Abstract
Background
The study aimed to examine the prevalence of gingival cancers compared to other oral mucosal sites, analyze patient profiles, and identify risk factors.
Materials and Methods
A retrospective monocentric study was conducted at the Department of Oral Medicine of Bretonneau Hospital in Paris, France. 32 patients diagnosed with oral mucosal cancer were included. Data extracted from electronic medical records encompassed patient demographics, cancer type, lesion location, and tobacco/alcohol use.
Results
46.9% were diagnosed with gingival cancer. Patients with gingival cancer had a mean age of 74.2 years old, higher than the mean age of 63.9 years old for those with non-gingival cancer. Men accounted for 60% of cases in the gingival cancer group. Squamous cell carcinoma was the predominant cancer type observed in both gingival and non-gingival cancers. Notably, 26.7% of gingival cancer patients used both alcohol and tobacco, all of them male. Among non-gingival cancer patients, 23.5% used both substances, with both sexes represented.
Conclusion
This study provides insights into the higher prevalence of oral squamous cell carcinoma among men with risk factors and highlights characteristics of gingival squamous cell carcinoma. Effective management strategies should include comprehensive clinical assessments to ensure early detection and intervention for improved outcomes.
Keywords: Anatomic location, Gingival cancer, Oral cancer, Oral squamous cell carcinoma
Introduction
In 2020, lips and oral cavity cancers ranked as the 16th most diagnosed cancer site [1]. Oral squamous cell carcinoma (OSCC) is the most frequently encountered histological subtype, accounting for over 90% of oral cavity cancers [2]. Overall, the 5-year survival rate for OSCC is 50%, although the prognosis depends on the location within the oral cavity, the presence of lymph node involvement, the presence of metastasis [3], the patient’s age, and patient comorbidities [4]. The high mortality rate of oral cancers is often attributed to a lack of awareness regarding these malignant tumors, which leads to delayed identification, diagnosis, and treatment, despite the easy accessibility of the oral cavity for clinical examination [5].
Oral cavity cancer primarily affects men over the age of 60, although the gender balance is shifting. Within younger age groups, women are experiencing an increasing incidence, mainly due to lifestyle changes [6]. The main risk factors for oral cavity cancer include tobacco and alcohol consumption [7, 8], betel quid use, and diet [9, 10]. A multicenter study conducted by Radoï et al., showed that 94% of oral cavity cancers in men can be explained by known or suspected risk factors, while the same statistic drops to 74% in women, suggesting the existence of unidentified factors [10]. Therefore, it is imperative to identify potentially malignant disorders and to conduct systematic screening for early diagnosis [11].
The type of cancer, location, TNM stage, and the patient’s condition determines therapeutic options. They include surgery, radiotherapy, chemotherapy, or a combination of the three [12].
Gingival squamous cell carcinoma (GSCC) is a less frequent location for OSCC, accounting for approximately 25% of cases, although results from studies range from less than 10–30% [13]. Fitzpatrick et al., examined the differential diagnoses submitted by practitioners when they encountered a gingival lesion that later turned out to be cancer. They found that 36% of the differential diagnoses did not include any form of malignancy [13]. Initially, GSCC can be mistaken for benign inflammatory conditions such as periodontal disease [14, 15] since patients are often asymptomatic, and GSCC related bone resorption may lead to tooth mobility, pocketing, and suppuration, which are nonspecific clinical signs that can result in misdiagnosis and delayed treatment [16]. In addition to periodontal diseases, differential diagnosis of GSCC also include benign tumors and immune-mediated diseases such as lichen planus.
Seoane et al., found that 75% of GSCC cases were diagnosed within 90 days of initial symptoms; however, the diagnosis was associated with advanced stages of disease, reflective of early bone invasion [17].
Verrucous carcinoma (VC) of the oral cavity is a rare variant of OSCC that exhibits less aggressive growth and less metastatic potential [18]. Early identification of VC is essential for improving patient prognosis.
The aims of this study were to examine, in our hospital, the prevalence of gingival cancers (GC) compared to other oral mucosal sites and the characteristics of patients with GC to demonstrate that this specific location is not uncommon, with the intention of encourage early detection.
Materials and methods
Ethical considerations
This retrospective monocentric cross-sectional study was conducted, and reported in accordance with the principles established by the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. All participants were verbally informed about the potential use of their anonymized medical data for research purposes and their non-objection was collected. All participants had the right to withdraw from the study at any time by completing a dedicated online form (http://recherche.aphp.fr/eds/droit-opposition). According to French law (loi Jardé), anonymous monocentric retrospective studies do not require institutional review board approval. The study protocol was approved by the French National Data Processing and Liberties Commission (CNIL) (N° 2217408 v0). The need for consent was deemed unnecessary according to the Paris Cite University Research Ethics Committee.
Study design and participants
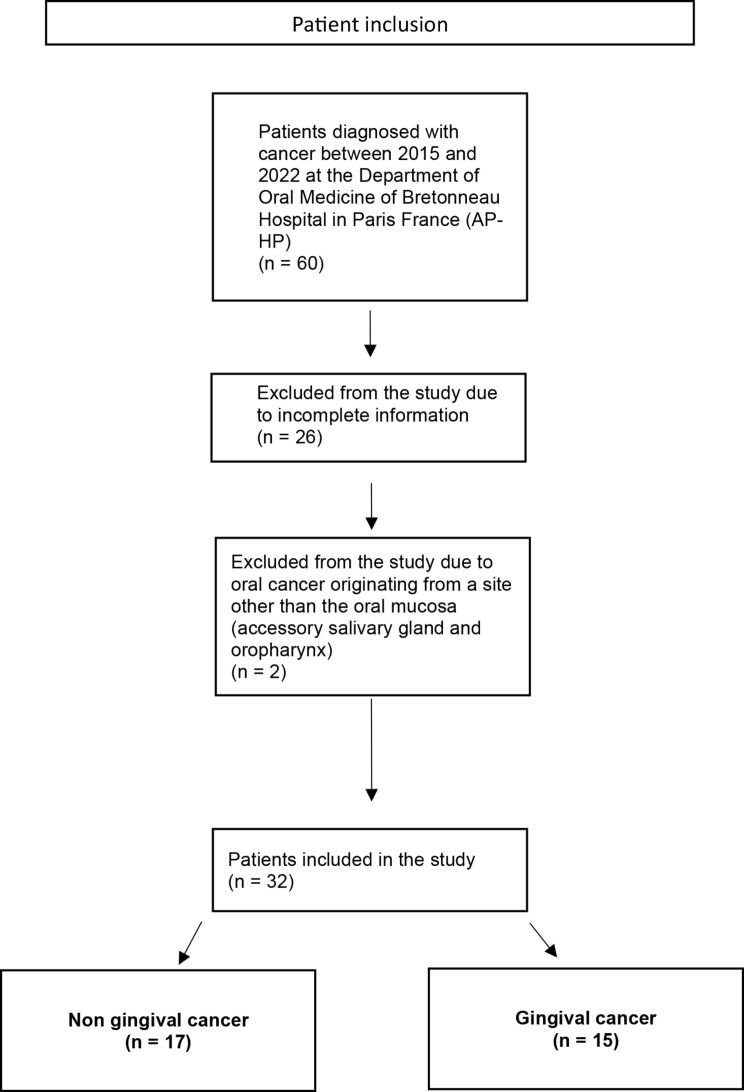
This study was conducted between 2015 and 2022 at the Department of Oral Medicine of Bretonneau Hospital in Paris, France (AP-HP). A total of 60 patients were diagnosed with oral cancers during this period and were referred and treated by a specialist department (Curie Institute, Otolaryngology department, Paris) where they are being monitored. We included 32 patients diagnosed with OSSC or VC cancer, confirmed by histological analysis. We excluded 28 patients : 26 due to incomplete medical records information, and 2 with oral cancer originating from a site other than the oral mucosa (one at an accessory salivary gland of the palate and the other at the level of the oropharynx) (Fig. 1). All included cases were confirmed to be primary oral cancers, with no evidence of metastatic origin.
Fig. 1.
Flow chart explaining how we include patients in the study
Grouping protocol
The included patients were divided into two groups based on tumor location:
Gingival Cancer Group (GC): This group consisted of 15 patients with histologically confirmed cancers originating from the attached gingiva or the free gingival margin or from the edentulous crest in the absence of teeth.
Non-Gingival Cancer Group (NGC): This group included 17 patients with histologically confirmed cancers originating from other oral mucosal sites: tongue, floor of mouth, buccal mucosa, hard and soft palate.
Data collection
Patient data were extracted from ORBIS, the electronic medical records software of the Assistance Publique des Hôpitaux de Paris (AP-HP). For each patient, we collected the following variables: date of birth, gender, date of diagnosis, the cancer type (histological diagnosis), lesion location (gingiva or other oral mucosal site), tobacco use (current or past) and alcohol consumption (current or past).
Data was reported following the STROBE guidelines.
Data analysis
Descriptive statistics were used to analyze the data. Frequencies and percentages were calculated for qualitative variables, whilemeans, standard deviations, minimums, and maximums were calculated for quantitative variables.
Study objectives
The primary objective of the study was to analyze the prevalence of GC compared to cancers of other oral mucosal sites. The secondary objectives were to compare the patient profiles and their risk factors between theNGC groups.
Statistical software
All statistical analyses and figure generation were performed using R software (R Core Team 2020).
Results
General characteristics of the cohort
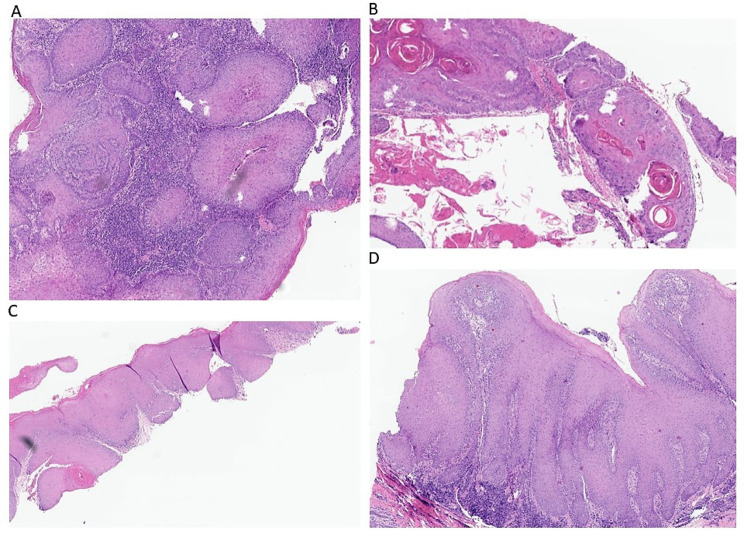
Data regarding the general characteristics of the cohort of 32 patients were summarized in Table 1. All patients had a diagnosis of oral mucosal cancer confirmed by histological analysis (Fig. 2A, B, C and D).
Table 1.
General characteristics of the cohort
| Age (Years) | Sex | Type of cancer | Extension | Alcohol | Tobacco |
|---|---|---|---|---|---|
| Patients with gingival cancers | |||||
| 71 | M | SCC | Yes | 1 | 1 |
| 59 | M | SCC | Yes | 1 | 1 |
| 71 | F | VC | No | 0 | 0 |
| 58 | F | SCC | No | 0 | 1 |
| 85 | M | SCC | No | 1 | 1 |
| 81 | F | SCC | No | NA | NA |
| 63 | M | SCC | Yes | NA | NA |
| 78 | M | SCC | No | 1 | 1 |
| 83 | M | SCC | No | 0 | 0 |
| 65 | M | SCC | No | 0 | 0 |
| 75 | M | VC | No | NA | 1 |
| 91 | F | VC | Yes | 0 | 0 |
| 85 | F | VC | Yes | 0 | 0 |
| 70 | F | VC | No | 0 | 0 |
| 36 | M | SCC | No | 0 | 0 |
| Mean : 74,2 | |||||
| Patients with non-gingival cancers | |||||
| 75 | M | SCC | Yes | 1 | 1 |
| 54 | F | VC | No | 0 | 1 |
| 67 | M | SCC | No | 0 | 1 |
| 61 | M | SCC | No | NA | NA |
| 59 | F | SCC | No | NA | 1 |
| 54 | M | SCC | No | NA | NA |
| 58 | F | SCC | No | 1 | 1 |
| 88 | F | SCC | No | 0 | 0 |
| 77 | F | VC | No | 0 | 0 |
| 75 | F | VC | No | NA | NA |
| 57 | F | SCC | No | NA | 1 |
| 90 | F | SCC | Yes | NA | NA |
| 63 | M | SCC | No | NA | NA |
| 59 | M | SCC | Yes | 0 | 1 |
| 62 | M | VC | No | NA | NA |
| 49 | M | SCC | No | 1 | 1 |
| 61 | F | VC | No | 1 | 1 |
| Mean : 63,9 | |||||
Summary table of the general data for patients with gingival and non-gingival cancers. The following parameters were analyzed for each patient: age, gender, cancer type, presence of extension to adjacent mucosal areas, and alcohol and tobacco consumption. M: Male, F: Female, SCC: Squamous cell carcinoma, VC: Verrucous carcinoma, NA: No answer
Fig. 2.
Histological sections of A : an SCC of the gingiva ((H&E stain, x 30). B: an SCC of the palate (H&E stain, x 40). C : an VC of the gingiva (H&E stain, x 30). D: an VC of the tongue (H&E stain, x 30)
Among the cohort, 46.9% were female and 53.1% were male (15 females/17 males), with an average age of 68.1 years old (+/- 13 years old; range 36–91) at the time of diagnosis. Notably, females in the cohort tended to be older, with a mean age of 71.7 (range 54–91) years old compared to 65 years old for males (range 36–83).
Regarding localization, 17 had non-gingival localization, and 15 had gingival localization.
In terms of cancer types, squamous cell carcinoma (SCC) represented 68.8% of diagnoses, of which 31.8% were women and 68.2% were men (7 females/15 males).
VC represented 31.2% of diagnoses, of which 80% were women and 20% were men (8 females/2 males).
Tumor extension to adjacent mucosal areas was observed in 25% of patients, while the cancer remained localized to a single anatomical site in 75% of cases.
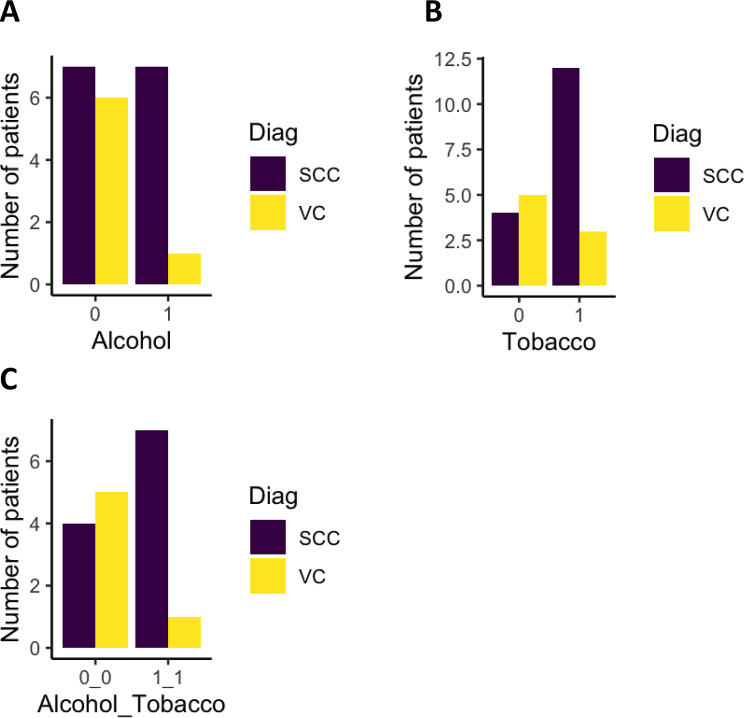
Tobacco and alcohol are the two primary risk factors for SCC and VC. Alcohol use was present in 25% of overall cases (Fig. 3A), with a prevalence of 25% in females and 75% in males. Among alcohol users, 87.5% were diagnosed with SCC and 12.5% with VC. Tobacco use was present in 46.9% of the cohort (Fig. 3B), with a prevalence of 40% in females and 60% in males. Among tobacco users, 80% developed SCC, and 20% developed VC.
Fig. 3.
General characteristics of the cohort in relation to risk factors. “Diag” corresponds to the diagnosis. SCC: Squamous cell carcinoma, and VC: Verrucous carcinoma. “0” indicates the absence of use of one or two risk factors, while “1” indicates the presence of use of these risk factors
Finally, the association between these two risk factors was analyzed. 25% of patients used both alcohol and tobacco (Fig. 3C), representing 25% of females and 75% of males. Among them, 87.5% developed SCC, and 12.5% developed VC.
Characteristics of gingival cancers compared to non-gingival cancer
Out of the 32 patients included in this study, 46.9% of oral cancers were present on the gingiva, while 53.1% had non-gingival localization.
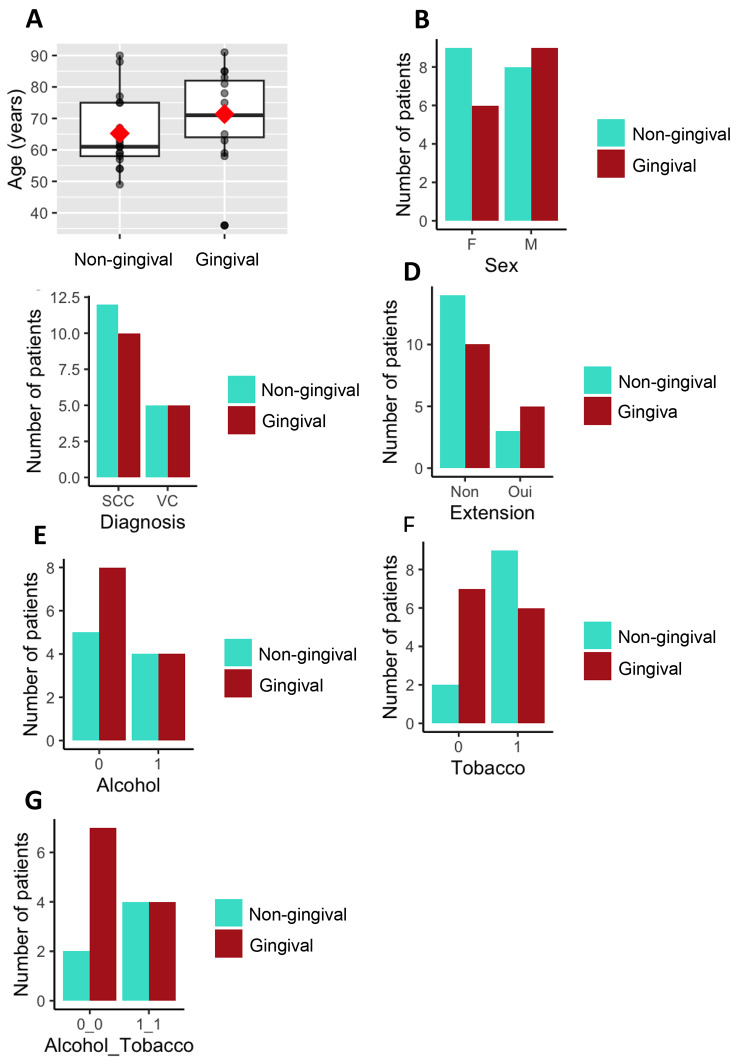
The mean age of patients with GC was 74.2 years old (+/- 13.9 years old; range 36–91), compared to 63.9 years old (+/- 11.8 years old; range 49–88) for NGC (Fig. 4A). Among patients with GC, females had a mean age of 76 years old (+/- 11.9 years old; range 58–91), while males had a mean age of 68.3 years old (+/- 15 years old; range 36–85). For NGC, females had a mean age of 68.8 years old (+/- 13.9 years old; range 54–90), and males had a mean age of 61.3 years old (+/- 7.9 years old; range 49–75).
Fig. 4.
Characteristics of patients diagnosed with gingival cancer. A: Box plot of age at diagnosis according to tumor localization. The red diamond represents the mean, and the horizontal bar in the box plots represents the median. B to G: Number of patients based on different characteristics. F stands for female, and M stands for male. In red, patients with gingival localization and in cyan, patients with non-gingival localization. “0” indicates no alcohol or tobacco consumption and “1” indicate the use of one of them. G: Number of patients with either alcohol and tobacco co-intoxication or no intoxication in the gingival cancer group (CG) and non-gingival cancer group (CNG)
In terms of gender distribution, 40% (6 out of 15) of patients diagnosed with GC were female, while 60% (9 out of 15) were male. Among patients with NGC, 53% (9 out of 17) were female, and 47% (8 out of 17) were male (Fig. 4B).
Within the group of GC patients, 66.7% (10 out of 15) had SCC, with females representing 20% (2 out of 10) and males representing 80% (8 out of 10). VC was present in 33.3% of the GC cases. Among the patients with gingival VC, 80% (4 out of 5) were females, and 20% (1 out of 5) were males (Fig. 4C).
Among patients with NGC, 70.6% (12 out of 17) had SCC, with 41.7% (5 out of 12) being females and 58.3% (7 out of 12) being males. Within this group, 29.4% (5 patients) had VC, with 80% (4 out of 5) were females and 20% (1 out of 5) were males (Fig. 4C).
Regarding tumor localization, 93% of the GC were diagnosed in the mandible, and 7% in the maxilla. Among the GC, 60% occurred in the premolar-molar region, while 40% occurred in the anterior region.
Tumor extension to adjacent mucosal areas was observed in 33.3% (5 out of 15) of the GC and 17.6% (3 out of 17) of the NGC (Fig. 4D).
Regarding the presence of risk factors, only 28.1% (9 out of 32) of the patients had not neither smoked nor used alcohol. Among this group, 66.7% were females and 33.3% were males (4 females and 3 males for GC, and 2 females for NGC).
Among patients with GC, 26.7% (4 out of 15) used alcohol, and all were male. In the NGC group, 23.5% (4 out of 17) used alcohol, with an equal distribution between females and males (2 each) (Fig. 4E). Among the GC patients with alcohol intoxication, all had SCC, while in the NGC group, 75% (3 out of 4) had SCC and 25% (1 out of 4) had VC.
Tobacco use was found in 40% (6 out of 15) of patients with GC, including 1 female and 5 males. In the NGC group, 52.9% (9 out of 17) of patients were tobacco users, including 5 females and 4 males (Fig. 4F). Among patients with GC and tobacco use, 83.3% developed SCC and 16.7% developed VC. For patients with NGC, 77.8% developed SCC and 22.2% developed VC.
Lastly, 26.7% (4 out of 15) of patients with GC had alcohol and tobacco co-intoxication, and all were male. Among the NGC patients, 23.5% (4 out of 17) had co-intoxication, including 2 females and 2 males (Fig. 4G). All GC patients who consumed both alcohol and tobacco developed SCC, while in the NGC group, 75% (3 out of 4) developed SCC and 25% (1 out of 4) developed VC.
Discussion
This study compared gingival and NGC in terms of age, gender distribution, cancer type, extension into adjacent tissues, and presence of risk factors.
A 2016 systematic review by Bark et al. [19] identified 61 studies on gingival cancers, concluding that these cancers are rare, understudied, and often grouped with other oral cavity cancers in research.
Our retrospective, observational, monocentric study focused on the prevalence and the profiles of patients who developed GC compared to other locations in the oral mucosa. 32 patients from the Department of Oral Medicine at Bretonneau Hospital in Paris (France) between 2015 and 2022 were included.
A multicentric study published in 2018 reported a prevalence of 16% for GC among individuals aged over 65 years old [20]. In our cohort, nearly three times more patients had GC (46.9%).
The incidence of oral cavity cancer is higher in individuals aged between 60 and 80 years old, and slightly higher in men than in women [20]. In our cohort, the average age was 68.1 years old, with 46.9% women and 53.1% men, which is consistent with the literature. Among the 32 patients, 46.9% had GC, with an average age of 74.2 years old, compared to 63.9 years old for NGC.
It is commonly accepted that GC more frequently affect the mandible than the maxilla, with 60% of cases occurring in the premolar-molar region [21], which aligns with our patient cohort. Ohyama et al. (2022) [22] further emphasized that maxillary GSCC located in the posterior region are associated with a significantly poorer prognosis, with a 5-year survival rate of only 63.8%.
SCC is the most common type of oral cavity cancer [21]. In their review, only 10% of SCCs were found on the gingiva, predominantly affecting elderly women, which is consistent with the findings of Koch et al., who also reported a higher frequency of VC in the gingiva. In this present study, 31.3% of GC were SCCs, with only 20% occurring in women. However, 66.7% of patients with GC were diagnosed with SCC, in disagreement with the results of Koch et al., and Bharanidharan et al. [21, 23]. This difference may be explained by the fact that the patient cohort of Koch et al. also includes the larynx, pharynx and hypopharynx [23].
Oral cavity cancer is multifactorial, with numerous risk factors, including alcohol consumption, tobacco use, chronic inflammation, ultraviolet radiation, human papillomavirus, immunosuppression, genetic predisposition, oral candidiasis, lichen planus, or any potentially transforming lesion [24]. It is well established that tobacco and alcohol are the two primary risk factors for oral cancer [9]. In our study, 26.7% of patients diagnosed with GC had alcohol intoxication, 40% had tobacco intoxication, and 26.7% had dual alcohol and tobacco intoxication, while 46.66% (7 patients) had no intoxication, which is in accordance with Amezaga-Fernandez (2024) [25]. Several hypotheses could explain the lower proportion of patients with intoxication in our study. First, these analyses were based on patient’s report, second, for some patients we did not have the information. Some authors suggest that dysbiosis of the oral microbiota could contribute to the development of OSCC [26–28], indicating the possibility of using certain bacteria as biomarkers for the onset of oral cavity cancer especially Fusobacterium.
In a study published in 2019 the gingival mucosa is one of the anatomical sites in the oral cavity where potentially malignant lesions are detected at a later stage. Indeed, these cancers are deeper according to the authors, resulting in a 6 to 10 times higher risk of developing cancer relative to lesions on the lips [29]impacting the prognosis and increasing mortality. In our study, extension of lesions to adjacent tissues was more significant in GC than NGC. Yoshida et al. (2018) [30] highlighted the prognostic significance of medullary bone invasion in gingival squamous cell carcinoma, showing it as an independent predictor of poorer overall survival. Moreover, the first signs are subtle and they are often misdiagnosed because they can initially mimic benign periodontal conditions [31]. Clinical examination alone is not sufficient for a definitive diagnosis, and additional histopathological examination is mandatory. Similarly, Arduino et al. (2021) [32] reported that the management of GSCC remains challenging, with a high proportion of late-stage diagnoses and poor survival outcomes. Their study showed that 70% of GSCC cases were diagnosed at stage IV, with a 5-year survival rate of only 43%, underscoring the need for earlier detection and intervention. This delay in diagnosis invariably results in a more advanced tumor stage, which negatively affects the survival rate (Niu et al. 2017) [33]. These authors have also proposed a prognostic index (classifying patients into low, moderate and high-risk groups) considering the tumor stage, the pathological neck status, the extracapsular spread, the perineural invasion and the differentiation of the tumor. Since then, other authors have been interested in the prognosis of cancers, particularly gingival cancers, trying to improve the current classification (TNM) using nomogram to predict the survival outcome of gingival cancer [34].
The importance of conducting a comprehensive clinical examination is crucial for the early detection of malignant or potentially malignant disorders. In our cohort, four oral cancers were diagnosed in patients with a history of oral lichen planus, one with verrucous leukoplakia, and one with Fanconi’s disease, a genetic condition known to be a risk factor for cancer development [35, 36].
The small number of patients included in our cohort may introduce biases in the results, although the selection of these patients was independent of cancer location. A second bias in this study is the choice of a monocentric model, but it provides an insight into the profile of patients with cancer that a single department may have to manage.
In the future, it would be beneficial to expand the cohort size by collaborating with other hospitals and other specialties such as otolaryngology and maxillofacial surgery.
Our cohort highlights a significant incidence of GC, which cannot be considered a rare entity. Due to the wide spectrum of clinical presentations, an ill-informed dentist may inadvertently misdiagnose a cancerous lesion as a manifestation of periodontal disease. To improve identification of oral malignancy, a more systematic diagnostic approach must be fostered. Clinician education should center on critical thinking and enumeration of differential diagnoses based on rigorous clinical examination. Educational models could refer to methods of teaching or physical models. Despite receiving appropriate treatment at Curie Institute (Paris, France), 5 patients have died to date and among them 4 had GC.
These findings underscore the importance of comprehensive clinical assessments to improve the management of patients with malignant gingival lesions.
Acknowledgements
We would like to thank Dr François Le Pelletier de Glatigny (Praxea Laboratory) who carried out the histological analyses and the photographs of histological slides.
Abbreviations
- OSCC
Oral Squamous Cell Carcinoma
- GSCC
Gingival Squamous Cell Carcinoma
- VC
Verrucous Carcinoma
- GC
Gingival Cancers
- NGC
Non-Gingival Cancer
- CNIL
French National Data Processing and Liberties Commission
- AP-HP
Assistance Publique des Hôpitaux de Paris
Author contributions
Concept and design: YS, ALE. Acquisition, analysis, and interpretation of data: YS, CML, ALE. Writing—original draft preparation: YS, ALE. Writing—review and editing: YS, ALE, MTC, CML. Statistical analyzes: YS. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The study was approved by the French National Data Processing and Liberties Commission (CNIL) (N° 2217408 v0). This study was approved by the Paris Cite University Research Ethics Committee (Reference: 2024-06-03 / N° IRB: IORG0010044). All participants were verbally informed about the potential use of their anonymized medical data for research purposes and their non-objection was collected. All participants had the right to withdraw from the study at any time by completing a dedicated online form (http://recherche.aphp.fr/eds/droit-opposition). According to French law (loi Jardé), anonymous monocentric retrospective studies do not require institutional review board approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Wallace ML, Neville BW. Squamous cell carcinoma of the gingiva with an atypical appearance. J Periodontol. 1996;67(11):1245–8. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T, Tsukahara K, Kawakita D, Yoshimoto S, Miura K, Sugasawa M, et al. Macroscopic and multiple metastases in sentinel lymph node biopsy are respectively associated with poor prognosis in early oral cancer. Int J Clin Oncol. 2023;28(4):512–20. [DOI] [PubMed] [Google Scholar]
- 4.Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015;33(29):3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16. [DOI] [PubMed] [Google Scholar]
- 6.Neville Brad W. Oral and maxillofacial pathology. 4th edition. Saint Louis: Elsevier; 2016. xiii + 912.
- 7.Radoï L, Paget-Bailly S, Guida F, Cyr D, Menvielle G, Schmaus A, et al. Family history of cancer, personal history of medical conditions and risk of oral cavity cancer in France: the ICARE study. BMC Cancer. 2013;13(1):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chieng CY, Dalal A, Ilankovan V. Occupational exposure and risk of oral and oropharyngeal squamous cell carcinoma: systematic review and 25-year retrospective cohort study of patients. Br J Oral Maxillofac Surg janv. 2023;61(1):39–48. [DOI] [PubMed] [Google Scholar]
- 9.Petti S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009;45(4–5):340–50. [DOI] [PubMed] [Google Scholar]
- 10.Radoï L, Menvielle G, Cyr D, Lapôtre-Ledoux B, Stücker I, Luce D. Population attributable risks of oral cavity cancer to behavioral and medical risk factors in France: results of a large population-based case–control study, the ICARE study. BMC Cancer. 2015;15(1):827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González‐Moles M, Kerr AR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating centre for oral Cancer. Oral Dis. 2021;27(8):1862–80. [DOI] [PubMed] [Google Scholar]
- 12.Sundermann BV, Uhlmann L, Hoffmann J, Freier K, Thiele OC. The localization and risk factors of squamous cell carcinoma in the oral cavity: a retrospective study of 1501 cases. J Cranio-Maxillofac Surg. 2018;46(2):177–82. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick SG, Neuman AN, Cohen DM, Bhattacharyya I. The clinical and histologic presentation of gingival squamous cell carcinoma: a study of 519 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(4):509–15. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Debnath N, Nayak PA, Khandelwal V. Gingival squamous cell carcinoma presenting as periodontal lesion in the mandibular posterior region. BMJ Case Rep. 2014;2014:bcr2013202511. 10.1136/bcr-2013-202511. PMID: 25139914; PMCID: PMC4139557. [DOI] [PMC free article] [PubMed]
- 15.Cabral LA, de Carvalho LF, Salgado JA, Brandão AA, Almeida JD. Gingival squamous cell carcinoma: a case report. J Oral Maxillofac Res. 2010;1(3):e6. 10.5037/jomr.2010.1306. PMID: 24421976; PMCID: PMC3886055. [DOI] [PMC free article] [PubMed]
- 16.Sloan P. Squamous cell carcinoma and precursor lesions: clinical presentation. Periodontol 2000. 2011;57(1):10–8. [DOI] [PubMed] [Google Scholar]
- 17.Seoane J, Warnakulasuriya S, Varela-Centelles P, Esparza G, Dios PD. Oral cancer: experiences and diagnostic abilities elicited by dentists in North-western Spain. Oral Dis. 2006;12(5):487–92. [DOI] [PubMed] [Google Scholar]
- 18.Walvekar RR, Chaukar DA, Deshpande MS, Pai PS, Chaturvedi P, Kakade A, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45(1):47–51. [DOI] [PubMed] [Google Scholar]
- 19.Bark R, Mercke C, Munck-Wikland E, Wisniewski NA, Hammarstedt-Nordenvall L. Cancer of the gingiva. Eur Arch Otorhinolaryngol. 2016;273(6):1335–45. [DOI] [PubMed] [Google Scholar]
- 20.Dhanuthai K, Rojanawatsirivej S, Thosaporn W, Kintarak S, Subarnbhesaj A, Darling M et al. Oral cancer: a multicenter study. Med Oral Patol Oral Cir Bucal. 2018;23(1):e23–9. 10.4317/medoral.21999. PMID: 29274153; PMCID: PMC5822535. [DOI] [PMC free article] [PubMed]
- 21.Bharanidharan R, Dineshkumar T, Raghavendhar K, Kumar A. Squamous cell carcinoma of the gingiva: a diagnostic enigma. J Oral Maxillofac Pathol. 2015;19(2):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohyama Y, Yamashiro M, Michi Y, Uzawa N, Myo K, Sonoda I, et al. Determination of significant prognostic factors for maxillary gingival squamous cell carcinoma in 90 cases. Indian J Otolaryngol Head Neck Surg. 2022;74(S3):5930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch BB, Trask DK, Hoffman HT, Karnell LH, Robinson RA, Zhen W, et al. National survey of head and neck verrucous carcinoma: patterns of presentation, care, and outcome. Cancer. 2001;92(1):110–20. [DOI] [PubMed] [Google Scholar]
- 24.Alnuaimi AD, Wiesenfeld D, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. Oral candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral Oncol. 2015;51(2):139–45. [DOI] [PubMed] [Google Scholar]
- 25.Amezaga-Fernandez I, Aguirre-Urizar JM, Suárez-Peñaranda JM, Chamorro-Petronacci C, Lafuente-Ibáñez de Mendoza I, Marichalar-Mendia X et al. Comparative clinicopathological study of the main anatomic locations of oral squamous cell carcinoma. Oral Dis. 2024. 10.1111/odi.14971 Epub ahead of print. PMID: 38693647. [DOI] [PubMed]
- 26.Yu YM, Zhou BH, Yang YL, Guo CX, Zhao J, Wang HW. Estrogen deficiency aggravates fluoride-induced liver damage and lipid metabolism disorder in rats. Biol Trace Elem Res. 2022;200(6):2767–76. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Huang JJ, Pan WL, Chan CP. Gingival mass as the primary manifestation of multiple myeloma: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod Juill. 1996;82(1):75–9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Liu Y, Zheng HJ, Zhang CP. The oral microbiota may have influence on oral cancer. Front Cell Infect Microbiol. 2020;9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su WWS, Su CW, Chang DC, Chuang SL, Chen SLS, Hsu CY, et al. Impact of varying anatomic sites on advanced stage and survival of oral cancer: 9-year prospective cohort of 27 717 cases. Head Neck. 2019;41(5):1475–83. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida S, Shimo T, Murase Y, Takabatake K, Kishimoto K, Ibaragi S, et al. The Prognostic implications of Bone Invasion in Gingival squamous cell carcinoma. Anticancer Res. 2018;38(2):955–62. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011;3(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arduino PG, Carbone M, Gambino A, Cabras M, Cannarsa F, Macciotta A et al. Challenging management of gingival squamous cell carcinoma: a 10 years single-center retrospective study on Northern-Italian patients. Med Oral Patol Oral Cir Bucal. 2021;26(1):e21–7. 10.4317/medoral.23913. PMID: 32851989; PMCID: PMC7806350. [DOI] [PMC free article] [PubMed]
- 33.Niu LX, Feng ZE, Wang DC, Zhang JY, Sun ZP, Guo CB. Prognostic factors in mandibular gingival squamous cell carcinoma: a 10-year retrospective study. Int J Oral Maxillofac Surg févr. 2017;46(2):137–43. [DOI] [PubMed] [Google Scholar]
- 34.Zheng S, Yang J, Li C, Han D, Xu F, Elishilia Kaaya R, et al. Prognostic exploration of all-cause death in gingival squamous cell carcinoma: a retrospective analysis of 2076 patients. Santhekadur P, éditeur. J Oncol. 2021;2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velleuer E, Dietrich R, Pomjanski N, De Santana Almeida Araujo I, Silva De Araujo BE, Sroka I, et al. Diagnostic accuracy of brush biopsy–based cytology for the early detection of oral cancer and precursors in fanconi anemia. Cancer Cytopathol. 2020;128(6):403–13. [DOI] [PubMed] [Google Scholar]
- 36.Moreau N, Renoux M, Mignerey J, Ejeil AL. Localized gingival erythroleukoplakia in a 57-year-old fanconi anemia patient: a case report. Médecine Buccale Chir Buccale. 2017;23(3):160–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.