Abstract
Backgrounds and Aims
The hypothesis that plants evolve features that protect accessible pollen from consumption by flower visitors remains poorly understood.
Methods
To explore potential chemical defence against pollen consumption, we examined the pollinator assemblage, foraging behaviour, visitation frequency and pollen transfer efficiency in Rhododendron molle, a highly toxic shrub containing rhodojaponin III. Nutrient (protein and lipid) and toxic components in pollen and other tissues were measured.
Key Results
Overall in the five populations studied, floral visits by butterflies and bumblebees were relatively more frequent than visits by honeybees. All foraged for nectar but not pollen. Butterflies did not differ from bumblebees in the amount of pollen removed per visit, but deposited more pollen per visit. Pollination experiments indicated that R. molle was self-compatible, but both fruit and seed production were pollen-limited. Our analysis indicated that the pollen was not protein-poor and had a higher concentration of the toxic compound rhodojaponin III than petals and leaves, this compound was undetectable in nectar.
Conclusion
Pollen toxicity in Rhododendron flowers may discourage pollen robbers (bees) from taking the freely accessible pollen grains, while the toxin-free nectar rewards effective pollinators, promoting pollen transfer. This preliminary study supports the hypothesis that chemical defence in pollen would be likely to evolve in species without physical protection from pollinivores.
Keywords: Bee foraging behaviour, butterfly pollination, chemical defence, exposed pollen presentation, Rhododendron molle, rhodojaponin III, toxic pollen
INTRODUCTION
Around 90 % of flowering plants rely on animals, commonly insects, to transport pollen (Ollerton et al., 2011; Tong et al., 2023). Pollen is protein-rich and widely consumed by diverse insects, particularly bees. There is often a conflict interest in pollen resource between pollen fertility in flowers and pollen parasitism of bees (Wang et al., 2019; van der Kooi et al., 2021). To reduce pollen exploitation, selection for physical and chemical protection for pollen grains would be favoured to achieve successful pollination (Hargreaves et al., 2009; Arnold et al., 2014; Trunz et al., 2020). If exposed pollen grains without physical protection are accessible and susceptible to exploitation, one would expect that such pollen should evolve chemical defence against pollen theft unless it is nutritionally poor. It has been hypothesized that evolution of repellent or toxic pollen as an anti-theft strategy is more likely in those species whose primary pollinators (e.g. nectar-feeding birds or lepidopteran insects) do not consume its pollen (Hargreaves et al., 2009), these species often simultaneously presenting amounts of pollen in relatively large flowers, for instance Rhododendron (see Song et al., 2019). However, the hypothesis that freely accessible pollen may be toxic, deterring pollen thieves, remains poorly understood in a given species (but see Hao et al., 2023).
Studies have shown that the pollen-feeding preferences of bees may be affected by pollen quality including nutritional components such as protein and lipid content, and secondary metabolites (Roulston et al., 2000; Arnold et al., 2014; Vaudo et al., 2016a, b; Stevenson, 2020). Bees tend to forage for pollen that has a relatively high protein (Roulston et al., 2000; Hanley et al., 2008; Garance et al., 2013; Russo et al., 2019) or low lipid content (Ruedenauer et al., 2020). High protein content in pollen diets can improve bumblebee colony growth (Génissel et al., 2002), whereas high lipid intake may be detrimental to both bumblebees and honeybees (Manning et al., 2007; Vaudo et al., 2016b; Ruedenauer et al., 2020). Secondary metabolites in pollen may also affect the foraging behaviour of bumblebees, influencing the time spent on pollen collection and the amounts of pollen collected (Muth et al., 2016). A comparative study on two species of Dipsacus (Caprifoliaceae) indicated that when pollen was passively placed on bumblebees’ bodies they groomed less into their corbiculae if it had a higher concentration of toxic saponin, a chemical defence deterring pollen exploitation. Therefore, more pollen grains remained on the bumblebees’ bodies, facilitating pollen transfer (Wang et al., 2019). Bumblebee pollinators (Bombus pascuorum and B. hortorum) of Aconitum napellus mainly collected nectar with a lower toxin concentration but rarely pollen, which had a high concentration of toxic compounds (Jacquemart et al., 2019). However, Bombus consobrinus which is specialized on Aconitum septentrionale positively fed on the pollen even with its defensive alkaloids (Gosselin et al., 2013).
Rhododendron (Ericaceae) species are evergreen ornamental woody plants of worldwide distribution with large brightly coloured flowers in spring (Fang et al., 2005). The hermaphroditic flowers are generally open-shaped with copious nectar at the base of a campanulate or tubular corolla and generally have five or ten stamens with poricidal anthers, each with two round apical pores. Pollen grains, generally fused into tetrads, are further connected together by viscin threads (Hesse, 1984). In open flowers, it is common to see pollen-thread tangles freely accessible to floral visitors (Song et al., 2019). Exposure of pollen grains without physical protection from thieves would involve a high risk of pollen loss to visitors that positively collect pollen. Therefore, these plants are expected to evolve strategies to attract effective pollinators but deter pollen thieves from collecting pollen.
Plant defence compounds in floral rewards have been hypothesized to filter out ineffective pollinators or discourage overexploitation by foragers (Adler, 2000; Rivest and Forrest, 2020; Stevenson, 2020). Secondary metabolites in Rhododendron species are known to be toxic to insects and even mammals (Popescu and Kopp, 2013). In 401 BC, ‘mad honey’ containing the diterpene grayanotoxin collected by bees from Rhododendron species (probably R. ponticum or R. luteum) was reported to be poisonous to humans in the Black Sea region (Sütlüpmar et al., 1993). Grayanotoxin I has been detected in the leaves, petals and nectar of seven Rhododendron species (Fattorini et al., 2023). Grayanotoxin I and III at natural concentrations in the nectar of R. ponticum flowers have been observed to deter Apis mellifera and Andrena carantonica (Tiedeken et al., 2016). Four Rhododendron species are reported to contain secondary metabolites in the pollen including terpenoids, alkaloids, flavonoids and phenylpropanoids (Palmer-Young et al., 2019; Yao et al., 2023). However, to our knowledge, possible impacts of these secondary compounds in pollen on pollinators or pollen feeders remain unexplored. To examine the above hypothesis that freely accessible pollen would have evolved chemical defence against pollen consumption, we investigated floral visitors and their foraging behaviour and pollination efficiency in Rhododendron molle, a famous insecticidal plant in Asia. In this toxic shrub, the diterpene compound rhodojaponin III was identified as the major insecticidal component in flowers and showed insecticidal activity against larvae of two species, namely Spodoptera frugiperda (the fall armyworm) and Leptinotarsa decemlineata (the Colorado potato beetle) (Klocke et al., 1991; Hu et al., 1993; Cai et al., 2018). We tested whether the rhodojaponin III occurred in nectar or pollen, and its potential effect on visitor foraging behaviour.
MATERIALS AND METHODS
Study species and sites
Rhododendron molle (Ericaceae) is a deciduous shrub up to 2 m tall, with terminal, racemose-umbellate inflorescences. The flowers open before or with the leaves. The corolla is broadly funnel-shaped, yellow or golden yellow (Fig. 1), with orange-red dots (nectar guides) on the upper petal (Fig. 1B) where nectar is secreted at the base. It contains five stamens and a sticky stigma above the anthers (Fig. 1B). Individuals generally flower from March to May and fruit from July to August. It is well known to be poisonous and also called ‘sheep harming flower’ because sheep often tremble and die after eating its leaves (Fang et al., 2005). Pollen grains in Rhododendron are fused into tetrads and further aggregated by viscin threads (Fig. 1F) in the poricidal anthers. Rhodojaponin III, the major insecticidal component in R. molle, is present in roots, petals and leaves (Cai et al., 2018) and probably in pollen.
Fig. 1.
Flowers, pollen traits and floral visitors in Rhododendron molle. (A) A flowering individual with several bright-yellow racemose umbels; note that two flowers are bagged with nylon nets to exclude visitors. (B) Side view of the funnel-shaped corolla with a hole (pink arrow) made by a robbing bumblebee, showing orange-red dots (nectar guides) on the upper petal, five poricidal anthers (white arrow) and a capitulate stigma (green arrow). (C) A bumblebee (Bombus breviceps) robbing nectar from the base of the corolla tube. (D) A bumblebee sucking nectar but not collecting pollen, with no pollen basket on the legs and pollen passively deposited on the body (white arrow) far from the stigma (green arrow). (E) A butterfly (Byasa mencius) sucking nectar while the hind wing carries pieces of pollen-thread tangles (white arrow), and the stigma contact site (green arrow). (F) Pollen grains attached to the hairs of a butterfly wing by viscin threads (white arrow) and a butterfly scale (pink arrow).
Three wild (Lechang, Siming Mountain and Dapan Mountain) and two transplanted (Jinggang Mountain and Kunming Botanical Garden) populations were investigated, each with at least 12 flowering individuals (see Supplementary Data Table S1, Fig. S1). All data were collected in 2018 and 2019.
Measurements of floral traits
We measured the size of sexual organs, nectar volume, sugar concentration, the length of each ‘pollen thread tangle’ (PTT), which was pulled gently away from the anther pore (Song et al., 2019), and pollen grain number per PTT in R. molle, i.e. the amount of pollen that can be maximally removed as a unit in a single visit by an effective pollinator. These floral traits were measured in 30 flowers (two flowers each on 15 plants) bagged with nylon nets before flowers open in Siming Mountain, the largest wild population. To see whether exposed pollen grains are susceptible to bees, we first measured the spectral reflectance of the corolla, anther, pollen and stigma in each of five flowers from the wild population in Siming Mountain at wavelengths from 300 to 700 nm, with a JAZ-EL200 spectrometer (Ocean Optics Inc., Dunedin, FL, USA) and a QR400-7-SR fibre optic reflection probe held at 45° to the surface of the measured parts (Xiong et al., 2019; Supplementary Data Fig. S2). Colour conspicuousness is estimated by chromatic (colour) and achromatic (intensity) contrast, detectable to the signal receivers (Van der Kooi and Kelber, 2022). We mapped the reflectance spectrum of corolla, anther, pollen and stigma into honeybee colour space and chromatic contrast was calculated based on the Euclidean distances (Chittka, 1992) between the anther/pollen/stigma and the corolla (Ohashi et al., 2015; Xiong et al., 2019). Achromatic contrast was calculated from the absolute difference of background spectra (the green photoreceptor, 0.5) minus the l-value for the corolla/anther/pollen/stigma from the vismodel output (Van der Kooi and Kelber, 2022). We performed these using the vismodel function in the package ‘pavo’ (Maia et al., 2019) in R v.4.3.2 (R Core Team, 2018) with the reflectance spectrum of green foliage as background and the D65 illumination spectrum as ambient illumination.
To examine whether pollen is bee-preferred (Vaudo et al., 2020), we measured the total protein and lipid content. Fresh pollen grains were collected from more than five individuals and the nitrogen content was measured using elemental analysis. Pollen samples were put in tin capsules in the oven for combustion at 950 °C with pure oxygen as the combustion gas and helium as the carrier gas. Nitrogen was measured via a thermal conductivity detection system (López et al., 2010). To get total protein content in pollen grains, the nitrogen content was multiplied by 6.25, a nitrogen-to-protein conversion factor (López et al., 2010). To investigate contents of different fatty acids in pollen, high-performance gas chromatography (Varian 450-GC, USA) mass spectrometry (Varian 320-MS, USA) (GC-MS) with internal standards was performed using a modified method from Ecker et al. (2012). To test for fatty acids, by methyl esterification of pollen, 3 mL methanol and 10 μL concentrated sulphuric acid were added to a 5-mL flask with about 50 mg of pollen grains, and after rotary evaporation for 4 h at 80 °C, the volume was made up to 5 mL with methanol for extraction. The solution was centrifuged for 10 min at 1000 r.p.m. after standing for 24 h, and the supernatant was sampled for GC-MS analyses with a VF-5 MS chromatographic column, and 2 μL was injected for the calibration and samples. The testing time for each sample was 46 min, and the injection split ratio was 0. Helium was used as the carrier gas at a flow rate of 1 mL min−1. Injector temperature was held at 280 °C. Column temperature was programmed to remain at 40 °C for 3 min and then rise to 290 °C at 40 °C min−1 and hold for 5 min (Hao et al., 2023). Five types of fatty acids, including linolenic acid, linoleic acid, palmitic acid, stearic acid and paullinic acid, were detected. The concentrations of linoleic acid, palmitic acid and stearic acid were calculated from the standard curves, linolenic acid from the internal standard of linoleic acid, and paullinic acid from the internal standard of stearic acid. Five replications were tested for protein content and one sample for fatty acids due to limitations on the amount of pure pollen available.
To measure the content of rhodojaponin III in different tissues, leaves, petals and pollen were collected from 20 individuals separately and dried to a constant mass at 50 °C. To gain sufficient amounts for analysis, samples of pollen and nectar from multiple flowers on each plant were collected as a unit. All samples were stored separately in centrifuge tubes at −20 °C until analysis. The concentration was analysed using high-performance liquid chromatography (HPLC; Supplementary Data Fig. S3). Leaves, petals and pollen were crushed into powder using a tissue homogenizer (Tissuelyser, Qiagen) at 30 Hz for 10 min in 2-mL centrifuge tubes. About 50 mg of leaves and petals, and 20 mg of pollen were weighed with an electronic balance (Sartorius BAS124S) and transferred to a 2-mL centrifuge tube with 15 % acetonitrile (Wang et al., 2019). The volume of acetonitrile for each sample was recorded. Samples of nectar (20 µL), each collected from one individual (after flowers had been bagged for 24 h) with microcapillaries, were used for testing. To extract as much rhodojaponin III as possible, the solution was extracted ultrasonically for 30 min (40 kHz, 100 W) and allowed to stand for 24 h before injection. Each 20-μL sample extract was then injected into an Acquity HPLC BEH C18 column (4.6 × 250 mm, 3 μm) (Waters, Milford, MA, US). The mobile phase was 15 % acetonitrile, the flow rate 1.0 mL min−1, the determination wavelength 191 nm and the detection signal was DAD (Diode Array Detector). All system operations and data analyses were conducted in the Center of Analysis and Test of Wuhan University, Wuhan, China.
Pollinator observations
To investigate potential pollinators of R. molle, we undertook a total of 155 30-min censuses (77.5 h of observations) in the five populations with at least ten censuses between 0900 and 1800 h in each population (Supplementary Data Table S1). After each census, open flowers in each observation area were counted, and visiting frequency was calculated as the number of visits per flower per hour (Table S4). To evaluate which floral visitors are effective in pollination, we recorded the sites of pollen placement on the body or legs and stigma contact on the body when the insect was foraging on a flower and which reward it was foraging for.
Pollination efficiency of two major insect visitors
To compare pollination efficiency between butterflies and bumblebees, we measured pollen removal and deposition by a single visit. For pollen removal, virgin (previously unvisited) inflorescences were bagged in bud until flowers on these plants opened, and each flower was allowed a single visit by one of the two major floral visitors. In total, we collected 26 butterfly-visited and 22 bumblebee-visited flowers, as well as 46 bagged flowers nearby that were used to estimate pollen production per flower. Then, pollen tetrads (each of four grains) within the anthers of these flowers were counted under a microscope. Pollen removal was calculated as pollen grains of the virgin flower minus those grains that remained in the once-visited flower. Given that visitation frequency of butterflies was low in the field, artificial simulation of floral visitation was performed with four captured butterfly individuals (Johnson et al., 2006). After each contact with one virgin flower, we gently cleaned off the PTT from the butterfly’s wings. The four butterflies used for examining pollen removal and deposition were released after each had contacted several (5–7) flowers. To estimate pollen deposition per visit, the stigma of a once-visited flower was removed and stored in a centrifuge tube with 75 % alcohol. Stigmas were softened with 8 mol L–1 NaOH before we counted deposited pollen grains under a light microscope. To estimate the amounts of pollen carried by the butterfly wings, pieces of the PTT attached on one wing were counted from the photos of seven butterfly individuals when they were collecting nectar in R. molle. To estimate pollination success, at least 38 late-phase open-pollinated flowers from at least 12 plants in each of five populations were randomly sampled. Stigmas were softened in 8 mol L–1 NaOH for 10 h in the lab, and each stigma was squashed on a slide with a cover slip, and pollen tetrads per stigma were counted to calculate stigmatic pollen loads per flower (Song et al., 2019).
Pollination treatments
To investigate whether R. molle is self-compatible and capable of autogamy and whether seed production is pollen-limited, we compared fruit and seed set in open-pollinated, bagged, cross- and self-pollinated flowers in 15 individuals (Huang et al., 2017). Outcross- and self-pollinated flowers were all emasculated carefully in flower buds and then bagged with nylon nets and bagged again after hand pollination. Open-pollinated flowers as control were randomly labelled and flowers with bagged treatments were bagged until fruits developed in August 2018. Each plant experienced all four treatments (details in Supplementary Data Table S7).
Data analysis
To compare differences in rhodojaponin III content in four plant tissues, we used a generalized linear model (GLM) with normal distribution and identity-link function. To compare intraspecific variation in pollen nutrient and rhodojaponin III content, we calculated the coefficient of variation (CV) within each tissue respectively. To compare visitation frequency of three types of insects among and within populations, we used a generalized linear mixed model (GLMM) with a normal distribution and identity-link function, with visitation frequency as the dependent variable, visitor type as a fixed factor and population as a random factor. The difference between pollen removal and deposition by butterflies and bumblebees was compared by a GLM with Poisson distribution and log-link function, with pollen grain number as the dependent variable, and butterflies and bumblebees as factors. Pollen loads on the stigmas of open-pollinated flowers from five investigated populations were compared by GLMs with Poisson distribution and log-link function, and the relationship with visitation frequency of different visitors was examined with Spearman’s rank correlation analysis. Fruit set and seed set among treatments were compared using a GLM with binomial distribution and logit-link function. The above analyses were performed in SPSS 27 (IBM Inc., New York, NY, USA).
RESULTS
Floral traits
The mean (±s.e.) distance from nectar to the stigma was 64.71 ± 1.12 mm (n = 30), to the longest stamen 53.72 ± 0.79 mm and to the shortest stamen 49.52 ± 0.82 mm. The volume of nectar secreted in 24 h was 1.36 ± 1.11 μL and sugar concentration (g sugar per 100 g solution) was 33.54 ± 1.03 %. Each anther had 3.0 ± 0.2 pieces of PTT, and one PTT was about 6.57 ± 0.17 mm long and contained 2232.8 ± 103.4 pollen grains (Supplementary Data Table S2).
Measurements of the reflectance spectrum showed that corolla reflectance peaked at wavelengths of 350–440 and 500–700 nm, the stigma at 500–670 nm, the anthers at 500–700 nm and pollen at 400–700 nm (Supplementary Data Fig. S2A). Bee receptor peaks are at 345, 440 and 575 nm (Briscoe and Chittka, 2001), indicating that pollen was visible to bees. Chromatic-contrast values between anther, pollen, stigma and corolla were 0.24 ± 0.05, 0.17 ± 0.03 and 0.22 ± 0.07 respectively (Fig. S2B), and achromatic-contrast values between corolla, anther, pollen, stigma and the background spectra (green photoreceptor) were 0.32 ± 0.01, 0.15 ± 0.03, 0.31 ± 0.01 and 0.19 ± 0.03 respectively (Fig. S2C), indicating that bees can perceive the pollen within flowers.
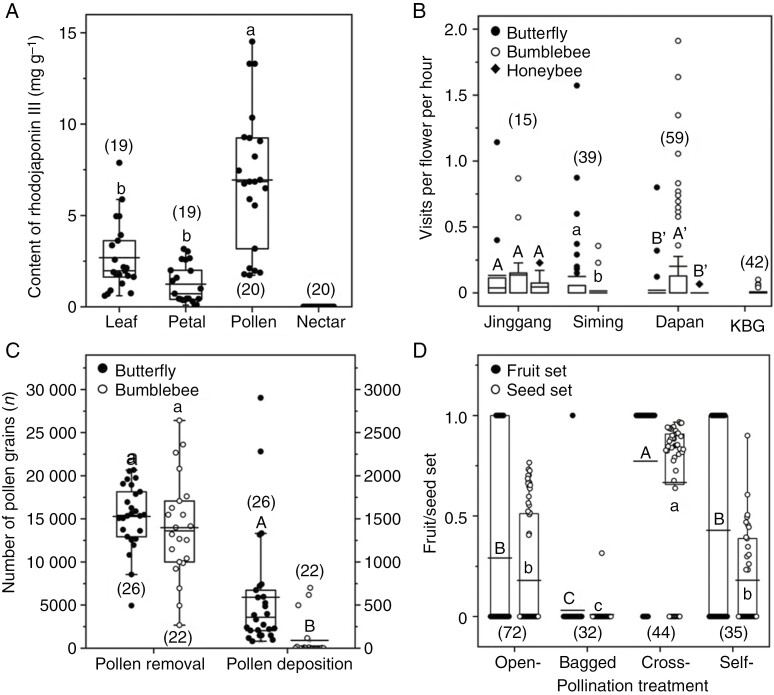
Total protein content in pollen was 356.58 ± 1.30 mg g−1 (mean ± s.e., n = 5) (35.7 %) and the CV was 0.8 % (Supplementary Data Table S3). Total lipid content was 77.70 mg g−1 (7.8 %), including five types of fatty acids: linolenic acid 28.13 mg g−1, linoleic acid 25.23 mg g−1, palmitic acid 20.90 mg g−1, stearic acid 1.55 mg g−1 and paullinic acid 1.89 mg g−1. The protein to lipid ratio (P : L) was 4.59. Measurements of the toxic compound rhodojaponin III in four tissues (Table S4) showed that it appeared in the leaves, petals and pollen, but not in the nectar (for chromatograms and standard curves see Fig. S3). The concentration of rhodojaponin III in pollen (6.63 ± 0.84 mg g−1) was significantly higher (Wald χ2 = 46.04, P < 0.001) than that in leaves (2.61 ± 0.44 mg g−1) and petals (1.33 ± 0.24 mg g−1) (Fig. 2A), and the CV was 56.9, 73.8 and 81.1 %, respectively.
Fig. 2.
Comparisons of concentrations of rhodojaponin III in four plant tissues (A), visitation frequency of floral visitors in four populations (B), pollen grains removed and deposited per flower per single visit by butterflies and bumblebees (C), and fruit set and seed set under four pollination treatments (D) in Rhododendron molle. Numbers of samples are given in parentheses. Different letters indicate significant differences at P < 0.01 based on generalized linear models. All panels display box plots based on raw data, indicating the median, mean value (horizontal line), interquartile range (hinges showing 25th and 75th percentiles), 1.5 times the interquartile range (whiskers) and outliers.
Visitor species and abundance
In five populations, three types of floral visitors were observed: butterflies (Byasa mencius and Papilio bianor), bumblebees (Bombus bicoloratus, B. flavescens and B. trifasciatus) and honeybees (Apis mellifera), except that no visitors were recorded in Lechang (Supplementary Data Table S5). All visitors foraged for nectar but not pollen, including bumblebees and honeybees in our 77.5 h of field observation. Ten bumblebees randomly collected had no corbiculae in the legs in the Dapan population, while two of eight bumblebees collected in the KBG population had corbiculae, only one with a few tetrads (20 grains of 4613 examined) and the other with no tetrads of R. molle but 3374 grains from other species. No corbiculae were observed in seven honeybees collected in the Jinggang population. In the five populations, results of GLMM showed that visitation frequency did not differ (P = 0.06) between butterflies and bumblebees, but both were significantly higher (P = 0.04 and P < 0.001, respectively) than honeybees, indicating that butterflies and bumblebees were major visitors. In Jinggang, visitation frequency did not differ (Wald χ2 = 1.49, d.f. = 2, P = 0.475) among butterflies (0.13 ± 0.08), bumblebees (0.14 ± 0.07) and honeybees (0.05 ± 0.02) (Fig. 2B). In Siming, the number of butterfly visits (0.13 ± 0.05) was significantly higher (Wald χ2 = 4.73, P = 0.030) than bumblebees (0.02 ± 0.01), and no honeybees were seen (Fig. 2B). In Dapan, bumblebees (0.20 ± 0.06) were relatively more frequent (Wald χ2 = 9.96 and 13.11, both P < 0.01) than butterflies (0.02 ± 0.01) and honeybees (0.001 ± 0.001), and the latter two did not differ (Wald χ2 = 1.85, P = 0.174) (Fig. 2B). In KBG, only the bumblebee B. trifasciatus acted as a potential pollinator (0.008 ± 0.003), and another bumblebee species (B. breviceps queen) served as the primary nectar robber (nectar robbing visits per flower per hour = 0.04 ± 0.01) (Fig. 1C) and honeybees (A. mellifera) were secondary robbers (0.05 ± 0.01). Robbing visits (Table S6) were excluded from the above visitation frequencies.
Pollination effectiveness of two major visitors
The number of pollen grains removed per visit did not differ significantly (Wald χ2 = 0.38, P = 0.538) between butterflies (15 275.2 ± 724.5) and bumblebees (13 969.8 ± 1265.4), whereas the number of grains deposited per visit by butterflies (591.4 ± 133.6, about one-quarter of the number of pollen grains contained in each PTT) was six times higher (Wald χ2 = 20.52, P < 0.001) than the number deposited by bumblebees (89.5 ± 45.4) (Fig. 2C; Supplementary Data Table S7), indicating that butterflies had higher pollen-transfer efficiency than bumblebees. Each wing of the seven butterfly individuals carried 11.1 ± 1.2 PTT, i.e. on average about 24 800 pollen grains, indicating that a single visit by butterflies theoretically can deposit more pollen than in natural stigmas. Stigmatic pollen loads of open-pollinated flowers did not differ (Wald χ2 = 0.40, P = 0.528) between Jinggang (2375.8 ± 165.4, n = 106) and Siming (2169.0 ± 295.4, n = 38), but both were significantly higher (Wald χ2 = 46.33 and 16.57, both P < 0.001) than in Dapan Mountain (1187.4 ± 70.9, n = 166), and four or six times higher (Wald χ2 = 83.20 and 29.94, both P < 0.001) than in Lechang (520.0 ± 110.6, n = 76) and six or three times higher (Wald χ2 = 117.73 and 47.56, both P < 0.001) than in KBG (379.9 ± 76.2, n = 67) (Table S8). Stigmatic pollen loads of open-pollinated flowers increased with the visitation frequency of butterflies (r = 0.975, P = 0.005), but not either of bumblebees (r = 0.700, P = 0.188) or honeybees (r = 0.671, P = 0.215), indicating that only the butterfly visits effectively contribute to pollen deposition in R. molle.
Pollination treatments
Both fruit set (0.43 ± 0.08) and seed set (0.18 ± 0.04) under self-pollination were significantly higher (Wald χ2 = 19.87 and 22.92 for fruit and seed set respectively, both P < 0.001) than in bagged flowers (0.03 ± 0.03, 0.01 ± 0.01) but lower (Wald χ2 = 10.78 and 99.91, both P < 0.001) than those from cross-pollination (0.77 ± 0.06, 0.67 ± 0.06), indicating that the species was self-compatible, but autonomous pollination contributed little to sexual reproduction (Fig. 2D; Supplementary Data Table S9). Fruit and seed set were significantly higher (Wald χ2 = 33.73 and 72.45, both P < 0.001) under cross-pollination than under open pollination (0.29 ± 0.05, 0.18 ± 0.03), suggesting that female reproductive success is likely to be limited by pollinator visits in wild populations (Fig. 2D; Table S9).
DISCUSSION
Our investigations of visitation frequency in five populations and measurements of pollen transfer efficiency by two major visitors indicated that butterflies were effective pollinators in R. molle, in which PTTs were freely accessible to pollen feeders. Although pollen of R. molle was protein-rich and expected to be nutritionally preferred by bees, high concentrations of the toxic compound rhodojaponin III appeared in pollen but not in nectar. This Rhododendron flower may offer toxin-free nectar to the butterfly pollinator and produce toxic pollen grains deterring consumption by pollen feeders, supporting the proposed hypothesis of chemical defence of open-access pollen.
Our estimation of pollen transfer efficiency indicated that butterflies were more efficient than bumblebees, given that they deposited more pollen grains per visit, consistent with the morphological fit between floral organs and pollinators. Pollen deposition by bumblebees was low, probably because their bodies rarely contacted the stigma when they were feeding on nectar at the corolla base because of the size mismatch of the pollinator and the floral sexual organ (Fig. 1D). For instance, the length of the largest bumblebee (from the tongue to abdomen tip <5 cm measured from the insect specimen) was much less than the distance from the nectar to the stigma (>6 cm). In five populations, visitation frequency of butterflies was significantly positively correlated with pollen grains deposited on the open-pollinated stigmas, suggesting that butterflies were more effective pollinators in R. molle. Clumped pollen grains adhering to the hairs of the butterfly wing (Fig. 1E) by viscin threads (Fig. 1F) were seen contacting the stigma when butterflies were feeding on nectar, as in Rhododendron calendulaceum, a butterfly-wing-pollinated shrub in North America (Epps et al., 2015). In Caesalpinia pulcherrima (Fabaceae), exposed pollen grains connected by threads were also effectively transferred by butterfly wings (Cruden and Hermann-Parker, 1979).
Hand pollination treatments showed that fruit and seed set in R. molle were limited by a scarcity of effective pollinators (Fig. 2D). Pollinator activity was unpredictable and variable in spring among five populations (Fig. 2B). It has been proposed that simultaneous pollen presentation should be favoured when pollinators are infrequent (Castellanos et al., 2006). The number of pollen grains per PTT (2233 in R. molle) falls within the range found in Lepidoptera-pollinated Rhododendron species (1678–7873 grains per PTT, see Song et al., 2019). This high degree of pollen aggregation could compensate for the low pollinator visitation frequency in that pollinators could remove more pollen grains in a single visit when pollen is aggregated. There is a risk of pollen loss in flowers with simultaneous pollen presentation that is freely accessible to pollen feeders. The importance of butterfly pollination here supports our prediction that aggregation of exposed pollen grains will be favoured when pollinators do not collect pollen as a reward.
Bees primarily obtain protein and lipid from pollen (Vaudo et al., 2016a). Recent surveys of pollen nutrients (proteins and lipids) have shown that bumblebees prefer to forage for pollen grains with high protein and/or low lipid content (Ruedenauer et al., 2020), especially favouring P : L ratios ranging from 5 : 1 to 10 : 1 (Vaudo et al., 2016a, 2020). Generalist bumblebees avoided collecting cucurbit pollen grains with a relatively low protein content (140.73 mg g−1), but relatively high lipid content (96.78 mg g−1), resulting in a low P : L ratio of 1.45 (Treanore et al., 2019; Brochu et al., 2020). Hao et al. (2023) found that pollen grains of Geranium delavayi (Geraniaceae), which were not collected by its effective pollinator bumblebees, also contained a low protein content (227.4 mg g−1), but high lipid content (138.8 mg g−1), a low P : L ratio (1.64), far lower than the P : L ratio preferred by bees. Our measurement showed that pollen grains of R. molle had a relatively high protein content (356.58 mg g−1) but low lipid content (77.75 mg g−1), and a high P : L ratio (4.6), close to the range that bumblebees prefer, suggesting that bumblebee rejection to collect its pollen was unlikely to be due to the nutritional component. Considering that most proteins in pollen are enzymes that function during pollen–stigma recognition, pollen tube growth and/or subsequent fertilization (Roulston et al., 2000), pollen protein content may be a consequence of selection from both pollen–pistil and plant–animal interactions to favour pollen fertility and reduce consumption.
A comparative survey of 31 species indicated that pollen had more diverse and higher concentrations of secondary metabolites than nectar. It included one Rhododendron species (R. prinophyllum), which showed a high concentration of terpenes (about 6 mg g−1) in the pollen (Palmer-Young et al., 2019). Pollen of another three Rhododendron species also contained terpenoids and other secondary metabolites including alkaloids, flavonoids and phenylpropanoids (Yao et al., 2023). Our results indicated that the concentration of the main secondary metabolite rhodojaponin III, which has been thought to deter herbivores (Popescu and Kopp, 2013), was highest in pollen but lowest in nectar and moderate in the petals and leaves in R. molle. We observed that the variation (CV) in rhodojaponin III concentration was lower in pollen than in leaves and petals, suggesting that chemical defence in pollen might have experienced more stabilizing selection from exploitation. The higher accumulation of toxin in pollen than in petals may function as chemical defence against pollen consumers rather than simply being a pleiotropic effect (Trunz et al., 2020). Our comparisons of different tissues including leaves, petals, pollen and nectar in over 20 Rhododendron species indicated that pollen (but not nectar) generally contained considerable amounts of secondary metabolites toxic to herbivores (Xiao-Wen Lv and Shuang-Quan Huang, unpubl. data), suggesting that species in which pollen is without physical protection and freely accessible in a mass presentation may have evolved chemical protection from pollen collection.
Pollen defence could reduce pollen consumption by non-specialists or less effective pollinators, while pollen-specialist bee pollinators could tolerate the toxicity of host pollen (Rivest and Forrest, 2020). For example, Bombus consobrinus as the only effective pollinator in the pollen-specialist species Aconitum septentrionale collected the pollen even with defensive alkaloids (Thøstesen and Olesen, 1996; Gosselin et al., 2013). The specialized pollinator Andrena astragali collected alkaloid-containing pollen from the host species Toxicoscordion paniculatum (Melianthiaceae) too (Cane, 2018). Our results showed that generalist bumblebees (B. bicoloratus, B. flavescens and B. trifasciatus) and honeybees (Apis mellifera) visited R. molle flowers mainly for nectar but not pollen, as evident in that its pollen tetrads were almost not collected into the bee’s corbiculae, suggesting that the high concentration of the secondary metabolite rhodojaponin III in pollen may deter ineffective pollinators from collecting pollen. Secondary metabolites in pollen can influence bumblebee behaviour in ways that reduce pollen collection for their larvae (Muth et al., 2016; Jacquemart et al., 2019) and pollen with more toxic saponins was less groomed into corbiculae and more pollen remained on the bumblebee bodies, which could maximize pollen transfer in two Dipsacus species (Wang et al., 2019). In flower arrays with different reward qualities in pollen and nectar, B. impatiens preferred flowers with nectar of higher sugar concentration even when their pollen had higher alkaloid content, suggesting that co-flowering species may evolve different reward qualities to filter pollinators (Francis et al., 2019).
In R. molle, bumblebees and honeybees collected toxin-free nectar (34 % sugar concentration) but not pollen with toxin. We observed that the bumblebee queen B. breviceps (but not B. trifasciatus) in the transplanted population KBG pierced the corolla to rob nectar (Fig. 1C) and honeybees followed, functioning as secondary nectar robbers, but no nectar-robbing from outside the corollas occurred in other wild populations. The tongue length of B. breviceps (about 10 mm) was similar to the other three bumblebee species (about 9 mm) and enabled them to access nectar at the bottom of the corolla from the normal entrance, but the body length of B. breviceps (about 22 mm) was larger than that of the other three bumblebee species (about 14 mm) (Fig. 1C and D). Therefore, B. breviceps should be more likely to contact anthers and PTTs passively placed on their bodies if they collect nectar as usual from the entrance of the corolla opening. We deduce that large bumblebees did not take an easy entrance to access nectar but pierce the corolla from outside, allowing the bees to avoid attachment of the unwanted toxic pollen grains. Given that bumblebees are able to discriminate and selectively forage based on pollen quality (Vanderplanck et al., 2014; Ruedenauer et al., 2015; Vaudo et al., 2020), the pollen toxicity could be an anti-theft strategy to protect pollen from being collected (Hargreaves et al., 2009; Wang et al., 2019).
CONCLUSION
Consistent with the hypothesis of toxic pollen deterring ineffective pollinators and/or pollen thieves in which pollen grains are freely accessible, our investigations on butterfly-pollinated R. molle showed that the concentration of the toxic compound rhodojaponin III was highest in pollen but not in nectar. Further studies are needed to examine whether other species with open access pollen have evolved toxicity to discourage pollen exploitation by ineffective pollinators while toxin-free nectar rewards effective pollinators to maximize pollen transfer.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: Locations of five populations of Rhododendron molle for the 2-year study. Figure S2: Reflectance spectrum of four floral parts in Rhododendron molle: corolla, anther, pollen and stigma (A), and chromatic contrast between anther, pollen and stigma against the background corolla viewed by Hymenoptera (B) and achromatic contrast of corolla, anther, pollen and stigma against the background green photoreceptor (C). Figure S3: Standard curves with concentration gradients for rhodojaponin III and chromatograms in the four tissues under HPLC analyses. Table S1: Locations and sizes of five populations of Rhododendron molle for a 2-year study. Table S2: Raw data of eight measured floral traits of 30 flowers of Rhododendron molle. Table S3: Total protein and lipid content (mg g−1) in pollen of Rhododendron molle. Table S4: Rhodojaponin III contents in four tissues of 20 individual plants of Rhododendron molle. Table S5: Records of floral visits to Rhododondron molle in five populations. Table S6: Records of nectar robbed by two types of bee in the transplanted population at Kunming Botanical Garden, Yunnan Province, Southwest China. Table S7: Number of pollen grains removed and deposited on the stigma by a single visit of the two major insect visitors to Rhododendron molle. Table S8: Stigmatic pollen loads of open-pollinated flowers in late phase in five investigated populations. Table S9: Fruit set, number of undeveloped ovules and developed ovules, and seed set per flower under four pollination treatments in Rhododendron molle.
ACKNOWLEDGMENTS
We thank Fei-Fei Chen, Wei Guo and local staff in the conservation areas for their help in the field, Dr Jia-Xing Huang from the Institute of Apicultural Research, CAAS, for the identification of bumblebees, Dr Ying-Ze Xiong for advice of data analysis, Jian-She He from the Detection Center of Wuhan University for advice on the analysis of chemical components, Sarah Corbet (University of Cambridge) and anonymous referees for helpful suggestions on early versions of the manuscript.
Contributor Information
Hui-Hui Feng, Institute of Evolution and Ecology, School of Life Sciences, Central China Normal University, Wuhan 430079, China; Department of Ecology, College of Life Sciences, Wuhan University, Wuhan 430072, China.
Xiao-Wen Lv, Institute of Evolution and Ecology, School of Life Sciences, Central China Normal University, Wuhan 430079, China.
Xiao-Chen Yang, College of Biology and Environmental Sciences, Jishou University, Jishou 416000, Hunan Province, China.
Shuang-Quan Huang, Institute of Evolution and Ecology, School of Life Sciences, Central China Normal University, Wuhan 430079, China.
ETHICS
There are no legal requirements for ethical approval for studies involving non-endangered plants and invertebrates in China.
DATA ACCESSIBILITY
All raw data associated with this project are available in the Supplementary Data including three figures (Figs S1–S3) and eight tables (Tables S1–S9).
AUTHOR CONTRIBUTIONS
S.-Q.H. designed the study. H.H.F., X.W.L and X.C.Y. performed the study and collected data, H.H.F. analysed the data, and H.H.F. and S.-Q.H. drafted the manuscript. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
COMPETING INTERESTS
We have no competing interests.
FUNDING
This work was supported by National Natural Science Foundation of China (grant nos. 31730012, 32030071).
LITERATURE CITED
- Adler LS. 2000. The ecological significance of toxic nectar. Oikos 91: 409–420. [Google Scholar]
- Arnold SE, Peralta Idrovo ME, Lomas Arias LJ, Belmain SR, Stevenson PC.. 2014. Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. Journal of Chemical Ecology 40: 878–881. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L.. 2001. The evolution of color vision in insects. Annual Review of Entomology 46: 471–510. [DOI] [PubMed] [Google Scholar]
- Brochu KK, Dyke M, Milano NJ, Petersen JD, Danforth BN.. 2020. Pollen defenses negatively impact foraging and fitness in a generalist bee (Bombus impatiens: Apidae). Scientific Reports 10: 3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Hu JH, Qin J, Sun T, Li XL.. 2018. Rhododendron molle (Ericaceae): phytochemistry, pharmacology, and toxicology. Chinese Journal of Natural Medicines 16: 401–410. [DOI] [PubMed] [Google Scholar]
- Cane JH. 2018. Co-dependency between a specialist Andrena bee and its death camas host, Toxicoscordion paniculatum. Arthropod-Plant Interactions 12: 657–662. [Google Scholar]
- Castellanos MC, Wilson P, Keller SJ, Wolfe AD, Thomson JD.. 2006. Anther evolution: pollen presentation strategies when pollinators differ. The American Naturalist 167: 288–296. [DOI] [PubMed] [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170: 533–543. [Google Scholar]
- Cruden RW, Hermann-Parker SM.. 1979. Butterfly pollination of Caesalpinia pulcherrima, with observations on a psychophilous syndrome. The Journal of Ecology 67: 155–168. [Google Scholar]
- Ecker J, Scherer M, Schmitz G, Liebisch G.. 2012. A rapid GC-MS method for quantification of positional and geometric isomers of fatty acid methyl esters. Journal of Chromatography. B. Analytical Technologies in the Biomedical and Life Sciences 897: 98–104. [DOI] [PubMed] [Google Scholar]
- Epps MJ, Allison SE, Wolfe LM.. 2015. Reproduction in flame azalea (Rhododendron calendulaceum, Ericaceae): a rare case of insect wing pollination. The American Naturalist 186: 294–301. [DOI] [PubMed] [Google Scholar]
- Fang MY, Fang RZ, Fang RC, et al. 2005. Rhododendron. In: Wu ZY, Raven PH, Hong DY. eds. Flora of China, Vol. 14. St. Louis ands Beijing: Science Press & Missouri Botanical Garden Press, 260–455. [Google Scholar]
- Fattorini R, Egan PA, Rosindell J, Farrell IW, Stevenson PC.. 2023. Grayanotoxin I variation across tissues and species of Rhododendron suggests pollinator–herbivore defence trade-offs. Phytochemistry 212: 113707. [DOI] [PubMed] [Google Scholar]
- Francis JS, Acevedo CR, Muth F, Leonard AS.. 2019. Nectar quality changes the ecological costs of chemically defended pollen. Current Biology: CB 29: R679–R680. [DOI] [PubMed] [Google Scholar]
- Garance DP, Marion S, Yves LC, et al. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One 8: e72016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Génissel A, Aupinel P, Bressac C, Tasei JN, Chevrier C.. 2002. Influence of pollen origin on performance of Bombus terrestris microcolonies. Entomologia Experimentalis et Applicata 104: 329–336. [Google Scholar]
- Gosselin M, Michez D, Vanderplanck M, Roelants D, Glauser G, Rasmont P.. 2013. Does Aconitum septentrionale chemically protect floral rewards to the advantage of specialist bumblebees? Ecological Entomology 38: 400–407. [Google Scholar]
- Hanley ME, Franco M, Pichon S, Darvill B, Goulson D.. 2008. Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Functional Ecology 22: 592–598. [Google Scholar]
- Hao K, Xu Q, Huang SQ.. 2023. Pollen-feeding behavior of diverse insects on Geranium delavayi, a flower with large, accessible pollen grains. American Journal of Botany 110: e16113. [DOI] [PubMed] [Google Scholar]
- Hargreaves AL, Harder LD, Johnson SD.. 2009. Consumptive emasculation: The ecological and evolutionary consequences of pollen theft. Biological Reviews of the Cambridge Philosophical Society 84: 259–276. [DOI] [PubMed] [Google Scholar]
- Hesse M. 1984. An exine architecture model for viscin threads. Grana 23: 69–75. [Google Scholar]
- Hu MY, Klocke JA, Chiu SF.. 1993. Response of five insects to botanical insecticides, Rhodojaponin-III. Journal of Economic Entomology 86: 706–711. [Google Scholar]
- Huang ZH, Song YP, Huang SQ.. 2017. Evidence for passerine bird pollination in Rhododendron species. AoB Plants 9: plx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemart AL, Buyens C, Hérent MF, et al. 2019. Male flowers of Aconitum compensate for toxic pollen with increased floral signals and rewards for pollinators. Scientific Reports 9: 16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M.. 2006. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 87: 2709–2716. [DOI] [PubMed] [Google Scholar]
- Klocke JA, Hu M, Choo S, Kubo I.. 1991. Grayanoid diterpene insect antifeedants and insecticides from Rhododendron molle. Phytochemistry 30: 1797–1800. [Google Scholar]
- López CVG, García MDCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF.. 2010. Protein measurements of microalgal and cyanobacterial biomass. Bioresource Technology 101: 7587–7591. [DOI] [PubMed] [Google Scholar]
- Maia R, Gruson H, Endler JA, White TE.. 2019. pavo2: New tools for the spectral and spatial analysis of colour in R. Methods in Ecology and Evolution 10: 1097–1107. [Google Scholar]
- Manning R, Rutkay A, Eaton L, Dell B.. 2007. Lipid-enhanced pollen and lipid reduced flour diets and their effect on the longevity of honey bees (Apis mellifera L.). Australian Journal of Entomology 46: 251–257. [Google Scholar]
- Muth F, Francis JS, Leonard AS.. 2016. Bees use the taste of pollen to determine which flowers to visit. Biology Letters 12: 20160356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Makino TT, Arikawa K.. 2015. Floral colour change in the eyes of pollinators: testing possible constraints and correlated evolution. Functional Ecology 29: 1144–1155. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S.. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Palmer-Young EC, Farrell IW, Adler LS, et al. 2019. Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecology Monographs 89: e01335. [DOI] [PubMed] [Google Scholar]
- Popescu R, Kopp B.. 2013. The genus Rhododendron: An ethnopharmacological and toxicological review. Journal of Ethnopharmacology 147: 42–62. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org (20 April 2019, date last accessed). [Google Scholar]
- Rivest S, Forrest JRK.. 2020. Defence compounds in pollen: why do they occur and how do they affect the ecology and evolution of bees? The New Phytologist 225: 1053–1064. [DOI] [PubMed] [Google Scholar]
- Roulston TAH, Cane JH, Buchmann SL.. 2000. What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecological Monographs 70: 617–643. [Google Scholar]
- Ruedenauer FA, Spaethe J, Leonhardt SD.. 2015. How to know which food is good for you: bumblebees use taste to discriminate between different concentrations of food differing in nutrient content. The Journal of Experimental Biology 218: 2233–2240. [DOI] [PubMed] [Google Scholar]
- Ruedenauer FA, Raubenheimer D, Beierlein DK, et al. 2020. Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecology Letters 23: 545–554. [DOI] [PubMed] [Google Scholar]
- Russo L, Vaudo AD, Fisher CJ, Grozinger CM, Shea K.. 2019. Bee community preference for an invasive thistle associated with higher pollen protein content. Oecologia 190: 901–912. [DOI] [PubMed] [Google Scholar]
- Song YP, Huang ZH, Huang SQ.. 2019. Pollen aggregation by viscin threads in Rhododendron varies with pollinator. The New Phytologist 221: 1150–1159. [DOI] [PubMed] [Google Scholar]
- Stevenson PC. 2020. For antagonists and mutualists: the paradox of insect toxic secondary metabolites in nectar and pollen. Phytochemistry Reviews 19: 603–614. [Google Scholar]
- Sütlüpmar N, Mat A, Satganoglu Y.. 1993. Poisoning by toxic honey in Turkey. Archives of Toxicology 67: 148–150. [DOI] [PubMed] [Google Scholar]
- Thøstesen AM, Olesen JM.. 1996. Pollen removal and deposition by specialist and generalist bumblebees in Aconitum septentrionale. Oikos 77: 77–84. [Google Scholar]
- Tiedeken EJ, Egan PA, Stevenson PC, et al. 2016. Nectar chemistry modulates the impact of an invasive plant on native pollinators. Functional Ecology 30: 885–893. [Google Scholar]
- Tong ZY, Wu LY, Feng HH, et al. 2023. New calculations indicate that 90% of flowering plant species are animal-pollinated. National Science Review 10: nwad219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanore ED, Vaudo AD, Grozinger CM, Fleischer SJ.. 2019. Examining the nutritional value and effects of different floral resources in pumpkin agroecosystems on Bombus impatiens worker physiology. Apidologie 50: 542–552. [Google Scholar]
- Trunz V, Lucchetti MA, Bénon D, et al. 2020. To bee or not to bee: the ‘raison d’être’ of toxic secondary compounds in the pollen of Boraginaceae. Functional Ecology 34: 1345–1357. [Google Scholar]
- van der Kooi CJ, Kelber A.. 2022. Achromatic cues are important for flower visibility to hawkmoths and other insects. Frontiers in Ecology and Evolution 10: 819436. [Google Scholar]
- van der Kooi CJ, Vallejo-Marín M, Leonhardt SD.. 2021. Mutualisms and (a)symmetry in plant–pollinator interactions. Current Biology 31: R91–R99. [DOI] [PubMed] [Google Scholar]
- Vanderplanck M, Moerman R, Rasmont P, et al. 2014. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS One 9: e86209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo AD, Patch HM, Mortensen DA, Tooker JF, Grozinger CM.. 2016a. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proceedings of the National Academy of Sciences of the United States of America 113: E4035–E4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo AD, Stabler D, Patch HM, Tooker JF, Grozinger CM, Wright GA.. 2016b. Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. The Journal of Experimental Biology 219: 3962–3970. [DOI] [PubMed] [Google Scholar]
- Vaudo AD, Tooker JF, Patch HM, et al. 2020. Pollen protein: lipid macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 11: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Tang J, Wu T, Wu D, Huang SQ.. 2019. Bumblebees rejection of toxic pollen facilitates pollen transfer. Current Biology 29: 1401–1406.e4. [DOI] [PubMed] [Google Scholar]
- Xiong YZ, Jia LB, Zhang C, Huang SQ.. 2019. Color-matching between pollen and corolla: Hiding pollen via visual crypsis? The New Phytologist 224: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Yao RX, Chen Y, Lv XQ, Wang JH, Yang FJ, Wang XY.. 2023. Altitude-related environmental factors shape the phenotypic characteristics and chemical profile of Rhododendron. Biodiversity Science 31: 22259. (In Chinese with English Abstract.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data associated with this project are available in the Supplementary Data including three figures (Figs S1–S3) and eight tables (Tables S1–S9).