Abstract
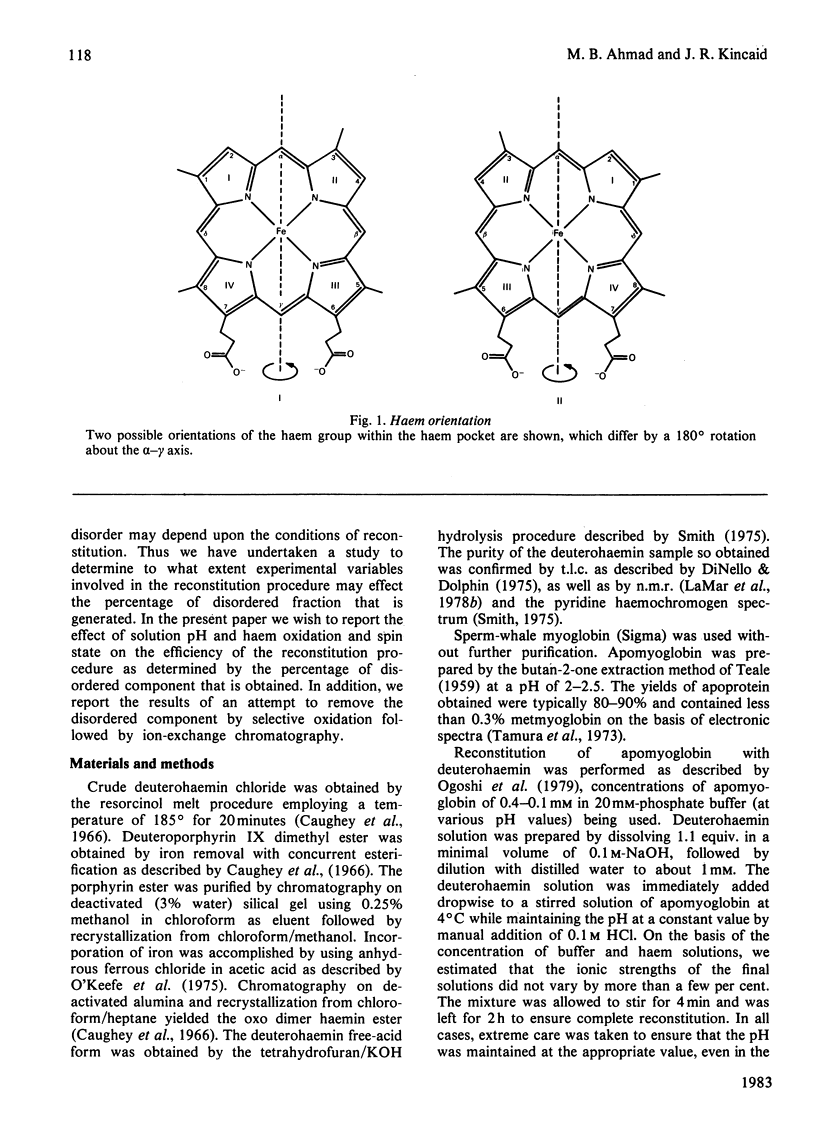
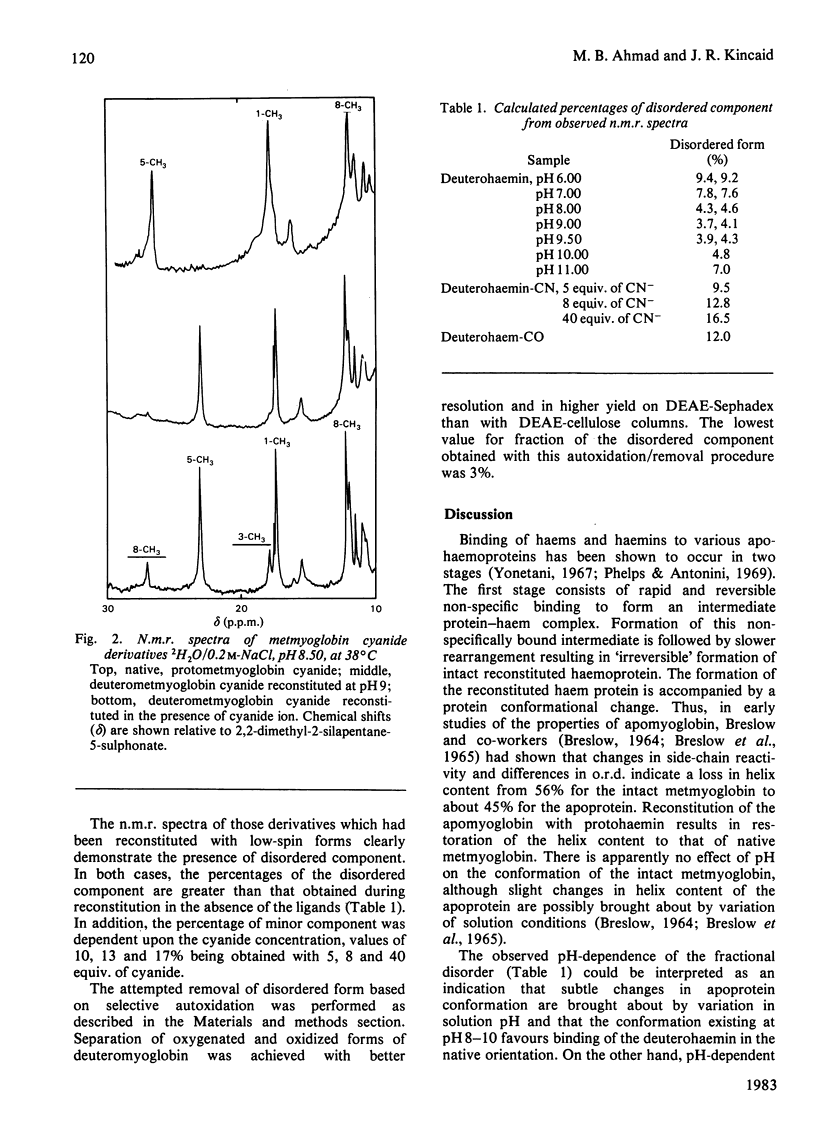
Apomyoglobin was reconstituted with deuterohaem derivatives under various conditions. The fraction of disordered component, which is characterized by a 180 degree rotation of the haem group, for the various preparations was determined by n.m.r. spectroscopy. By using the procedures described, it was shown that the fraction of disordered component is minimized if the reconstitution is carried out with high-spin ferric haem derivatives within an experimentally determined optimum pH range of 8-9.5. Use of low-spin derivatives in either the ferrous or ferric forms leads to substantial increases in the fraction of disordered form. Attempted removal of the disordered form by selective oxidation and chromatographic purification was not effective.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW E., BEYCHOK S., HARDMAN K. D., GURD F. R. RELATIVE CONFORMATIONS OF SPERM WHALE METMYOGLOBIN AND APOMYOGLOBIN IN SOLUTION. J Biol Chem. 1965 Jan;240:304–309. [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Brown S. B., Hatzikonstantinou H. The dimerization of ferrihaems. II. Equilibrium and kinetic studies of mesoferrihaem dimerization. Biochim Biophys Acta. 1978 Mar 20;539(3):352–363. doi: 10.1016/0304-4165(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Hatzikonstantinou H. The dimerization of ferrihaems. III. Equilibrium and kinetic studies on the dimerization of coproferrihaem. Biochim Biophys Acta. 1978 Dec 1;544(2):407–417. doi: 10.1016/0304-4165(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Hatzikonstantinou H. The dimerization of ferrihaems. IV. Studies on haematoferrihaem and a general appraisal of the nature and implications of dimerization. Biochim Biophys Acta. 1979 Jun 1;585(1):143–153. doi: 10.1016/0304-4165(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobin molecules. Proc Natl Acad Sci U S A. 1966 Sep;56(3):974–978. doi: 10.1073/pnas.56.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Caughey W. S., Alben J. O., Fujimoto W. Y., York J. L. Substituted deuteroporphyrins. I. Reactions at the periphery of the porphyrin ring. J Org Chem. 1966 Aug;31(8):2631–2640. doi: 10.1021/jo01346a042. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Barlow C. H., Maxwell J. C., Volpe J. A., Wallace W. J. Reactions of oxygen with hemoglobin, cytochrome c oxidase and other hemeproteins. Ann N Y Acad Sci. 1975 Apr 15;244:1–9. doi: 10.1111/j.1749-6632.1975.tb41517.x. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Foulds J., McClain W. H. Transfer ribonucleic acid nucleotidyl-transferase plays an essential role in the normal growth of Escherichia coli and in the biosynthesis of some bacteriophage T4 transfer ribonucleic acids. J Biol Chem. 1974 Oct 25;249(20):6696–6699. [PubMed] [Google Scholar]
- DiNello R. K., Dolphin D. H. Analytical chromatography of hemins on silica gel. Anal Biochem. 1975 Apr;64(2):444–449. doi: 10.1016/0003-2697(75)90452-2. [DOI] [PubMed] [Google Scholar]
- Docherty J. C., Brown S. B. Haem disorder in reconstituted human haemoglobin. Biochem J. 1982 Dec 1;207(3):583–587. doi: 10.1042/bj2070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman K. D., Eylar E. H., Ray D. K., Banaszak L. J., Gurd F. R. Isolation of sperm whale myoglobin by low temperature fractionation with ethanol and metallic ions. J Biol Chem. 1966 Jan 25;241(2):432–442. [PubMed] [Google Scholar]
- Ikeda-Saito M., Iizuka T., Yamamoto H., Kayne F. J., Yonetani T. Studies on cobalt myoglobins and hemoglobins. Interaction of sperm whale myoglobin and Glycera hemoglobin with molecular oxygen. J Biol Chem. 1977 Jul 25;252(14):4882–4887. [PubMed] [Google Scholar]
- Keller R., Groudinsky O., Wüthrich K. Contact-shifted resonances in the 1H NMR spectra of cytochrome b5. Resonance identification and spin density distribution in the heme group. Biochim Biophys Acta. 1976 Apr 14;427(2):497–511. doi: 10.1016/0005-2795(76)90192-6. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Budd D. L., Viscio D. B., Smith K. M., Langry K. C. Proton nuclear magnetic resonance characterization of heme disorder in hemoproteins. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5755–5759. doi: 10.1073/pnas.75.12.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Mar G. N., Burns P. D., Jackson J. T., Smith K. M., Langry K. C., Strittmatter P. Proton magnetic resonance determination of the relative heme orientations in disordered native and reconstituted ferricytochrome b5. Assignment of heme resonances by deuterium labeling. J Biol Chem. 1981 Jun 25;256(12):6075–6079. [PubMed] [Google Scholar]
- La Mar G. N., Smith K. M., Gersonde K., Sick H., Overkamp M. Proton nuclear nagnetic resonance characterization of heme disorder in monomeric insect hemoglobins. J Biol Chem. 1980 Jan 10;255(1):66–70. [PubMed] [Google Scholar]
- O'Keeffe D. H., Barlow C. H., Smythe G. A., Fuchsman W. H., Moss T. H., Lilienthal H. R., Caughey W. S. Magnetic and spectroscopic probes for FeOFe linkages in hemin systems. Bioinorg Chem. 1975;5(2):125–147. doi: 10.1016/s0006-3061(00)80056-3. [DOI] [PubMed] [Google Scholar]
- Ogoshi H., Kawabe K., Mitachi S., Yoshida Z. I., Imai K., Tyuma I. Influence of steric factors on oxygen binding. I. Studies on 2,4-diisopropyldeuteroheme-myoglobin. Biochim Biophys Acta. 1979 Dec 14;581(2):266–275. doi: 10.1016/0005-2795(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Structure and mechanism of haemoglobin. Br Med Bull. 1976 Sep;32(3):195–208. doi: 10.1093/oxfordjournals.bmb.a071363. [DOI] [PubMed] [Google Scholar]
- Phelps C., Antonini E. The combination of carbon monoxide-haem with apoperoxidase. Biochem J. 1969 Oct;114(4):719–724. doi: 10.1042/bj1140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Reversible splitting of human myoglobin; physico-chemical properties and oxygen equilibrium of reconstituted proto- and deuteromyoglobin. Arch Biochem Biophys. 1957 Nov;72(1):243–246. doi: 10.1016/0003-9861(57)90195-9. [DOI] [PubMed] [Google Scholar]
- SMITH M. H., GIBSON Q. H. The preparation and some properties of myoglobin containing meso- and deutero- haem. Biochem J. 1959 Sep;73:101–106. doi: 10.1042/bj0730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sono M., Asakura T. Decrease in oxygen affinity of myoglobin by formylation of vinyl groups of heme. J Biol Chem. 1975 Jul 10;250(13):5227–5232. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Tamura M., Asakura T., Yonetani T. Heme modification studies of myoglobin. I. Purification and some optical and EPR characteristics of synthesized myoglobins containing unnatural hemes. Biochim Biophys Acta. 1973 Feb 21;295(2):467–479. [PubMed] [Google Scholar]
- Tucker P. W., Phillips S. E., Perutz M. F., Houtchens R., Caughey W. S. Structure of hemoglobins Zürich [His E7(63)beta replaced by Arg] and Sydney [Val E11(67)beta replaced by Ala] and role of the distal residues in ligand binding. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1076–1080. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. X. Crystalline apo-and reconstituted holoenzymes. J Biol Chem. 1967 Nov 10;242(21):5008–5013. [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Woodrow G. V., 3rd Studies on cobalt myoglobins and hemoglobins. I. Preparation and optical properties of myoglobins and hemoglobins containing cobalt proto-, meso-, and deuteroporphyrins and thermodynamic characterization of their reversible oxygenation. J Biol Chem. 1974 Feb 10;249(3):682–690. [PubMed] [Google Scholar]


