Abstract
Drug-carrying nanoparticles can be recognized and captured by macrophages and cleared away by the immune system, resulting in reduced drug efficacy and representing the main drawbacks. Biomimetic nanoparticles, which are coated with cell membranes from natural resources, have been applied to address this problem. This type of nanoparticle maintains some specific biological activities, allowing them to carry drugs reaching designated tissues effectively and have a longer time in circulation. This review article aims to summarize recent progress on biomimetic nanoparticles based on cell membranes. In this paper, we have introduced the classification of biomimetic nanoparticles, their preparation and characterization, and their applications in inflammatory diseases and malignant tumors. We have also analyzed the shortcomings and prospects of this technology, hoping to provide some clues for basic researchers and clinicians engaged in this field.
Keywords: Biomimetic nanoparticle, Cell membrane, Inflammatory diseases, Cancer
Introduction
Inflammatory diseases are a group of disorders, which can be categorized as acute and chronic inflammatory diseases. The former refers to trauma, acute infection, and acute immune rejection, while the latter includes disorders such as atherosclerosis, arthritis, inflammatory bowel disease, and cancers [1]. Inflammation is closely related to human diseases and plays a fundamental role in the progress of many diseases, including cancer [2]. Therefore, it is critical to properly manage these inflammatory disorders.
As far as we know, cancer is a type of disease that seriously threatens human health, and its growth process is multifactorial. Scholars believe that the occurrence of tumors is related to chronic inflammation [3–6]. Although traditional radiotherapy and chemotherapy have relieved the tumor burden for patients with unresectable or metastatic cancer, they also indiscriminately damage normal cells, leading to a series of adverse reactions and causing significant physiological and psychological impacts [7]. Despite this, chemotherapeutic drugs with cytotoxicity remain a prioritized clinical strategy, though they cannot selectively target cancer cells after systemic administration. Furthermore, adverse reactions restrict the dosage of the drug, resulting in a low efficacy of chemotherapy. Therefore, there is an urgent need for a new treatment approach that can enhance the efficacy and reduce adverse events of antitumor drugs.
Nanoparticles provide a useful tool for effectively delivering therapeutic drugs in the treatment of inflammatory diseases and tumors. Nanoparticles can achieve high-dose loading of drugs by physical characteristics. In addition, the large pore size of newly formed blood vessels inside the tumor makes it easier for nanoparticles to gather at the tumor site for specific accumulation, which is called the enhanced permeability and retention (EPR) effect [8]. Although scientists have tried their best to modify the surface of nanoparticles to induce more “active targeting” capabilities, most of the current targeting strategies based on the EPR effect are “passive”, which means that the strategy of nano-delivery of drugs relies almost entirely on the physical properties of the nanoparticles and the tumor itself [9]. However, drug-carrying nanoparticles also have other drawbacks, such as cytotoxicity, immunogenicity, and poor targeting, limiting their wide clinical application [10]. In particular, these nanoparticles can be recognized, captured, and cleared away by the immune system, reducing its drug delivery efficacy. It has been reported that 30–99% of the nanoparticles will be eliminated by the liver after systematic administration, and only less than 5% of the dose will be delivered to the targeted tumor lesion, which makes the antitumor effect very limited [11]. Fortunately, a novel type of nanoparticles called biomimetic nanoparticles based on cell membranes (BNCM) prepared from natural resources was recently developed. These kinds of nanoparticles are characterized by their specific tissue targeting, low toxicity, higher histocompatibility, and prolonged half-life time in circulation, rendering them a potential candidate for effective drug delivery in the treatment of inflammatory diseases and tumors [12]. Besides, some scholars also point out that cholesterol levels can regulate the activity of nanoparticle enzymes wrapped in cell membranes. They reported that reducing membrane cholesterol levels effectively enhanced cell membrane permeability, which may be attributed to the increased enzymatic activity. The result suggested that the enzyme activity of cell membrane-wrapped nanoparticles can be easily adjusted by tailoring the level of membrane cholesterol, thereby adjusting the cell membrane permeability and enhancing the release of drugs inside the membrane.
Therefore, the authors reviewed recently published literature and sought to summarize the latest advances in this field, particularly the application of BNCM in the treatment of inflammations and malignant tumors.
Features of BNCM and underlying mechanisms
Low immunogenicity and long half-time in circulation
Immune cells, such as macrophages, T cells, dendritic cells, and neutrophils, can be rapidly recruited to specific locations during the initial immune response. The underlying mechanism for the prolonged circulation of biomimetic nanoparticles based on immune cells is opsonization and self-recognition, delaying elimination by phagocytes [13].
BNCM contains molecular proteins similar to those found in source cells, which help evade the recognition, ingestion, and elimination of the macrophage phagocytic system. In nanoparticles without cell membrane encapsulation, the lack of surface-specific molecules makes them prone to phagocytosis by the immune system, leading to a significant reduction in half-life in the circulation. For instance, red blood cell membranes coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles (RBC-NPs) had an elimination half-life of 39.6 h, which was significantly longer than polyethylene glycol (PEG)-coated lipid–PLGA hybrid nanoparticles with an elimination half-life of only 15.8 h [14].
RBCs, responsible for transporting oxygen to tissues and organs, have a lifespan of approximately 120 days within the human body [15]. The transmembrane protein CD47, expressed on the erythrocyte membrane, selectively binds to signal regulatory protein (SIRP) α expressed on the macrophage membrane, subsequently releasing the “don’t eat me” signal. The activation of this signal assists in preventing macrophage uptake, thus avoiding immune system attacks, rendering RBC-NPs a longer circulation time in vivo [15, 16]. Platelets, fragments originating from the cytoplasmic lysis of mature megakaryocytes in bone marrow, play a crucial role in the body’s hemostatic function [17]. CD47 is also expressed on the surface of platelets, inhibiting macrophage uptake. Additionally, CD55 and CD59 are expressed on platelets, and the presence of these two proteins inhibits the immune complement system, preventing immune system attacks [18].
While normal cells in the body often express low levels of CD47, some cancer cells notably overexpress CD47 on their membranes. When CD47 binds to SIRP α on the surface of phagocytes, cancer cells successfully evade attacks by the immune system. PD-L1 on the surface of cancer cells, acting as an immunosuppressive molecule, interferes with the host immune system’s recognition, serving as a significant factor enabling cancer cells to persist and proliferate indefinitely in the body [19–21]. Consequently, biomimetic nanoparticles derived from cancer cells can also achieve a prolonged time in circulation [22].
Carrying elements to boost immune response
Immunotherapy has garnered significant attention, particularly in the realm of anticancer therapies. Some specific cell membranes possess immune-activating characteristics, such as expressing immunogenic antigens that can stimulate an immune response. Thus, they can be used for developing vaccines. Therefore, BNCM holds an inherent advantage in immunotherapy. When nanoparticles are encapsulated in cancer cell membranes, abundant membrane-binding proteins, and tumor-associated antigens can be delivered to antigen-presenting cells (APC), thereby inducing an immune response against tumors [22]. Similar to biomimetic nanosystems based on eukaryotic cells, bacterial membranes containing specific proteins can also be combined with functional nano-carriers for drug delivery and immunostimulation. Moreover, bacterial membranes feature a multitude of pathogen-associated molecular patterns (PAMPs), including lipopolysaccharides, peptidoglycan, mannose, porin, and lectin. These PAMPs can induce the recognition and binding of pattern recognition receptors, especially toll-like receptors (TLRs), which are highly expressed on the surface of innate immune cells, thus activating the immune system [23]. Therefore, bacterial nanoparticles with antigen presentation capabilities exhibit potent antibacterial effects.
Selective targeting
BNCM can achieve selective targeting by mimicking the recognition and interaction mechanisms of source cells. The mechanism largely depends on molecules present on the cell membrane, which can help the immune system recognize and bind to specific cells or tissues [24]. Due to the diverse types and quantities of molecules expressed on the cell surface, BNCM from different sources exhibit distinct targeting abilities and can be used to target selective cells or tissues [25]. In contrast, nanoparticles lacking membrane coating exhibit a poorer selectivity due to the absence of specific molecules. Thus, surface modification or functionalization is often required to achieve this function.
Neutrophils are the first immune cell population recruited toward the inflammation site, so drug-loaded biomimetic nanoparticles derived from neutrophils possess a natural advantage in active targeting. This advantage largely relies on the lymphocyte function-associated antigen (LFA-1) molecule expressed on cell membranes, which binds to intercellular adhesion molecule-1 (ICAM-1) on the endothelial of inflamed tissues [25]. Additionally, neutrophils can directly target circulating tumor cells through the binding CD44 to L-selectin [25]. Similarly, P-selection overexpressed on platelets also exhibits an affinity for excessive CD44 on the surface of cancer cells, which promotes the active targeting of platelets to tumors and the selective release of loaded drugs carried by BNCM-based on platelets [7].
Tumor cells possess the ability to proliferate indefinitely, resist the immune system, and target homologous cells. Thomsen–Friedenreich antigen and E-cadherin provide the necessary conditions for the interaction between homologous tumor cells and their homologous aggregation [26]. It is worth noting that during the process, the cancer cell membrane can be damaged by membrane extraction technology, making it a challenge to maintain the integrity of the cell membrane and resulting in limited immune escape capabilities for tumor cell-derived nanoparticles. To address this limitation, researchers have integrated cell membranes from different sources to create hybrid membranes. The hybrid membrane inherits the characteristics of the source cells and enhances immune escape capabilities [24]. The hybridization of cells from different sources serves to amplify the characteristics of the original source cells, thus improving the antitumor effect. For instance, Jiang et al. fused erythrocyte membranes with human breast cancer MCF-7 cells to create Melanin@RBC-M when encapsulating melanin nanoparticles [27]. It significantly enhanced the targeting ability of melanin nanoparticles because the hybrid cell membrane contained a fragment of MCF-7 cell membrane, which carried self-recognition biomarkers of homologous cancer cells such as N-cadherin, galectin-3 and epithelial cellular adhesion molecule (EpCAM).
Preparation and characterization of nanoparticles
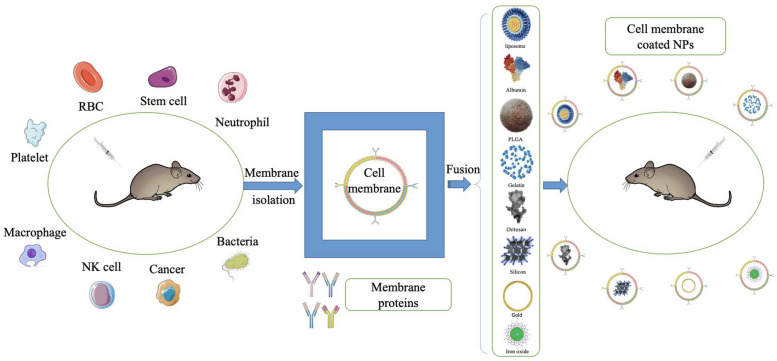
The preparation of BNCM mainly under through three steps, including extraction of cell membranes, synthesis of core nanoparticles, and fusion of cell membrane and core nanoparticles (Fig. 1).
Fig. 1.
Schematic diagram of the preparation of cell membrane-coated nanoparticles. Different cell membranes are isolated from their source cells and then fused with core nanoparticles to form cell membrane-coated nanoparticles
Cell membrane extraction
The cell membrane consists of a phospholipid bilayer that is an elastic semi-permeable structure primarily composed of phospholipids and proteins. It is responsible for regulating the movement of substances into and out of cells and organelles, which is vital in the life process. Cell membrane extraction includes two steps, namely membrane dissolution and membrane purification. For enucleated cells such as mature red blood cells and platelets, the cell membrane can be disrupted through hypotonic treatment, followed by repeated cycles of freezing and thawing. Subsequently, differential centrifugation is used to eliminate soluble proteins, and the process concludes with repeated extrusion to create target cell vesicles [28]. However, for eukaryotic cells such as leukocytes and macrophages, the extraction process is more complicated. Cells are separated from the surrounding tissue or blood and then cultured. The nuclei of cells are removed through hypotonic lysis, mechanical membrane destruction, and sucrose gradient centrifugation, and the cell membrane is isolated [29]. Finally, the separated cell membrane is thoroughly cleansed with a plasma buffer and extruded using a porous polycarbonate membrane to form nano-vesicles [30].
It is necessary to mention here that some scholars point out that cholesterol levels can regulate the activity of nanoparticle enzymes wrapped in cell membranes. They reported that reducing membrane cholesterol levels effectively enhanced cell membrane permeability, which may be attributed to the increased enzymatic activity [31]. The result suggested that the enzyme activity and permeability of cell membrane-wrapped nanoparticles can be easily adjusted by tailoring the level of membrane cholesterol, providing a new method in the phase of cell membrane extraction for considering how to control BNCM to improve permeability and release the carried drugs.
Types of nanoparticle core
The nanoparticle core occupies a central position and key role in the drug delivery system. Some materials are used to make nanoparticles, which include metals (gold, Fe3O4, metal–organic frameworks, and hollow copper sulfide), polymers (PLGA, liposomes, and albumin), gels (deoxycholic acid chitosan derivatives and cholesterol-modified polysaccharides, such as dextran, mannan, pullulan and polyaminoacids), and mesoporous silica, among others [25, 32, 33]. Recently, Zou’s team synthesized two mesenchymal stem cell (MSC) membrane-coated silica nanoparticles (MCSNs), which have similar sizes but distinctly different stiffness values (MPa and GPa), and finally, they found that soft MCSNs had much lower macrophage uptake and enabled the forming of a more protein-rich membrane coating compared with the stiff MCSNs [34]. The result suggested that the elasticity of the nanoparticle core can regulate the formation of BNCM and their nano-bio interaction.
Due to the high specific surface area, nanoparticles are prone to mutual attraction and aggregation, which can alter their physical and chemical properties [35]. Therefore, maintaining colloidal stability can prevent the occurrence of aggregation, thereby preserving the uniformity and dispersion of the particles. The Hammett constant, as an indicator of colloidal stability, can be used to assess and optimize the design and application of nanoparticles [35]. In the domain of inorganic nanoparticles, nanogels possess a Hamaker constant akin to water, which guarantees exceptional stability in biological fluids, primarily due to their limited propensity to aggregate [36]. Nanogels possess biocompatibility, drug-loading capacity, and multifunctional stimulative properties and can also penetrate human skin while preserving the potency of encapsulated drugs [37]. These attributes collectively render nanogels an ideal platform for encapsulating diverse drugs or nucleic acids [38]. Nanogels exhibit more potent anti-inflammatory effects than organic gels when they are locally applied in a mouse ear swelling model, though both are prepared at a PH of 7 [39]. Mesoporous silica nanoparticles (MSN) have garnered attention as valuable drug carriers for achieving targeted drug delivery and controlled release, attributing to their highly ordered pore structure, uniform and controllable pore size, substantial specific surface area, pore volume, ease of surface modification with organic functional groups, and exceptional biocompatibility [40]. Coating the surface of MSN with cyclodextrin-modified hyaluronic acid (HA) could effectively prevent premature leakage of doxorubicin (DOX) loaded in MSN, leading to enhanced antitumor efficacy [41]. The underlying principle is that CD44, which is overexpressed by tumor cells, serves as a high-affinity receptor for HA, and consequently, the HA-CD44 receptor-mediated endocytosis facilitates increased uptake of DOX by tumor cells. Metal nanomaterials are widely applied due to their favorable biocompatibility and physicochemical properties. For example, glutathione-modified gold nanoclusters (Au29SG27) rapidly suppressed the inflammatory response and effectively mitigated bone tissue damage in treating rheumatoid arthritis [42].
Due to the advantages of PLGA, like good biodegradability, biocompatibility, and non-toxic nature [43], it has been approved by the FDA for various drug delivery systems, such as liposomal doxorubicin, Albumin-bound paclitaxel, and Ferumoxytol (iron oxide nanoparticle compound) [44–46]. In a study [47], nano-carriers co-loaded with hemoglobin and doxorubicin were camouflaged with cancer cell membranes through repeated extrusion. This innovative approach aimed to overcome chemotherapy resistance induced by hypoxia and achieve targeted delivery of antineoplastic drugs. Due to the high drug-loading capacity and ability to simultaneously deliver hydrophilic and hydrophobic drugs, liposomes are frequently employed as the core materials in biomimetic nanosystems. Another noteworthy development involves macrophage membrane-encapsulated and pH-responsive liposomes loaded with the anticancer drug emtansine. This formulation allows for targeted delivery to lung tumors and has demonstrated a significant inhibition of pulmonary metastasis in breast cancer [48].
Nanoparticle fusion
The fusion of nanoparticle cores with cell membranes is the final step, primarily achieved through extrusion, ultrasound, or electroporation [49]. Membrane-derived vesicles are generated via porous polycarbonate membrane extrusion, and membrane biomimetic core–shell nanodrugs are obtained by co-extruding these vesicles with nano-core carriers [50]. Extrusion is a straightforward and effective approach, but it is unsuitable for large-scale production, representing a significant obstacle in the clinical application [51]. Alternatively, the ultrasonic method involves co-incubating cell membrane vesicles with core carriers under ultrasound to facilitate the encapsulation of membrane vesicles onto the core carriers [52]. During this process, the appearance uniformity and drug-loading efficiency of biomimetic nanoparticles can be influenced by ultrasonic frequency, duration, and intensity. Furthermore, the uneven distribution of biomimetic nanoparticles represents another limitation of ultrasonic preparation, as it can lead to localized high temperatures that affect the activity of membrane proteins [53]. With the advancement of microcurrent technology, electroporation technology based on microfluidic chips has emerged as a standout method for preparing biomimetic nanoparticles due to its high repeatability and throughput [54]. The microfluidic chip system comprises five components including a sample injection port, a Y-type merging channel, an S-type mixing channel, an electroporation zone, and a sample outlet. The fundamental process involves the introduction of membrane vesicles and nano-carriers into the system via the injection port, followed by their merging in the Y-channel, completion of the mixing task in the S-channel, and entry into the electroporation area. Under the influence of an electrical pulse, the cell membrane envelops the core material [54–56]. The parameters, such as pulse voltage, duration, and flow rate, can be adjusted to achieve nanoparticles with predetermined coatings and excellent stability. However, it is worth noting that the high cost associated with electroporation technology should be considered in nanoparticle preparation.
Nanoparticle characterization
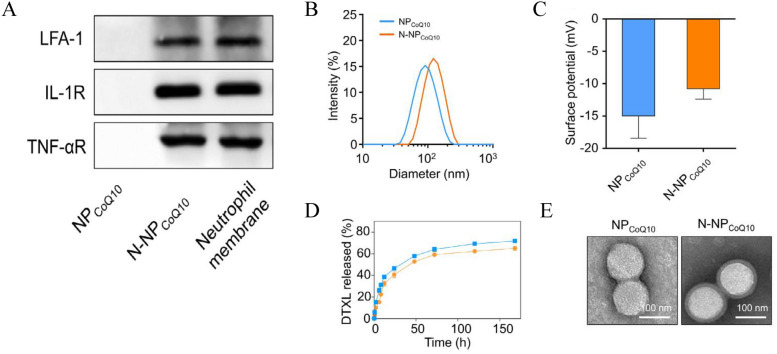
After biomimetic nanoparticles are prepared, it becomes essential to characterize their physical, chemical, and biological properties to verify the presence of the cell membrane coating on their surface, which serves as the foundation for subsequent experiments. Some characterization methods and relevant results are shown in Fig. 2. In general, the efficiency of cell membrane coating depends on nanoparticle size, surface charge, and protein composition. The cell membrane introduces changes in both particle size and Zeta potential, which are typically assessed through transmission electron microscopy (TEM), scanning electron microscopy (SEM), and dynamic light scattering (DLS). TEM is commonly employed to detect alterations in particle size. Typically, biomimetic nanoparticles have an increased size by approximately 10–20 nm compared to uncoated nanoparticles. This size increase aligns with the thickness of the phospholipid bilayer. Moreover, SEM can visualize the characteristic shell–core structure of biomimetic nanoparticles. For instance, the average size of nanoparticles increased from 225 to 247 nm after encapsulating the PLGA core with erythrocyte membranes [57]. DLS is frequently utilized to measure the Zeta potential of nanoparticles. The surface properties of nanoparticles change after cell membrane coating, resulting in shifts in the surface charge. Given that the in vivo performance of biomimetic nanoparticles hinges on the biological activity of the cell membrane and the expression of specific molecules on that membrane, the assessment of these indicators holds particular significance. To evaluate the biological functionality of membrane-coated nanodrugs and the expression of specific membrane molecules, techniques such as sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting, enzyme-linked immunosorbent assay (ELISA), and immunofluorescence are employed. For example, Western blotting analysis was employed to confirm the presence of the erythrocyte membrane characteristic protein CD47 on the surface of melanin nanoparticles camouflaged with erythrocyte membrane (Melin@RBC), and this presence was shown to reduce tumor burden in A549 tumor-bearing mice [16]. Similarly, by immunofluorescence was used to confirm that nanoparticles derived from cancer cells had homologous targeting to human breast cancer MDA-MB-435 cells [58].
Fig. 2.
Characterization of nanoparticles. A Characteristic protein bands of NPCoQ10 and N-NPCoQ10 were examined by Western blotting. The neutrophil cell lysate was set as a positive control. B, C The hydrodynamic size (B) and surface zeta potential (C) of NPCoQ10 and N-NPCoQ10 examined dynamic light scattering (DLS). D Release profile of NPCoQ10 and N-NPCoQ10. E Representative images of NPCoQ10 and N-NPCoQ10 detected by transmission electron microscopy (TEM). Samples were stained with uranyl acetate. Scale bar, 100 nm.
Adapted from Ref. [65] with permission
Exploratory applications of BNCM for drug delivery in inflammatory diseases
Acute inflammation
Ischemia–reperfusion injury
Adequate blood perfusion is critical to maintaining the supply of oxygen and nutrients. However, a decrease in tissue blood perfusion due to various reasons can lead to cellular damage, a condition known as ischemia injury. Paradoxically, restoring blood perfusion and oxygen supply to ischemic organs can exacerbate this damage, a phenomenon referred to as ischemia–reperfusion injury [59]. This type of injury can result from various pathological processes, including myocardial infarction, ischemic stroke, acute renal injury, trauma, circulatory arrest, and multiple organ injuries, such as those occurring in the heart, brain, and kidney following blood flow restoration after organ transplantation [60]. Ischemia–reperfusion injury is an inevitable occurrence in transplantation, and reperfusion often causes more damage than ischemia. The mechanisms underlying ischemia–reperfusion injury involve mitochondrial damage, calcium overload, and inflammatory cell infiltration [61].
As of now, there is no effective solution to ischemia–reperfusion injury, but attempts have been made by using BNCM techniques to rise to the challenges. Macrophage membranes encapsulated PLGA nanoparticles (M-NPs) were synthesized in a study [62]. These M-NPs possessed the characteristics of macrophage membranes and the ability to carry drugs and were able to reduce hepatic ischemia–reperfusion injury in a rat model of liver transplantation when they were injected via tail vein pre-operatively. The serum levels of inflammatory factors were significantly decreased, and the survival time was significantly prolonged by the administration of M-NPs. The mechanism underlying this protection by M-NPs might be the upregulation of TLR4, which binds to lipopolysaccharide (LPS), reducing the serum levels of LPS and sequentially the release of cytokines stimulated by LPS [62].
A substantial amount of reactive oxygen species and inflammatory mediators are generated during cerebral ischemia–reperfusion injury, leading to severe tissue and cell damage, even necrosis. The blood–brain barrier (BBB) prevents harmful substances from entering brain tissue, but it also hinders drug delivery, posing a significant obstacle to brain injury treatment. In a study, nano-vesicles derived from neutrophil membranes containing resolvin (RvD)2 were designed and were shown to protect against cerebral ischemia–reperfusion injury [63]. These nano-vesicles accumulated significantly in ischemic brain tissues after intravenous injection, leading to the effective removal of pro-inflammatory factors at the injury site, a reduction in the infarct area of the middle cerebral artery, and alleviation of neuroinflammation, suggesting these nanoparticles could pass through the BBB and release drugs to exert anti-inflammatory effects. In another study, monocyte membranes-coated rapamycin nanoparticles (McM/RNPs could pass through the BBB and successfully deliver rapamycin to inhibit the proliferation of inflammatory cells and significantly improve neurological scores and cerebral infarction volume in a rat model of cerebral ischemia–reperfusion injury [64]. Similarly, coenzyme Q10 (CoQ10) nanoparticles (N-NP CoQ10) fused with neutrophil membranes were able to specifically target kidneys in a rat model of renal ischemia–reperfusion injury [65]. N-NP CoQ10 possessed potent anti-inflammatory and antioxidant properties, effectively inhibited renal cell apoptosis, promoted the recovery of renal function, and mitigated renal ischemia–reperfusion injury. These findings may underscore the potential of BNCM as a promising approach for addressing ischemia–reperfusion injury in clinical settings.
Pneumonia
During acute inflammatory reactions, the release of inflammatory mediators can lead to tissue hypoxia and tissue injury. Up to date, no in vivo targeting anti-inflammatory drugs are available. Thus, the precise delivery of drugs to inflammatory tissues represents a promising approach for treating inflammatory diseases. This was accomplished by integrating therapeutic drugs into denatured albumin nanoparticles, which successfully target pulmonary vascular endothelium [66]. This approach reduced pulmonary neutrophil infiltration and the release of inflammatory mediators in a mouse model of acute pneumonia and also reversed acute lung injury induced by lipopolysaccharide. However, this type of nanoparticle is prone to detachment from neutrophils, potentially resulting in drug misdelivery and reduced efficacy. D-series resolvin (RvD) is a lipid mediator known for its ability to promote the resolution of tissue inflammation. It has been reported that RvD2 could diminish the adhesion between neutrophils and endothelial cells, reduce the release of inflammatory factors, and inhibit neutrophil infiltration, expediting the regression of inflammation in sepsis-afflicted mice [67]. However, RvD has poor targeting and is susceptible to enzymatic degradation in vivo. In a study, neutrophil membrane-derived nanocapsules (NMVs) were developed to address these issues [68]. RvD1 and ceftazidime (CEF) were loaded onto the surface and inside of these NMVs to create CEF-RvD1-NMVs, which exhibited remarkable targeting ability towards peritoneal inflammatory vessels in a mouse model of peritonitis induced by pseudomonas aeruginosa. Notably, CEF-RvD1-NMVs significantly enhanced anti-inflammatory and bacteriostatic effects compared to CEF-RvD1, CEF, or RvD1 alone.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered a global pandemic of coronavirus disease 2019 (COVID-2019) globally [69]. The infectivity of SARS-CoV-2 relies heavily on its binding to protein receptors on target cells. Two types of nanosponges derived from the membranes of human lung epithelial type II cells (Epithelial-NS) and macrophages (MΦ-NS), respectively, were developed for specific targeting [70]. These nanoparticles demonstrated the ability to neutralize COVID-2019 in a concentration-dependent manner, though their effectiveness in treating SARS-CoV-2 infection still requires validation in appropriate animal models and clinical trials. Nonetheless, these findings offer new insights into potential solutions for COVID-2019 and other infections in the future.
Acute pancreatitis
Acute pancreatitis is an inflammatory disorder due to trypsin activation, leading to the digestion, edema, bleeding, and potentially necrosis of pancreatic tissues [71]. Severe acute pancreatitis can cause pancreatic tissue necrosis, inducing the systemic inflammatory response syndrome or multiple organ dysfunction syndrome. Unfortunately, there are no effective drugs available for the treatment or prevention of acute pancreatitis. The existing drug treatments primarily focus on symptom relief, such as inhibiting trypsin secretion, providing spasmolysis and analgesia, preventing infections, and improving circulatory perfusion [72].
The blood–pancreatic barrier (BPB) hinders the effective transport of drugs from the bloodstream to the site of inflammation in the pancreas. The advent of the BNCM techniques has successfully solved this issue. Celastrol (CLT) is a compound derived from wilfordii roots, known for its ability to inhibit inflammation by targeting nuclear transcription factor kappaB (NF-κB) [73]. CLT coated with neutrophil membrane (NNPs/CLT) could cross the BPB and selectively accumulate more in inflamed pancreatic tissues than uncoated CLT in a rat model of acute pancreatitis [74]. Administration of NNPs/CLT reduced serum amylase and pancreatic myeloperoxidase levels and also alleviated lung injury in the rats (Fig. 3A–C). Additionally, NNPs/CLT could reduce the production of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), by blocking the signaling pathway and inhibiting the expression of NF-κB (Fig. 3D, E).
Fig. 3.
Therapeutic efficacy of NNPs/CLT on inflammation in the AP rat model. A–C Serum amylase level (A), weight of ascites (B), pancreatic wet/dry ratio (C), Serum TNF-α (D) and serum IL-6 (E) levels in the AP rats. *p < 0.05 versus sham group, #p < 0.05 versus model group, &p < 0.05 versus CLT solution group, and $p < 0.05 versus NPs/CLT group. Data represent mean ± SD (n = 6).
Adapted from Ref. [74] with permission
Sepsis
Sepsis results from infections in various body sites and is associated with severe infectious diseases such as severe pneumonia, peritonitis, cholangitis, urinary tract infections, and more. Endotoxins secreted by Gram-negative bacteria play a crucial role in the pathogenesis of sepsis by activating the inflammatory response, leading to the release of inflammatory mediators, which can ultimately cause multiple organ dysfunction or failure [75, 76]. Therefore, a crucial part of sepsis treatments is the neutralization and clearance of endotoxin. Endotoxin can bind to immune cells’ pattern recognition receptors (PRRs) [77]. PLGA core nanoparticles coated with macrophage membrane (MΦ-NPs) were used to treat sepsis [78]. MΦ-NPs inherited the biological characteristics of the source cell membrane, enabling them to neutralize endotoxins through interactions with TLR4 and CD14 expressed on cell membranes. MΦ-NPs showed reduced levels of pro-inflammatory cytokines, including IL-6, TNF-α, and IFN-γ. MΦ-NPs could also inhibit the activation of TLR4 in a free endotoxin environment, while synthetic polyethylene glycol (PEG-NPs) and erythrocyte membrane nanoparticles co-incubated with lipopolysaccharide could not, which highlighted the unique advantage of macrophage membrane in neutralizing lipopolysaccharide [78].
Others
Except for the inflammation diseases mentioned above, BNCM is also applied in periodontitis. Pan combined minocycline-loaded polydopamine nanoparticles (PM) with cRGD-modified cell membrane (RCM) to prepare a novel type of PM@RCM, which rescues impaired human periodontal ligament stem cells through antioxidant, anti-ferroptosis, and anti-inflammation, exhibiting favorable antibacterial bioactivity both in vivo and in vitro [79].
Chronic inflammation
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a type of chronic, non-specific inflammatory condition characterized by joint damage, which can result in cartilage and bone degradation, ultimately leading to joint deformities and loss of normal function. While biological agents have been employed to treat RA, many patients still experience suboptimal clinical outcomes. Thus, new treatments are needed to address this challenge [80].
RA is regarded as an autoimmune disease and the invasion of immune cells into the synovial joint space plays a pivotal role. Neutrophils contribute to the progression of RA because they can stimulate synoviocytes in the joint to produce chemokines, attracting more neutrophils to the inflammatory site [81]. Activated neutrophils degranulate and release their contents, leading to cartilage destruction [82]. In addition to conventional drug therapies, biomimetic nanoparticle delivery strategies based on cell membranes have shown promise in treating RA. Researchers have developed a novel approach to treat rheumatoid arthritis in mouse models, including the collagen-induced mouse model and human transgenic mouse arthritis model [83]. This approach involves the preparation of new nanoparticles coated with polymers and wrapped in neutrophil membranes [84]. Due to the adhesion between neutrophils and chondrocytes, these nanoparticles not only have the ability to neutralize pro-inflammatory cytokines and suppress inflammatory responses in arthritic mice but also possess the capability to aggregate and penetrate the cartilage matrix in the distal part of the femoral head, providing robust protection for cartilage to reduce joint damage [85].
FK506 is an effective immunosuppressant for RA patients who have not responded well to methotrexate. In a study [86], nanoparticles encapsulated in platelet membranes carrying FK506 were tested in a mouse model of collagen-induced arthritis (CIA). These nanoparticles were found to be localized in synovial tissues by binding to Glycoprotein VI, which is expressed on the platelet membrane and interacts with type IV collagen. FK506-loaded nanoparticles significantly reduced the degree of bone erosion in the ankle joint and effectively controlled the progression of RA. In another study conducted by the same research group, similar results were obtained using macrophage-derived microvesicles encapsulated with nanoparticles for RA treatment [87]. These findings underscore the feasibility and effectiveness of biomimetic nanoparticles in treating RA.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a group of chronic conditions that primarily affect the gastrointestinal tract, leading to inflammation of the intestines and the most common types are Crohn’s disease and ulcerative colitis [88]. Current treatments for IBD focus on inflammation control, symptom management, and surgical intervention for severe cases with complications. Anti-inflammatory drugs in IBD treatment include aminosalicylic acid preparations, corticosteroids, and immunosuppressants. Keratinocyte growth factor (KGF) is secreted by stromal cells to stimulate epithelial proliferation [89]. The expression of endogenous KGF is increased in IBD, and KGF could promote mucosal repair in gastrointestinal injuries and colitis. However, the clinical application of KGF in managing IBD has been limited due to poor stability, production difficulties, and non-specific distribution [90].
It has been found in a study [91] that the encapsulation of KGF in liposomes (KGF-Lips) could enhance its stability. In subsequent studies, KGF-Lips were embedded into neutrophil membranes (NEM) isolated from mice and activated by lipopolysaccharide to create neutrophil-like liposomes (KGF-Neus), which exhibited remarkable stability in vivo. Intravenous administration of KGF-Neus significantly reduced intestinal mucosal ulcers and neutrophil infiltration and promoted the recovery of intestinal morphology and function in a mouse model with UC induced by sodium dextran sulfate [91].
Macrophages can undergo polarization into two distinct phenotypes, M1 and M2, in the context of inflammatory conditions within the colon. Patients with UC often exhibit an increase in M1 macrophages and a decrease in M2 macrophages in their inflamed colon tissues. This imbalance between M1 and M2 macrophages has been associated with the progression of UC [92]. Exogenous administration of M2 macrophages could effectively suppress colitis, representing a potential therapeutic strategy for UC [93]. Nanoparticles encapsulating rosiglitazone, a compound capable of inducing macrophages to polarize towards M2, within macrophage membranes were able to target inflamed colon tissues [94]. These nanoparticles could release rosiglitazone, promote macrophage polarization towards M2, and absorb the inflammatory mediators once they arrived in the inflamed colon tissues [94]. This approach holds promise as a potential strategy for treating UC by modulating macrophage polarization and mitigating inflammation.
Atherosclerosis
Atherosclerosis is a chronic vascular inflammatory disease characterized by the deposition of atherosclerotic plaques beneath the arterial endothelium, which can lead to lumen stenosis or occlusion, resulting in ischemic events and potentially life-threatening vascular conditions such as acute myocardial infarction. Platelet has an inherent affinity with atherosclerotic plaques and can be considered a potential candidate cell targeting atherosclerotic sites, so platelet-coated nanoparticles (PNPs) have been utilized for developing atherosclerosis treatments [95]. Human platelets were fused with PLGA nanoparticles to create PNPs with physical and chemical properties similar to platelets, and these PNPs expressed key molecules of platelets, including immunomodulatory proteins, integrins, and other transmembrane proteins [96]. Docetaxel-loaded PNPs (PNP-DtX1) were tested in a rat model of coronary artery restenosis, and the results revealed that PNP-DtX1-treated rats had the average intima-to-media ratio of 0.18 ± 0.06 and the lumen occlusion rate of 8%, which were significantly lower than free docetaxel-treated rats that had an average intima-to-media ratio of 0.76 ± 0.18 and a lumen occlusion rate of 33.6% [96].
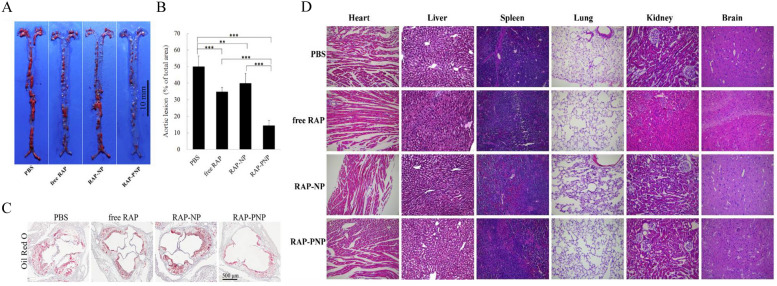
Similarly, platelet membrane-coated rapamycin-loaded PLGA nanoparticles (RAP-PNP) were tested for atherosclerosis in an apolipoprotein E-deficient mouse atherosclerosis model [97]. The results showed that platelet membrane coating enhanced drug loading and entrapment efficiency of rapamycin and inhibited plaque development (Fig. 4A). The aorta stained with Sudan red quantitative results showed that the average plaque decreased from 50.1 ± 6.3% to 34.8 ± 2.6% after free rapamycin treatment (Fig. 4B). However, RAP-PNP therapy could further the proportion of atherosclerotic lesions to 14.5 ± 2.9% (Fig. 4A, B). Oil-red O staining of the aortic root revealed that RAP-PNP significantly reduced the plaque area and increased the lumen area (Fig. 4C). Notably, the results of H&E staining sections demonstrated no apparent injury in the major organs of mice treated with RAP-PNP for 4 weeks, including the heart, liver, spleen, lung, kidney, and brain (Fig. 4D). Further experimental results revealed that RAP-PNP could substantially slow the progression of atherosclerosis by reducing the area of necrotic foci and the number of macrophages, increasing smooth muscle cells, enhancing collagen content, and stabilizing plaques [98].
Fig. 4.
In vivo anti-atherosclerosis efficacy of RAP-PNP (n = 6). A Representative photographs of en face Sudanese red-stained total aortas from each group. Scale bar = 10 mm. B Quantitative percentage of plaque area compared with total luminal surface area. C Oil-Red O detection on the section of aortic roots from mice after different treatments. Scale bar, 500 μm. D H&E staining of major organs of ApoE−/− mice treated with RAP-PNP for 4 weeks (magnification = 200 ×).
Adapted from Ref. [97] with permission
Applications of BNCM for drug delivery in cancer treatments
The relationship between cancer and inflammation
German pathologist Virchow first reported the presence of white blood cells in tumor tissues in 1863 and proposed the relationship between cancer and inflammation [3], getting support from other scholars [4–6]. Inflammatory responses can cause gene mutations and stimulate cell proliferation. Activated inflammatory cells produce reactive oxygen species, nitrogen species, and inflammatory factors, inducing DNA oxidative damage [99].
In chronic inflammation, tissue damage and repair occur continuously in an environment rich in reactive oxygen species, leading to genomic changes, such as point mutations, deletions, or rearrangements, which are key driving forces behind tumorigenesis [99, 100]. Moreover, chronic inflammation can promote cancer growth as it stimulates the formation of new tumor blood vessels by activating vascular endothelial growth factor, providing essential nutrients for tumor growth [92].
Inflammation also facilitates the process of tumor metastasis. Cancer metastasis is a complex, multi-step, dynamic process involving interactions among cancer cells, immune cells, inflammatory cells, and surrounding tissue components. The inflammatory environment creates favorable conditions for tumor metastasis [101]. During tumor progression, cancer cells secrete cytokines like TNF-α, IL-6, IL-8, and chemokines to recruit more inflammatory cells, such as neutrophils, lymphocytes, and natural killer cells, inducing the formation of the tumor inflammatory microenvironment [102]. Given the association of inflammatory cells with tumor growth and metastasis, BNCM can potentially deliver antitumor drugs.
Chemotherapy
Paclitaxel-loaded polymer nanoparticles (PPNs) were fused with membranes of 4T1 breast cancer cells to create cancer cell membrane-modified PPNs (CPPNs) [26]. In mice with orthotopic breast tumor model and lung metastasis, CPPNs selectively accumulated in primary tumors and metastatic lymphoid nodules of the lung, significantly inhibited the growth of primary and metastatic tumors, demonstrating better antitumor efficiency than free paclitaxel.
Inspired by loading DOX into MSN, Huang et al. subsequently encapsulated MSN/DOX with iRGD, a cyclic tumor-penetrating peptide, to modify erythrocyte membranes, creating composite nanoparticles called iRGD-RM-(DOX/MSN) [103]. In an orthotopic breast cancer transplantation model, iRGD-RM-(DOX/MSN) effectively targeted tumors and reduced macrophage phagocytosis, leading to a remarkable tumor growth inhibition rate of 86.29 ± 5.12%.
Photothermal therapy
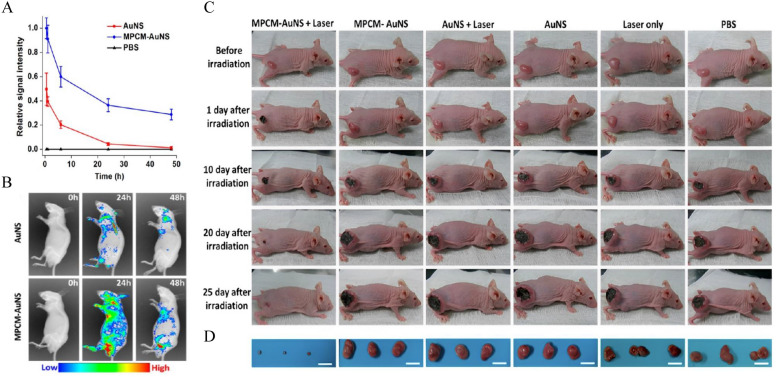
A novel photothermal conversion agent based on macrophage membrane (MPCM)-camouflaged gold nano-shells (AuNS) was designed for the photothermal therapy of cancer [104]. Compared with the bare AuNS, MPCM-AuNS showed significantly enhanced blood retention time, while the bare AuNS was almost eliminated from the blood after 24 h in mice bearing 4T1 tumors (Fig. 5A). In addition, fluorescence imaging confirmed that MPCM-AuNS was significantly accumulated in tumor tissues and had a longer circulation time (Fig. 5B). Further experimental results revealed that tumor tissues in the MPCM-AuNS group were ablated obviously after near-infrared irradiation, and only a black scar was left at the primary tumor site 25 days after irradiation, while tumor volumes in the control groups kept increasing (Fig. 5C, D). Similarly, graphene quantum dots (GQDs) and DOX were encapsulated with glioma homologous cancer cell membranes to synthesize GQDs/DOX@CCM nanoparticles [105]. GQDs/DOX@CCM nanoparticles within actively targeted to mouse glioma cells in vitro. Under laser stimulation, cell membranes on the surface of these nanoparticles began to cleave and release DOX, which significantly increased cell uptake and showed a high efficiency in killing tumor cells.
Fig. 5.
In vivo circulation time, accumulation and antitumor effects of MPCM-AuNs in the 4T1-tumor-bearing mice. In vivo circulation time of the MPCM-AuNSs and AuNSs and their accumulation at the tumor sites. A Relative fluorescence signal intensity of nanoparticles in blood after intravenous injection. B In vivo fluorescence time-lapse imaging of nude mice bearing 4T1 tumor after the intravenous injection of nanoparticles during 48 h. C Photographs of the tumor region were time-lapse acquired and systematically administrated after 5 min of NIR irradiation. D Photographs of relevant tumors originated from each group in C. Scale bar = 2 cm.
Adapted from Ref. [104] with permission
Tumor vaccine
A poly PLGA nano-vaccine encapsulated within erythrocyte membranes (Man-RBC-NPhgp) was developed and loaded with the antigen peptide (hgp10025-33) and monophosphoryl lipid (MPLA), an agonist of TLR4, and embedded mannose on cell membranes [106]. Man-RBC-NPhgp could target the mannose receptors on lymphocytes and enhance lymphocyte uptake. Man-RBC-NPhgp enhanced IFN-γ secretion and CD8 + T cell response in melanoma immunotherapy. Man-RBC-NPhgp induced cytotoxic T lymphocytes with an impressive content of 36.6%, which was significantly higher than free peptide + MPLA (hgp + M) (27.6%) and PLGA-NPhgp + M (28.4%), which highlighted the superiority of nano-tumor vaccines.
Summary and prospect
Although the design and application of BNCM in disease control is still in its infancy, this new type of drug delivery is expected to become a new generation of drug delivery methods. These nanoparticles can be better used in disease diagnosis and treatment according to the biological characteristics of nano-carriers. This technique was initially developed by using erythrocyte membrane coating to prolong the circulation time of nanoparticles. With the deepening of research, various cell membrane types have been used as sources of membranes, including white blood cells, platelets, cancer cells, NK cells, and others.
According to the different biological characteristics of different cell membranes and the flexibility of nano-core materials, the development of BNCM has a broad application prospect. For example, in inflammatory diseases, BNCM represented by neutrophil membranes reaches the focus of inflammation under chemotaxis and releases the drugs carried by nanoparticles after interaction with tissue cells. In the context of cancers, nanoparticles enveloped in cancer cell membranes inherit the unique characteristics of their source cells. This homologous targeting ability allows them to seek out tumor tissues once they enter the bloodstream actively. The continued research and development of BNCM hold great promise for revolutionizing drug delivery and improving disease diagnosis and treatment across various medical fields.
BNCM represents a promising drug delivery method rooted in natural cells, which can enhance drug utilization, mitigate adverse reactions, and enable precise drug targeting. Nonetheless, several issues, including the high receptor affinity, in vivo circulation, cytotoxicity, and clinical transformation, necessitate further optimization to advance this technology. For instance, using many immune cells to prepare BNCM for treating inflammatory diseases could induce or exacerbate inflammation through interactions with the immune system. In the context of mesenchymal stem cells, while their excreted components exhibit anti-inflammatory and immune-related effects, they may also have the unintended consequence of directly or indirectly promoting tumorigenesis or accelerating the progression of existing tumors.
Additionally, the complex process of extracting cell membranes and the need for repeatability pose significant challenges for industrial-scale production. For instance, blood cells extracted from the bloodstream undergo purification, drug loading, and reintegration. During the separation process, it is crucial to maintain their cellular activity. Disruption of the cell structure can trigger the expression of signals like “find me” and “eat me”, leading to phagocytosis. This extraction method presents a substantial challenge.
Moreover, a considerable gap exists between laboratory research and clinical application, and numerous obstacles must be surmounted. While substantial progress has been made in laboratory research, the safety and efficacy of BNCM still need to be verified by high-quality, large-scale data. Therefore, conducting more comprehensive biological investigations is imperative to explore the mechanisms, biological effects, and strategies for mitigating potential toxicity.
Finally, it is essential to mention the establishment of rigorous quality control standards to ensure the absence of contamination during nanoparticle preparation, preventing potential harm arising from immune responses against these particles. While challenges persist in the application of BNCM, it is undeniable that their natural advantages and potential applications hold great promise. This drug delivery system based on natural cell membranes is poised to pioneer a new frontier in drug delivery, sparking renewed interest in nanomedicine design and application.
Acknowledgements
Thanks for all the authors’ contribution to this article.
Author contributions
Shijie Qiu and Feifan Zhu wrote the main manuscript text and Shijie Qiu and Feifan Zhu prepared figures 1–5. Liquan Tong reviewed the manuscript.
Funding
This work was supported by the Fifth Affiliated Hospital of Harbin Medical University in China (2023-001).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript has been approved by all co-authors for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–85. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- 4.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- 5.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian BZ. Inflammation fires up cancer metastasis. Semin Cancer Biol. 2017;47:170–6. [DOI] [PubMed] [Google Scholar]
- 7.Vijayan V, Uthaman S, Park IK. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers (Basel). 2018;10:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Choi Y, Chang H, Um W, Ryu JH, Kwon IC. Alliance with EPR effect: combined strategies to improve the epr effect in the tumor microenvironment. Theranostics. 2019;9:8073–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons TD, Singh R, Sorolla A, Chaudhari N, Hubbard A, Iyer KS. Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir ACS J Surfaces Colloids. 2018;34:15343–9. [DOI] [PubMed] [Google Scholar]
- 10.Najahi-Missaoui W, Arnold RD, Cummings BS. Safe Nanoparticles: are we there yet? Int J Mol Sci. 2020;22:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–48. [DOI] [PubMed] [Google Scholar]
- 12.Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9:675–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, Luo Z, Men Y, Yang P, Peng H, Guo R, Tian Y, Pang Z, Yang W. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials. 2017;143:29–45. [DOI] [PubMed] [Google Scholar]
- 17.Kunde SS, Wairkar S. Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm. 2021;598: 120395. [DOI] [PubMed] [Google Scholar]
- 18.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eladl E, Tremblay-LeMay R, Rastgoo N, Musani R, Chen W, Liu A, Chang H. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y, Sun W, Lin J, Wang T, Fine J, Cheng H, Dotti G, Huang P, Gu Z. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater. 2018;30: e1707112. [DOI] [PubMed] [Google Scholar]
- 22.Xie W, Deng WW, Zan M, Rao L, Yu GT, Zhu DM, Wu WT, Chen B, Ji LW, Chen L, Liu K, Guo SS, Huang HM, Zhang WF, Zhao X, Yuan Y, Dong W, Sun ZJ, Liu W. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano. 2019;13:2849–57. [DOI] [PubMed] [Google Scholar]
- 23.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao Y, Zhang Y, Blum NT, Lin J, Huang P. Biomimetic hybrid membrane-based nanoplatforms: synthesis, properties and biomedical applications. Nanoscale Horiz. 2020;5:1293–302. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Liu Y, He R, Xu D, Zang J, Weeranoppanant N, Dong H, Li Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8:552–68. [DOI] [PubMed] [Google Scholar]
- 26.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang P, Zhang Z, Yu H, Wang S, Li Y. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28:9581–8. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Liu Y, Guo R, Yao X, Sung S, Pang Z, Yang W. Erythrocyte–cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. [DOI] [PubMed] [Google Scholar]
- 28.Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou J, Kim HW, Gao W, Lu W, Zhang L. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials. 2016;111:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, Peng R, Liu Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–9. [DOI] [PubMed] [Google Scholar]
- 30.Rao L, He Z, Meng QF, Zhou Z, Bu LL, Guo SS, Liu W, Zhao XZ. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res A. 2017;105:521–30. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Kai M, Duan Y, Zhou Z, Fang RH, Gao W, Zhang L. Membrane cholesterol depletion enhances enzymatic activity of cell-membrane-coated metal-organic-framework nanoparticles. Angew Chem Int Ed Engl. 2022;61: e202203115. [DOI] [PubMed] [Google Scholar]
- 32.George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm. 2019;561:244–64. [DOI] [PubMed] [Google Scholar]
- 33.Sivaram AJ, Rajitha P, Maya S, Jayakumar R, Sabitha M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:509–33. [DOI] [PubMed] [Google Scholar]
- 34.Zou D, Wu Z, Yi X, Hui Y, Yang G, Liu Y, Tengjisi, Wang H, Brooks A, Wang H, Liu X, Xu ZP, Roberts MS, Gao H, Zhao CX. Nanoparticle elasticity regulates the formation of cell membrane-coated nanoparticles and their nano-bio interactions. Proc Natl Acad Sci U S A. 2023;120:e2214757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore TL, Rodriguez-Lorenzo L, Hirsch V, Balog S, Urban D, Jud C, Rothen-Rutishauser B, Lattuada M, Petri-Fink A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem Soc Rev. 2015;44:6287–305. [DOI] [PubMed] [Google Scholar]
- 36.Yallapu MM, Jaggi M, Chauhan SC. Design and engineering of nanogels for cancer treatment. Drug Discov Today. 2011;16:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi WI, Lee JH, Kim J-Y, Kim J-C, Kim YH, Tae G. Efficient skin permeation of soluble proteins via flexible and functional nano-carrier. J Control Release. 2012;157:272–8. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Maciel D, Rodrigues J, Shi X, Tomas H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem Rev. 2015;115:8564–608. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Ortega LA, Acosta-Osorio AA, Grube-Pagola P, Palmeros-Exsome C, Cano-Sarmiento C, Garcia-Varela R, Garcia HS. Anti-inflammatory activity of curcumin in gel carriers on mice with atrial edema. J Oleo Sci. 2020;69:123–31. [DOI] [PubMed] [Google Scholar]
- 40.Lee AL, Gee CT, Weegman BP, Einstein SA, Juelfs AR, Ring HL, Hurley KR, Egger SM, Swindlehurst G, Garwood M, Pomerantz WCK, Haynes CL. Oxygen sensing with perfluorocarbon-loaded ultraporous mesostructured silica nanoparticles. ACS Nano. 2017;11:5623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Wang J, Gou K, Kang W, Guo X, Zhu K, Li S, Li H. pH/H(2)O(2) dual-responsive chiral mesoporous silica nanorods coated with a biocompatible active targeting ligand for cancer therapy. ACS Appl Mater Interfaces. 2021;13:35397–409. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Q, Zhao Y, Cai P, He Z, Gao F, Zhang J, Gao X. Dose-dependent efficacy of gold clusters on rheumatoid arthritis therapy. ACS Omega. 2019;4:14092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jan N, Madni A, Khan S, Shah H, Akram F, Khan A, Ertas D, Bostanudin MF, Contag CH, Ashammakhi N, Ertas YN. Biomimetic cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for biomedical applications. Bioeng Transl Med. 2023;8: e10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. [DOI] [PubMed] [Google Scholar]
- 45.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4. [DOI] [PMC free article] [PubMed]
- 46.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian H, Luo Z, Liu L, Zheng M, Chen Z, Ma A, Liang R, Han Z, Lu C, Cai L. Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance. Adv Funct Mater. 2017;27:1703197. [Google Scholar]
- 48.Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, Li Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10:7738–48. [DOI] [PubMed] [Google Scholar]
- 49.Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raza F, Zafar H, Zhang S, Kamal Z, Su J, Yuan WE, Mingfeng Q. Recent advances in cell membrane-derived biomimetic nanotechnology for cancer immunotherapy. Adv Healthc Mater. 2021;10: e2002081. [DOI] [PubMed] [Google Scholar]
- 51.Liu T, Shi C, Duan L, Zhang Z, Luo L, Goel S, Cai W, Chen T. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J Mater Chem B. 2018;6:4756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris JC, Scully MA, Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers (Basel). 2019;11:1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He W, Frueh J, Wu Z, He Q. Leucocyte membrane-coated Janus microcapsules for enhanced photothermal cancer treatment. Langmuir. 2016;32:3637–44. [DOI] [PubMed] [Google Scholar]
- 54.Choi S-E, Khoo H, Hur SC. Recent advances in microscale electroporation. Chem Rev. 2022;122:11247–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao L, Cai B, Bu LL, Liao QQ, Guo SS, Zhao XZ, Dong WF, Liu W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–505. [DOI] [PubMed] [Google Scholar]
- 56.Silva AK, Di Corato R, Pellegrino T, Chat S, Pugliese G, Luciani N, Gazeau F, Wilhelm C. Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials. Nanoscale. 2013;5:11374–84. [DOI] [PubMed] [Google Scholar]
- 57.Fan Z, Li PY, Deng J, Bady SC, Cheng H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018;11:5573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez AR, Sanchez-Tarjuelo R, Cravedi P, Ochando J, Lopez-Hoyos M. Review: ischemia reperfusion injury-a translational perspective in organ transplantation. Int J Mol Sci. 2020;21:8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zang X, Zhou J, Zhang X, Han Y, Chen X. Ischemia reperfusion injury: opportunities for nanoparticles. ACS Biomater Sci Eng. 2020;6:6528–39. [DOI] [PubMed] [Google Scholar]
- 61.Chen D-Q, Guo Y, Li X, Zhang G-Q, Li P. Small molecules as modulators of regulated cell death against ischemia/reperfusion injury. Med Res Rev. 2022;42:2067–101. [DOI] [PubMed] [Google Scholar]
- 62.Ou Z, Zhong H, Zhang L, Deng M, Zhang W, Wang J, Feng H, Gong J, Miao C, Yi Z. Macrophage membrane-coated nanoparticles alleviate hepatic ischemia-reperfusion injury caused by orthotopic liver transplantation by neutralizing endotoxin. Int J Nanomedicine. 2020;15:4125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13:1272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Wang Y, Li S, Cui Y, Liang X, Shan J, Gu W, Qiu J, Li Y, Wang G. Functionalized nanoparticles with monocyte membranes and rapamycin achieve synergistic chemoimmunotherapy for reperfusion-induced injury in ischemic stroke. J Nanobiotechnology. 2021;19:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, Liu X, Yang Q, Yu L, Chang Y, Qu M. Neutrophil membrane-enveloped nanoparticles for the amelioration of renal ischemia-reperfusion injury in mice. Acta Biomater. 2020;104:158–66. [DOI] [PubMed] [Google Scholar]
- 66.Chu D, Gao J, Wang Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano. 2015;9:11800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao J, Dong X, Su Y, Wang Z. Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021;123:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang X, Zheng YW, Bao S, Zhang H, Chen R, Yao Q, Kou L. Drug discovery and formulation development for acute pancreatitis. Drug Deliv. 2020;27:1562–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–34. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M, Chen Y, Yang MJ, Fan XR, Xie H, Zhang L, Nie YS, Yan M. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-kappaB pathway. Phytother Res. 2019;33:1191–8. [DOI] [PubMed] [Google Scholar]
- 74.Zhou X, Cao X, Tu H, Zhang ZR, Deng L. Inflammation-targeted delivery of celastrol via neutrophil membrane-coated nanoparticles in the management of acute pancreatitis. Mol Pharm. 2019;16:1397–405. [DOI] [PubMed] [Google Scholar]
- 75.Liu D, Huang S-Y, Sun J-H, Zhang H-C, Cai Q-L, Gao C, Li L, Cao J, Xu F, Zhou Y, Guan C-X, Jin S-W, Deng J, Fang X-M, Jiang J-X, Zeng L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel S, Puri N, Dellinger RP. Sepsis management for the nephrologist. Clin J Am Soc Nephrol. 2022;17:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gauthier AE, Rotjan RD, Kagan JC. Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biol. 2022;12: 220146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, Zhang L. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114:11488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan S, Zhong W, Lan Y, Yu S, Yang L, Yang F, Li J, Gao X, Song J. Pathology-guided cell membrane-coated polydopamine nanoparticles for efficient multisynergistic treatment of periodontitis. Adv Funct Mater. 2024;34:2312253. [Google Scholar]
- 80.Huang J, Fu X, Chen X, Li Z, Huang Y, Liang C. Promising therapeutic targets for treatment of rheumatoid arthritis. Front Immunol. 2021;12: 686155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 82.Wright HL, Cox T, Moots RJ, Edwards SW. Neutrophil biomarkers predict response to therapy with tumor necrosis factor inhibitors in rheumatoid arthritis. J Leukoc Biol. 2017;101:785–95. [DOI] [PubMed] [Google Scholar]
- 83.Dolati S, Sadreddini S, Rostamzadeh D, Ahmadi M, Jadidi-Niaragh F, Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed Pharmacother Biomedecine & Pharmacotherapie. 2016;80:30–41. [DOI] [PubMed] [Google Scholar]
- 84.Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell’Accio F, Pitzalis C, Oliani SM, Jan LY, Perretti M. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7:315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, Fang RH, Gao W, Zhang L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182–90. [DOI] [PubMed] [Google Scholar]
- 86.He Y, Li R, Liang J, Zhu Y, Zhang S, Zheng Z, Qin J, Pang Z, Wang J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018;11:6086–101. [Google Scholar]
- 87.Li R, He Y, Zhu Y, Jiang L, Zhang S, Qin J, Wu Q, Dai W, Shen S, Pang Z, Wang J. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19:124–34. [DOI] [PubMed] [Google Scholar]
- 88.Wright EK, Ding NS, Niewiadomski O. Management of inflammatory bowel disease. Med J Aust. 2018;209:318–23. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi M, Ota S, Nishimura S, Ogura K, Maeda S, Toda N, Hamada E, Terano A, Omata M. Keratinocyte growth factor is an endogenous stimulant of rabbit gastric epithelial cell proliferation and migration in primary culture. J Gastroenterol Hepatol. 1996;11:1089–96. [DOI] [PubMed] [Google Scholar]
- 90.Sadeghi S, Kalhor H, Panahi M, Abolhasani H, Rahimi B, Kalhor R, Mehrabi A, Vahdatinia M, Rahimi H. Keratinocyte growth factor in focus: a comprehensive review from structural and functional aspects to therapeutic applications of palifermin. Int J Biol Macromol. 2021;191:1175–90. [DOI] [PubMed] [Google Scholar]
- 91.Zhao YZ, ZhuGe DL, Tong MQ, Lin MT, Zheng YW, Jiang X, Yang WG, Yao Q, Xiang Q, Li XK, Xu HL. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J Control Release. 2019;299:90–106. [DOI] [PubMed] [Google Scholar]
- 92.Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F, Zhang XL. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest. 2014;43:638–52. [DOI] [PubMed] [Google Scholar]
- 93.Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, McKay DM. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405. [DOI] [PubMed] [Google Scholar]
- 94.Sun T, Kwong CHT, Gao C, Wei J, Yue L, Zhang J, Ye RD, Wang R. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10:10106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, Zang G, Li J, Li X, Li Y, Zhao Y. Cell-derived biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: predecessors and perspectives. Regen Biomater. 2020;7:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song Y, Huang Z, Liu X, Pang Z, Chen J, Yang H, Zhang N, Cao Z, Liu M, Cao J, Li C, Yang X, Gong H, Qian J, Ge J. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE(-/-)) mice. Nanomedicine. 2019;15:13–24. [DOI] [PubMed] [Google Scholar]
- 98.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–32. [DOI] [PubMed] [Google Scholar]
- 99.Kay J, Thadhani E, Samson L, Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst). 2019;83: 102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell ALM, Sweasy JB. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49:116–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hibino S, Kawazoe T, Kasahara H, Itoh S, Ishimoto T, Sakata-Yanagimoto M, Taniguchi K. Inflammation-induced tumorigenesis and metastasis. Int J Mol Sci. 2021;22:5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou S, Lu J, Liu S, Shao J, Liu Z, Li J, Xiao WA. Role of the tumor microenvironment in malignant melanoma organoids during the development and metastasis of tumors. Front Cell Dev Biol. 2023;11:1166916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang J, Lai W, Wang Q, Tang Q, Hu C, Zhou M, Wang F, Xie D, Zhang Q, Liu W, Zhang Z, Zhang R. Effective triple-negative breast cancer targeted treatment using iRGD-modified RBC membrane-camouflaged nanoparticles. Int J Nanomedicine. 2021;16:7497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–8. [DOI] [PubMed] [Google Scholar]
- 105.Ren Y, Miao C, Tang L, Liu Y, Ni P, Gong Y, Li H, Chen F, Feng S. Homotypic cancer cell membranes camouflaged nanoparticles for targeting drug delivery and enhanced chemo-photothermal therapy of glioma. Pharmaceuticals (Basel). 2022;15:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, Kong M, Qi Y, Tan S, Zhang Z. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.