Abstract
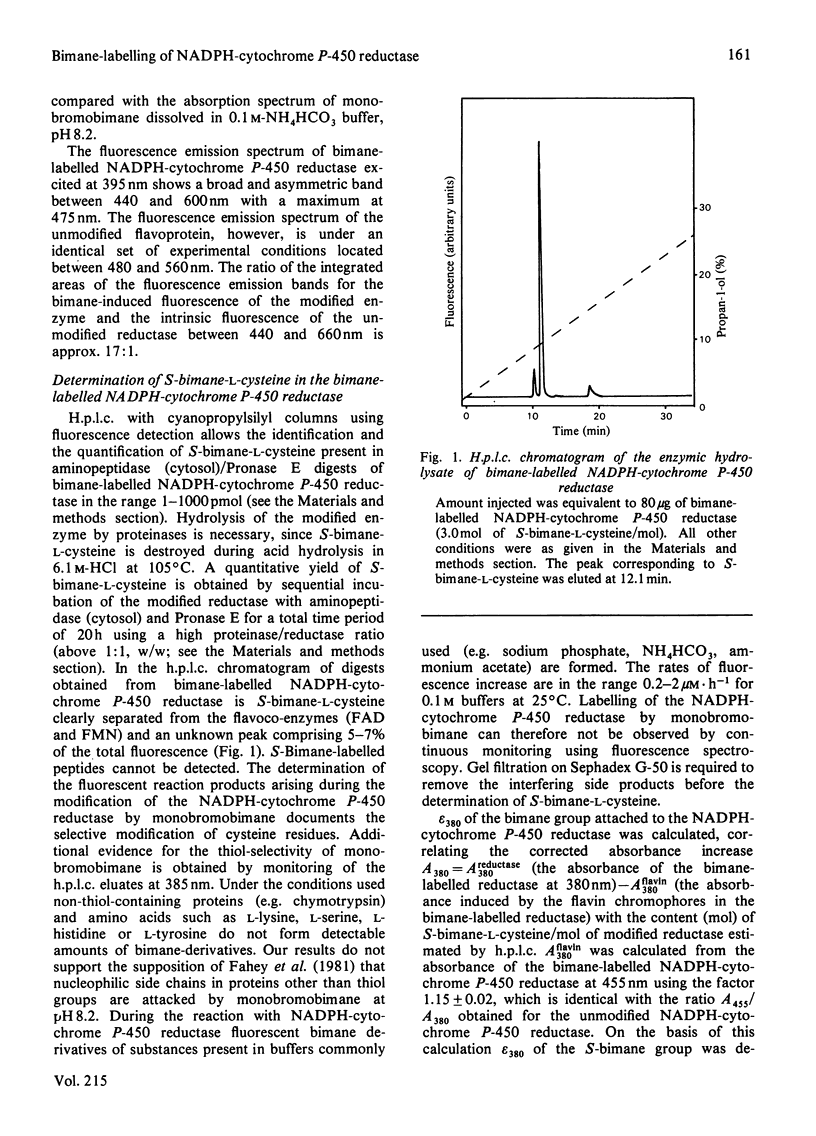
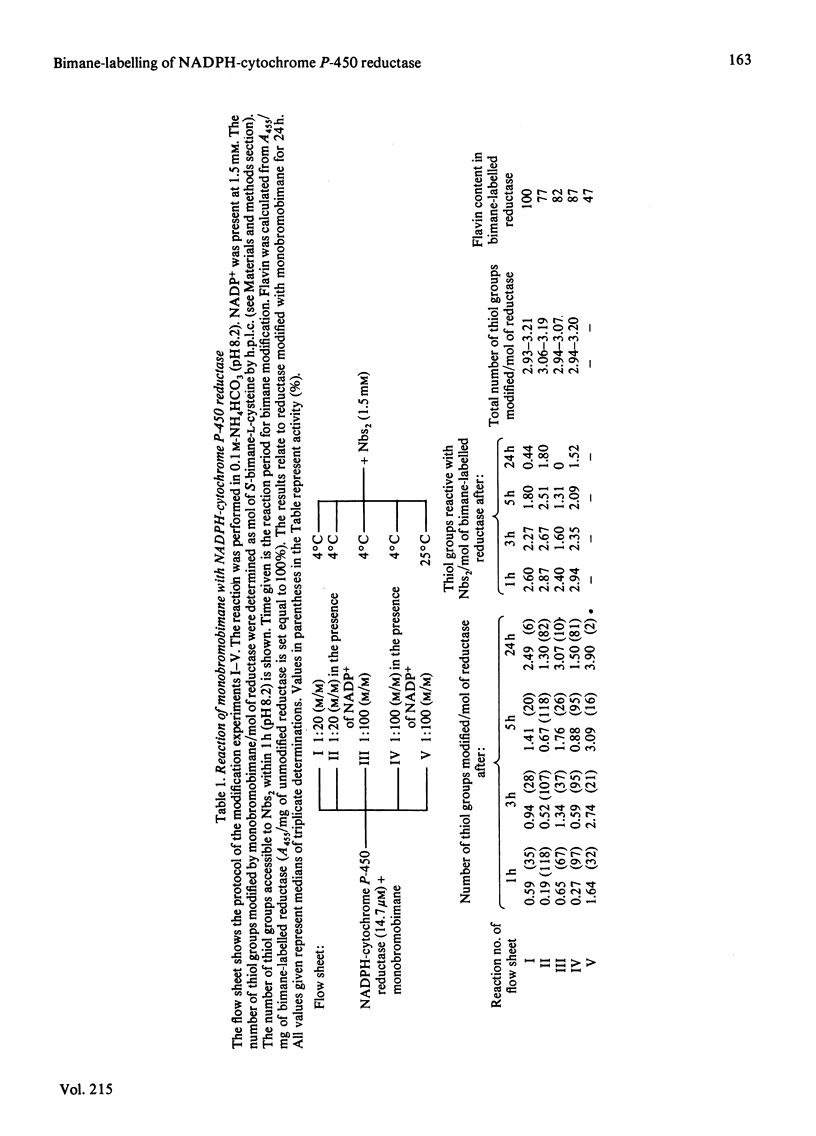
The kinetics of thiol-group alkylation in NADPH-cytochrome P-450 reductase during its inactivation by monobromobimane has been studied using the fluorimetric determination of S-bimane-L-cysteine by high-performance liquid chromatography. Loss of activity during the reaction of NADPH-cytochrome P-450 reductase with monobromobimane is caused by the alkylation of one single critical cysteine residue, which can be protected against thiol-specific reagents by NADP(H). The chemical stability of the bimane group allows the digestion of bimane-labelled NADPH-cytochrome P-450 reductase by CNBr. The critical cysteine residue could be located in a CNBr-cleaved peptide purified to homogeneity with Mr 10 500 +/- 1 000 and valine as N-terminus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. D., Coon M. J. Structural features of liver microsomal NADPH-cytochrome P-450 reductase. Hydrophobic domain, hydrophilic domain, and connecting region. J Biol Chem. 1982 May 25;257(10):5929–5938. [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. The sequence determination of a protein in a micro scale: the sequence analysis of ribosomal protein L34 of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):873–886. doi: 10.1515/bchm2.1976.357.1.873. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Makino N., Mason H. S. Redox properties of the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 and reduced nicotinamide adenine dinucleotide-cytochrome b5 reductases. Biochemistry. 1974 Apr 9;13(8):1701–1710. doi: 10.1021/bi00705a023. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Newton G. L., Ranney H. M. Bimane fluorescent labels: labeling of normal human red cells under physiological conditions. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3382–3386. doi: 10.1073/pnas.76.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazar T., Ehrig H., Lumper L. The functional role of thiol groups in protease-solubilized NADPH-cytochrome c reductase from pork-liver microsomes. Eur J Biochem. 1977 Jun 15;76(2):365–371. doi: 10.1111/j.1432-1033.1977.tb11604.x. [DOI] [PubMed] [Google Scholar]
- Lumper L., Busch F., Dzelić S., Henning J., Lazar T. Studies on the cosubstrate site of protease solubilized NADPH-cytochrome P450 reductase. Int J Pept Protein Res. 1980 Jul;16(1):83–96. doi: 10.1111/j.1399-3011.1980.tb02940.x. [DOI] [PubMed] [Google Scholar]
- Nishibayashi-Yamashita H., Sato R. Vitamin K3-dependent NADPH oxidase of liver microsomes. Purification, properties, and identity with microsomal NADPH-cytochrome c reductase. J Biochem. 1970 Feb;67(2):199–210. doi: 10.1093/oxfordjournals.jbchem.a129243. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y., Shibata Y. Studies on FAD- and FMN-binding domains in NADPH-cytochrome P-450 reductase from rabbit liver microsomes. J Biol Chem. 1982 Nov 10;257(21):12532–12539. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Cinti D. L. Preparation of microsomes with calcium. Methods Enzymol. 1978;52:83–89. doi: 10.1016/s0076-6879(78)52008-9. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. The occurrence of molecular interactions among NADPH-cytochrome c reductase, heme oxygenase, and biliverdin reductase in heme degradation. J Biol Chem. 1982 Jul 10;257(13):7786–7793. [PubMed] [Google Scholar]