Tetraena and Roepera (Zygophyllaceae). Molecular Phylogenetics and Evolution 97: 129–144. [DOI] [PubMed] [Google Scholar]
- Legner N, Fleck S, Leuschner C.. 2014. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees - Structure and Function 28: 263–280. [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M.. 2000. The evolutionary ecology of seed size. In: Fenner M. ed. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, United Kingdom: CAB International, 31–57. [Google Scholar]
- Leroy J-F. 1961. Coffeae Novae Madagascarienses. Journal d’Agriculture Tropicale et de Botanique Appliquée 8: 1–20. [Google Scholar]
- Liu W, Zheng L, Qi D.. 2020. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecology and Evolution 10: 8166–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB. 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecology Letters 11: 995–1003. [DOI] [PubMed] [Google Scholar]
- Maurin O, Davis AP, Chester M, Mvungi EF, Jaufeerally-Fakim Y, Fay MF.. 2007. Towards a phylogeny for Coffea (Rubiaceae): identifying well-supported lineages based on nuclear and plastid DNA sequences. Annals of Botany 100: 1565–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M.. 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185. [DOI] [PubMed] [Google Scholar]
- Meeus S, Van den Bulcke J, Wyffels F.. 2020. From leaf to label: a robust automated workflow for stomata detection. Ecology and Evolution 10: 9178–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles JE, Cavender-Bares J, Townsend PA, et al. 2020. Leaf reflectance spectra capture the evolutionary history of seed plants. New Phytologist 228: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell N, Carlson JE, Holsinger KE.. 2018. Correlated evolution between climate and suites of traits along a fast–slow continuum in the radiation of Protea. Ecology and Evolution 8: 1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. 2005. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the USA 102: 10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münkemüller T, Boucher FC, Thuiller W, Lavergne S.. 2015. Phylogenetic niche conservatism – common pitfalls and ways forward. Functional Ecology 29: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U. 2010. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research 25: 693–714. [Google Scholar]
- Nowak MD, Haller BC, Yoder AD.. 2014. The founding of Mauritian endemic coffee trees by a synchronous long-distance dispersal event. Journal of Evolutionary Biology 27: 1229–1239. [DOI] [PubMed] [Google Scholar]
- O’Meara BC, Ané C, Sanderson MJ, Wainwright PC.. 2006. Testing for different rates of continuous trait evolution using likelihood. Evolution 60: 922–933. [PubMed] [Google Scholar]
- Onstein RE, Jordan GJ, Sauquet H, et al. 2016. Evolutionary radiations of Proteaceae are triggered by the interaction between traits and climates in open habitats. Global Ecology and Biogeography 25: 1239–1251. [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2018. caper: comparative analyses of phylogenetics and evolution in R (1.0.1). Retrieved November 10, 2023 from https://cran.r-project.org/package=caper. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K.. 2019. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Paź-Dyderska S, Dyderski MK, Nowak K, Jagodziński AM.. 2020. On the sunny side of the crown – quantification of intra-canopy SLA variation among 179 taxa. Forest Ecology and Management 472: 118254. [Google Scholar]
- Peel JR, Mandujano Sanchez MC, Lopez Portillo J, Golubov J.. 2017. Stomatal density, leaf area and plant size variation of Rhizophora mangle (Malpighiales: Rhizophoraceae) along a salinity gradient in the Mexican Caribbean. Revista de Biología Tropical 65: 701–712. [Google Scholar]
- Pennell MW, Eastman JM, Slater GJ, et al. 2014. Geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30: 2216–2218. [DOI] [PubMed] [Google Scholar]
- Peppe DJ, Royer DL, Cariglino B, et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytologist 190: 724–739. [DOI] [PubMed] [Google Scholar]
- Perez TM, Rodriguez J, Heberling JM.. 2020. Herbarium‐based measurements reliably estimate three functional traits. American Journal of Botany 107: 1457–1464. [DOI] [PubMed] [Google Scholar]
- Poole D, Miller P.. 1981. The distribution of plant water stress and vegetation characteristics in Southern California Chaparral. American Midland Naturalist 105: 32–43. [Google Scholar]
- Poorter L, Bongers F.. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87: 1733–1743. [DOI] [PubMed] [Google Scholar]
- Price CA, Wright IJ, Ackerly DD, Niinemets U, Reich PB, Veneklaas EJ.. 2014. Are leaf functional traits ‘invariant’ with plant size and what is ‘invariance’ anyway? Functional Ecology 28: 1330–1343. [Google Scholar]
- R Core Team. 2023. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/. [Google Scholar]
- Rae JWB, Zhang YG, Liu X, Foster GL, Stoll HM, Whiteford RDM.. 2021. Atmospheric CO2 over the past 66 million years from marine archives. Annual Review of Earth and Planetary Sciences 49: 609–641. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. Journal of Plant Sciences 164: 143–164. [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods in Ecology and Evolution 1: 319–329. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Revell LJ, Harmon LJ, Collar DC.. 2008. Phylogenetic signal, evolutionary process, and rate. Systematic Biology 57: 591–601. [DOI] [PubMed] [Google Scholar]
- Robbrecht E, Manen JF.. 2006. The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. a new classification in two subfamilies, Cinchonoideae and Rubioideae. Systematics and Geography of Plants 76: 85–146. [Google Scholar]
- Royer DL, Wilf P, Janesko DA, Kowalski EA, Dilcher DL.. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 92: 1141–1151. [DOI] [PubMed] [Google Scholar]
- Ruffo CK, Birnie A, Tengnäs B.. 2002. Edible wild plants of Tanzania. In: Technical Handbook No. 27. Nairobi, Kenya: Regional Land Management Unit RELMA/Sida. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statista. 2023. Coffee - Worldwide. Statista market forecast. Retrieved October 15, 2023 from https://www.statista.com/outlook/cmo/hot-drinks/coffee/worldwide. [Google Scholar]
- Stoffelen P. 1998. Coffea and Psilanthus (Rubiaceae) in tropical Africa: a systematic and palynological study, including a revision of the West and Central African species. PhD Thesis, Katholieke Universiteit Leuven, Belgium.
- Stoffelen P, Anthony F, Janssens SB, Noirot M.. 2021. A new coffee species from South-West Cameroon, the principal hotspot of diversity for Coffea L. (Coffeeae, Ixoroideae, Rubiaceae) in Africa. Adansonia 43: 277–285. [Google Scholar]
- Thuiller W, Lavorel S, Midgley G, Lavergne S, Rebelo T.. 2004. Relating plant traits and species distributions along bioclimatic gradients for 88 Leucadendron taxa. Ecology 85: 1688–1699. [Google Scholar]
- Vandelook F, Janssens SB, Probert RJ.. 2012. Relative embryo length as an adaptation to habitat and life cycle in Apiaceae. New Phytologist 195: 479–487. [DOI] [PubMed] [Google Scholar]
- Vandelook F, Janssens SB, Matthies D.. 2018. Ecological niche and phylogeny explain distribution of seed mass in the central European flora. Oikos 127: 1410–1421. [Google Scholar]
- Verbeeck H, Boeckx P, Steppe K.. 2011. Tropical forests: include Congo basin. Nature 479: 179. [DOI] [PubMed] [Google Scholar]
- Verbeeck H, Betehndoh E, Maes WH, et al. 2014. Functional leaf trait diversity of 10 tree species in Congolese secondary tropical forest. Journal of Tropical Forest Science 26: 409–419. [Google Scholar]
- Violle C, Navas M-L, Vile D, et al. 2007. Let the concept of trait be functional! Oikos 116: 882–892. [Google Scholar]
- Wang R, Yu G, He N, et al. 2014. Elevation-related variation in leaf stomatal traits as a function of plant functional type: evidence from Changbai Mountain, China. PLoS One 9: e115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ, Graham CH.. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics 36: 519–539. [Google Scholar]
- Wigley BJ, Slingsby JA, Díaz S, Bond WJ, Fritz H, Coetsee C.. 2016. Leaf traits of African woody savanna species across climate and soil fertility gradients: evidence for conservative versus acquisitive resource-use strategies. Journal of Ecology 104: 1357–1369. [Google Scholar]
- Wright IJ, Westoby M, Baruch Z, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Dong N, Maire V, et al. 2017. Global climatic drivers of leaf size. Science 357: 917–921. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhou G.. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59: 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Niu H, Wang S, et al. 2012. Gene or environment? Species-specific control of stomatal density and length. Ecology and Evolution 2: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WL, Chen YJ, Brodribb TJ, Cao KF.. 2016. Weak co-ordination between vein and stomatal densities in 105 angiosperm tree species along altitudinal gradients in Southwest China. Functional Plant Biology 43: 1126–1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.