Abstract
Hemp (Cannabis sativa L.) is an annual, and dioecious herb belonging to the Cannabaceae family. This plant is native to Central and Southeast Asia. The wild races of this species are commonly growing in Khyber Pakhtunkhwa and Punjab provinces, as well as in Islamabad, Pakistan. This study provides crucial insights into how environmental variables influence the wild hemp populations, which can be utilized in for conservation and breeding. The present study was aimed at evaluating the effects of key environmental factors such as altitude, geographical location, precipitation, relative humidity, maximum, minimum, and average temperature on 16 morpho-agronomic traits of a wild population of hemp growing in the Potohar Plateau and Lesser Himalayas. Our findings indicated that high relative humidity (> 64%), low average temperature (< 15 °C), intermediate average temperature (19–22 °C), and high average temperature (> 22 °C) played significant roles in determining the distribution pattern of the wild hemp. Correlation analysis demonstrated that average annual temperature contributed a higher percentage of variation in phenotypic diversity than geographic variables. Additionally, cluster analysis indicated three groups for the selected 35 populations. Clustering and Principal Component Analysis (PCA) of the morpho-agronomic traits indicated that group 1 from the Lesser Himalayas showed high relative humidity (> 64%) and low average temperature (< 15 °C). Conversely, Group 2 populations from the Potohar Plateau demonstrated intermediate average temperature (19–22 °C). There is an existence of Group 3 in the Potohar Plateau with a high average temperature (> 22 °C) compared to Group 1 and Group 2. Our examination highlights the complex interplay between ecological factors, and morphological attributes in native landraces of Cannabis sativa, giving significant insight into knowledge for preservation and breeding initiatives. A study of genetic diversity could complement morpho-agronomic traits in future research to learn more about how genetic variation affects environmental adaptation.
Keywords: Morpho-agronomic traits, Population diversity, Environmental variables, Himalayas, Wild hemp, Cannabis sativa
Introduction
Cannabis sativa L. is a notable plant that has been growing since before the dawn of mankind and adapted to environmental conditions [1, 2]. It represents a monospecific genus in the family Cannabaceae [3, 4]. Humans have utilized it for more than 5,000 years and it is one of the oldest plants used for the production of food and fiber [5]. Various cultivars and landraces of hemp exist worldwide that contrast in chemical content growth habits, agronomic necessities, and processing [6, 7]. Nevertheless, extensive studies on the effect of environmental elements on the phenotypic diversity of wild hemp (Cannabis sativa L.) populations are lacking which can provide significant insight into preservation and breeding strategies. According to [8, 9], this plant is endowed with worthwhile natural, agronomical, and pharmaceutical properties used for extracting unrefined substances for different conventional (fiber, food, oil, medicine) or inventive industrial applications (new biomaterials and biofuels).
Cannabis sativa is considered native to Asia, explicitly the southern Caspian area, Siberia, China, Pakistan, India, and the Himalayas [3, 10–13]. In Pakistan, this plant is found in the upland of the country in wild forms. It is naturally growing in the Potohar Plateau, the Khyber Pakhtunkhwa, and Azad Jammu & Kashmir [14]. Nonetheless, because of far and wide transportation and change by humans throughout recent years, it is challenging to decide its unique geographic range or whether a plant gathered in nature is native or has been impacted by genetic introgression from modern cultivars [15]. In addition, Pre-human history shows that the oldest fossils of Cannabis sativa, dating back 19.6 million years, were found on the Northeastern Tibetan Plateau. From there, it spread to Europe and East Asia [16, 17]. In terms of pre-colonial history, its domestication is deemed to have occurred across Eurasia. In post-colonial history, cannabis spread globally, leading to the development of modern hemp-type and drug-type cultivars. It tends to be found in different places such as fields, rubbish stacks, vacant lots, pastures, trenches, springs, and open woods [2]. It is an herbaceous multi-purpose plant that can reach up to 5 m in height. It has erect stems with palmate leaves comprising 5-7-lanceolate leaflets. It is generally dioecious with male and female reproductive organs on separate individuals [4]. Inflorescences consist of highly branched compound raceme that can be tracked down on lengthy, leafy stems from each leaf axil. The staminate flower consists of five light green, bristly sepals around 2.5–4 mm long, and five pendulous stamens, with thin fibers and stamen. The pistillate flowers are practically sessile and are two by two. The fruit is an achene, contains a solitary seed with a hard shell firmly covered by the slender mass of the ovary, and it is ellipsoid, somewhat compacted, smooth, around 2–5 mm long, by and large, tarnish and mottled [18].
Cannabis sativa possesses two subspecies viz., C. sativa subsp. sativa, and C. sativa subsp. Indica. According to them, C. sativa subsp. sativa contains < 0.3% THC, while, C. sativa subsp. indica contains ≥ 0.3% THC [19]. Various countries have consolidated the 0.3% rule in guidelines overseeing fiber-type (hemp) plants, and above that, it is considered drug-type (marijuana) plants [20, 21]. Ecological variables are supposed to assume a critical part in hemp improvement by incorporating some factors like height, temperature, humidity, and soil piece coupled with anthropogenic influences. These variables can altogether affect the growth, morphology, improvement, conveyance, and agronomic attributes of hemp plants [22, 23]. Fluctuations in these environmental circumstances can prompt critical modifications in the degrees of cannabinoids, terpenes, alkaloids, phenolic compounds and other bioactive compounds present in the plant [24, 25]. Formisano et al. [26] examined the reaction of hemp plants to saline water systems and plant-based biostimulant applications. They find that the saline water system essentially influences biomass. In addition, Massuela et al. [27] examined the effect of nutrient stress at the flowering stage on biomass production.
Afridi Tirah, now known as Wadi Tirah, is close to the Afghanistan border in Pakistan and is home to the Afridi clan, renowned for quite a long time for hemp cultivation. The locally created charas, known as black gold, are exceptionally strong. It is generally consumed locally and also distributed to different areas [28]. Shahwar and Tayyab [29] studied phytochemical characterization of charas, a bioproduct of local hemp from four districts of Pakistan revealed minor variations in composition and reported predominant psychoactive and non-psychoactive compounds such as tetrahydrocannabinol, and Cannabidiol. Recently other researchers such as Jehangir et al. [22] stated that elevation, temperature, relative humidity, human interference, and soil attributes were responsible for hemp distribution and prevalence in the Western Himalayas, Pakistan. Owing to restrictions imposed by the legislation, no detailed research has been conducted in the country previously. This research is the initial part of the screening hemp germplasm for piloting and executing hemp cultivation in the country. Keeping in view, this study was aimed at assessing a correlation of 16 morpho-agronomic traits of 35 different wild populations of native hemp with different environmental variables such as high relative humidity (> 64%) and low average temperature (< 15 °C), intermediate average temperature (19–22 °C), and high average temperature (> 22 °C).
Materials and methods
Study area
In Pakistan, the Himalayan range can be divided into three zones such as (1) the Central or Great Himalayas, averaging 6,061 m in level, lodging tops like Nanga Parbat; (2) the Lesser Himalayas or the middle Himalayas, 2000–3000 m high, covering Hazara division, Islamabad-Rawalpindi, and Azad Jammu and Kashmir, with an area of around 23,295 km², situated between 33.44°N and 35.35°N slope and between 72.33°E and 74.05°E longitude and (3) Sub-Outer Himalayas, 8–48 km wide with a height of 909-1212 m, spanning Hazara-Murree slopes, Rawalpindi slopes, and Pabbi slopes [30, 31]. The common precipitation of Lesser Himalayas ranges from 70 to 90 mm in southern parts and 100–130 mm in northern parts. The parts of the north get little rain but experience weighty snowfall during winter [30, 32]. The Potohar Plateau, covering roughly 22,254 km² falls into semiarid regions including Attock, Jhelum, Chakwal, and Rawalpindi. Attock faces droughts despite the presence of the Jhelum River, while Jhelum battles with irrigation challenges because of the rising distinction between land and river levels. Chakwal encounters minimal precipitation and is especially defenseless to droughts, while Rawalpindi appreciates moderate temperatures all through the year as a tropical region of the Potohar Plateau [33].
Sampling of germplasm
The sampling for morphometric analysis of wild hemp germplasm was carried out in the mid of June and July 2023. Thirty-five growing localities (Fig. 1) were selected from two major study sites including the Potohar Plateau and the Lesser Himalayas of Pakistan. The germplasm collection was covered from a wide range of latitudes and longitudes from 32.57°N to 34.22°N and from 72.02°E to 73.72°E. The height of these areas fluctuates extraordinarily across the study area, which ranges from 201.5 m (Pind Dadan Khan, Punjab) in the Potohar region to 2413.8 m (Natya Gali, Abbottabad, Khyber Pakhtunkhwa) in the Lesser Himalayas. The precipitation, maximum, minimum, and average temperature, and relative humidity are shown in Table 1(https://power.larc.nasa.gov/data-access-viewer/).
Fig. 1.
Location of the collected samples, covering the geographical distribution of hemp (Cannabis sativa L.) in the Himalayas, Pakistan
Table 1.
The geographical and climatic variables of the 35 wild germplasm of hemp in the Lesser Himalayas and Potohar Plateau, Pakistan
| Sites | Location | Latitude E° | Longitude N° | Altitude (m) | Relative Humidity (%) | Max Temperature (°C) | Min Temperature (°C) | Average Temperature (°C) | Precipitation (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sohawa | 33.08° | 73.23° | 518.5 | 60.56 | 33.05 | 12.83 | 22.94 | 4.08 |
| 2 | Jehlum City | 32.92° | 73.72° | 227.4 | 56.9 | 33.81 | 14.93 | 24.37 | 4.09 |
| 3 | Mangla Cant | 33.12° | 73.62° | 284.1 | 57.82 | 33.81 | 13.2 | 23.50 | 4.18 |
| 4 | Pind Dadan Khan | 32.57° | 72.02° | 205.5 | 54.65 | 35.49 | 13.81 | 24.65 | 2.86 |
| 5 | Mulhal Mughul | 32.99° | 73.14° | 527 | 60.56 | 33.05 | 12.83 | 22.94 | 4.08 |
| 6 | Chakwal City | 32.91° | 72.87° | 517.3 | 60.56 | 33.05 | 12.83 | 22.94 | 4.08 |
| 7 | Choa Sedan Shah | 32.72° | 72.98° | 624.4 | 54.65 | 35.49 | 13.93 | 24.71 | 2.86 |
| 8 | Kalar Kahar | 32.78° | 72.70° | 642 | 57.97 | 33.645 | 12.45 | 23.04 | 3.46 |
| 9 | Talagang | 32.92° | 72.43° | 455 | 56.47 | 33.23 | 12.77 | 23.001 | 3.12 |
| 10 | Attock City | 33.77° | 72.70° | 450.6 | 56.82 | 32.42 | 13.78 | 23.10 | 4.7 |
| 11 | Kamra | 33.87° | 72.43° | 319.3 | 56.84 | 32.42 | 13.78 | 23.10 | 4.70 |
| 12 | Fateh Jhang | 33.56° | 72.64° | 499.3 | 60.39 | 32.45 | 12.16 | 22.30 | 4.23 |
| 13 | Pindigheb | 33.23° | 72.27° | 327.1 | 59.74 | 32.87 | 13.14 | 22.98 | 4.12 |
| 14 | Jhand | 33.43° | 72.02° | 350.1 | 57.56 | 33.37 | 12.16 | 22.76 | 3.66 |
| 15 | Arid University | 33.64° | 73.08° | 514 | 60.16 | 32.16 | 12.63 | 22.39 | 4.95 |
| 16 | Mandra | 33.36° | 73.24° | 527.2 | 60.16 | 32.166 | 12.63 | 22.39 | 4.95 |
| 17 | Kotli Satian | 33.79° | 73.51° | 1328.2 | 58.13 | 30.98 | 11.15 | 21.06 | 4.31 |
| 18 | Taxila | 33.71° | 72.81° | 524 | 60.39 | 32.45 | 12.16 | 22.30 | 4.23 |
| 19 | Pulgran | 33.76° | 73.21° | 620.1 | 60.16 | 32.16 | 12.63 | 22.39 | 4.95 |
| 20 | Banigala | 33.71° | 73.16° | 541 | 60.45 | 31.87 | 12.32 | 22.09 | 4.43 |
| 21 | Quaide-a-Azam university | 33.74° | 73.13° | 612 | 60.16 | 32.16 | 12.63 | 22.39 | 4.95 |
| 22 | Muree city | 33.96° | 73.38° | 2126.1 | 64.56 | 24.42 | 4.76 | 14.59 | 3.92 |
| 23 | Lower Topa | 33.89° | 73.43° | 1976.3 | 64.54 | 24.42 | 4.76 | 14.59 | 3.92 |
| 24 | Bhorban Pc | 33.95° | 73.45° | 1902.2 | 64.88 | 23.98 | 4.76 | 14.37 | 3.54 |
| 25 | Kohala | 34.10° | 73.49° | 650.2 | 64.25 | 21.38 | 4.21 | 12.79 | 3.79 |
| 26 | Pagwari | 33.98° | 73.49° | 1301.1 | 64.54 | 24.42 | 4.76 | 14.59 | 3.92 |
| 27 | Patriata | 33.87° | 73.45° | 1631 | 64.58 | 24.42 | 4.76 | 14.59 | 3.92 |
| 28 | Haripur City | 34.02° | 72.85° | 480 | 60.34 | 28.48 | 10.31 | 19.39 | 4.81 |
| 29 | Khanpur | 33.80° | 72.91° | 578.2 | 60.54 | 28.48 | 10.31 | 19.39 | 4.81 |
| 30 | Hattar | 33.78° | 72.91° | 479.7 | 60.49 | 28.48 | 10.31 | 19.39 | 4.81 |
| 31 | Ghazi | 34.01° | 72.66° | 343.1 | 56.82 | 32.42 | 13.78 | 23.10 | 4.70 |
| 32 | Abbottabad City | 34.16° | 73.28° | 1401.1 | 60.94 | 28.57 | 10.82 | 19.69 | 4.53 |
| 33 | Havelian | 34.05° | 73.16° | 872 | 60.49 | 28.48 | 10.31 | 19.39 | 4.81 |
| 34 | Nathia Gali | 34.22° | 73.72° | 2434.8 | 60.53 | 28.78 | 10.22 | 19.52 | 4.79 |
| 35 | Thandiani | 34.22° | 73.37° | 2076.1 | 60.89 | 28.85 | 10.13 | 19.49 | 4.23 |
Data collection
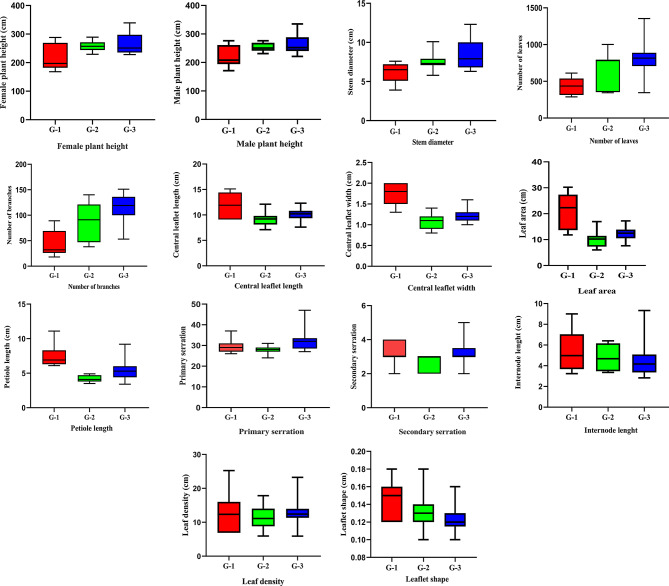
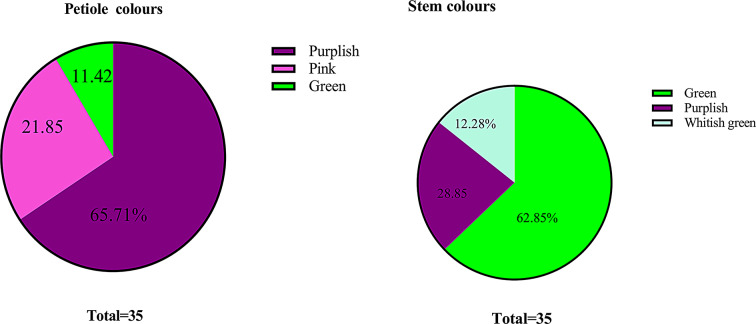
The sampling was carried out during the field trips from June 14 to July 29, 2023. The quantitative traits of female hemp plants (Fig. 2) were taken (in cm) for selected morphometric traits such as female plant height, male plant height, stem diameter, number of leaves, number of branches, central leaflet length, central leaflet width, leaf area, internode length, leaflet shape, leaf density, petiole length, primary serration, secondary serration; qualitative traits such as stem color, petiole color via visual observation (Figs. 3 and 4).
Fig. 2.
Wild hemp plant, A) female plant with trichrome, B) different population, C) stem diversity and D) compound leaf
Fig. 3.
Phenotypic divergence of wild Cannabis between Lesser Himalayas (G1) and Potohar Plateau (G2 and G3). The box and whisker plot represents the observed measures for each region, with the centre bar indicating the median value
Fig. 4.
Diversity of Stem and petiole colors observed in the wild population of Hemp
Limitation of research
This research work is geographically bound areas from Potohar Plateau to the Lesser Himalayas of Pakistan, and it may not fully represent the varietal variations of wild hemp across the country. Future research ought to incorporate a more extensive scope of locations where wild hemp grows naturally and consider extra environmental factors to give a more comprehensive understanding of the phenotypic variety and environmental flexibility of native hemp. Besides, molecular characterization could additionally clarify the genetic premise of observed phenotypic variation.
Statistical analysis
Microsoft Office Excel 2016 was used to calculate the mean, median, maximum, and minimum values, standard deviations, and correlation coefficient of selected morpho-agronomic data. R software (ver. 4.3.2) was used for One-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test for seeking differences in morpho-agronomic traits. The significance level at P < 0.05 was settled for testing the significant variation. The coefficient of variance was sought to indicate the degree of dispersion of phenotypic characteristics [34]. Pearson’s coefficient of correlation was used using Origin Pro 2022 to relate the relationship among the various environmental variables that affect the phenotypic diversity of wild hemp [35]. The cluster analysis and principal component analysis (PCA) were drawn for different morpho-agronomic traits using SPSS 25.0 [36]. Graph pad version 8 was used to exhibit variations among different groups by drawing box plots with mean points of the data. The significance level was assessed after 9999 permutations, and the simple Mantel test was performed with the R package “Vegan” [37].
Results
Association and variation characteristics among morpho-agronomic traits
Statistical analysis was carried out to measure the phenotypic variation among 35 different wild populations of hemp (Cannabis sativa L.) in the Lesser Himalayas and Potohar Plateau (Fig. 3). The variation in plant height for females ranged between 168 cm and 339 cm and for males was 171 to 335 cm with a stem diameter of 3.9 cm to 12.3 cm. The number of leaves of the female plant ranged from 288 to 1354, and the number of branches ranged from 18 to 114 (Fig. 3). The central leaflet length ranged from 8.1 cm to 15.1 cm with its width from 0.9 to 2 cm. The leaf area ranged from 7.29 to 28.2 cm and the leaflet length varied from 0.10 to 0.17 cm. Leaf density was measured at 5.91 to 25.24 cm. Internode length ranged from 3 to 9 cm and petiole length ranged from 3.4 to 11.1 cm. Primary serration ranged from 24 to 47 and secondary serration from 2 to 4 (Fig. 3; Table 2). The petiole and stem color were green, whitish green, pink, and purple (Fig. 4).
Table 2.
Descriptive statistics of morpho-agronomic traits
| Traits | N | Mean | SD | Sum | Min | Max | CV |
|---|---|---|---|---|---|---|---|
| “htf.pl” | 35 | 241.02 | 40.77 | 8436 | 162 | 323 | 16.91 |
| “htm.pl” | 35 | 271.17 | 42.78 | 9491 | 184 | 352 | 15.77 |
| “nm.lf” | 35 | 687.65 | 300.31 | 24,068 | 288 | 1354 | 43.67 |
| “nm.br” | 35 | 56.31 | 21.65 | 1971 | 18 | 103 | 38.45 |
| “len. Intr” | 35 | 4.84 | 1.62 | 169.56 | 3.13 | 9.33 | 33.49 |
| “dia.st” | 35 | 7.28 | 2.15 | 254.9 | 3.9 | 12.3 | 29.58 |
| “len.pet” | 35 | 5.73 | 2.02 | 200.8 | 3.4 | 11.1 | 35.35 |
| “len.cl” | 35 | 10.37 | 1.60 | 363 | 8.1 | 15.1 | 15.51 |
| “wid.cl” | 35 | 1.37 | 0.32 | 48.1 | 0.9 | 2 | 23.43 |
| “ara.lf” | 35 | 14.64 | 5.63 | 512.65 | 7.29 | 30.2 | 38.47 |
| “lfl.shp” | 35 | 0.13 | 0.01 | 4.61 | 0.10 | 0.17 | 14.86 |
| “ser.pr” | 35 | 30.8 | 4.99 | 1078 | 24 | 47 | 16.22 |
| “ser.sc” | 35 | 3.02 | 0.74 | 106 | 2 | 4 | 24.66 |
| “den.lf” | 35 | 12.48 | 4.29 | 436.95 | 5.91 | 25.24 | 34.38 |
Legends: N: Number of samples, SD: Standard deviation, Minimum value of a trait, Max: Maximum value of a trait, CV: Coefficient of variation. htf.pl: Plant height female, htm.pl: Plant height male dia.st: stem diameter, nm.lf: number of leaves, nm.br: Number of branches, len.cl: Central leaflet length, wid.cl: Central leaflet width, ara. lf: leaf area, len.pet: Petiole Length, ser.pr: Primary serration, ser.sc: Secondary Serrattion, len. Intr: Internode length, lfl. shp: leaflet shape, den.lf: leaf density
There was a coefficient of variation of 16.91% for plant height (females) and 15.77 for plant height (males) observed among different populations of hemp with a 29.58% stem diameter. Other morpho-agronomic traits such as the number of leaves, number of branches, central leaflet length, central leaflet width, leaf area, leaflet shape, leaf density, internode length, petiole length, primary serration, and secondary serration; stem and petiole color, the coefficient of variation was 43.67, 38.45, 15.51, 23.43, 38.47, 14.86, 34.38, 33.49, 35.35, 16.22, 24.66, 39.20 and 39.69, respectively (Table 2).
The result of analysis of variance (ANOVA) for each trait shown in Table 3. There was a variation in studied parameters except for the number of leaf density. Likewise, statistically, there were significant differences among different groups. Almost all morpho-agronomic traits exhibited significant associations with each other except leaf density. The highest correlation considering all populations was found between plant height female, plant height male, stem diameter, and number of leaves and branches. Meanwhile, a parallel response was observed among primary and secondary serration; and leaf density. In addition, a significant correlation was also observed in petiole length, central leaflet length, central leaflet width, leaflet shape, and leaf area.
Table 3.
One-way ANOVA followed by Tukey’s multiple comparison tests for different quantitative traits of hemp germplasm
| Traits | Group no | Mean diff | df | F | p-value |
|---|---|---|---|---|---|
| htf.pl | G-1 vs. G-2 | 41.49 | 2 | 5.57 | 0.0434 |
| G-1 vs. G-3 | 50.56 | 32 | 0.0064 | ||
| G-2 vs. G-3 | 9.070 | 34 | 0.7719 | ||
| 0.0084 | |||||
| dia.st | G-1 vs. G-2 | 1.404 | 2 | 5.02 | 0.2077 |
| G-1 vs. G-3 | 2.468 | 32 | 0.0068 | ||
| G-2 vs. G-3 | 1.064 | 34 | 0.2423 | ||
| 0.0810 | |||||
| nm.lf | G-1 vs. G-2 | 198.5 | 2 | 8.41 | 0.1504 |
| G-1 vs. G-3 | 384.1 | 32 | 0.0010 | ||
| G-2 vs. G-3 | 185.6 | 34 | 0.0799 | ||
| 0.0012 | |||||
| nm.br | G-1 vs. G-2 | 45.53 | 2 | 15.31 | 0.0088 |
| G-1 vs. G-3 | 73.07 | 32 | < 0.0001 | ||
| G-2 vs. G-3 | 27.53 | 34 | 0.0558 | ||
| < 0.00013 | |||||
| len.cl | G-1 vs. G-2 | 2.777 | 2 | 7.55 | 0.0014 |
| G-1 vs. G-3 | 1.850 | 32 | 0.0238 | ||
| G-2 vs. G-3 | 0.9262 | 34 | 0.2539 | ||
| 0.0021 | |||||
| wid.cl | G-1 vs. G-2 | 0.6753 | 2 | 28.29 | < 0.0001 |
| G-1 vs. G-3 | 0.5277 | 32 | < 0.0001 | ||
| G-2 vs. G-3 | 0.1476 | 34 | 0.1296 | ||
| < 0.0001 | |||||
| ara.lf | G-1 vs. G-2 | 11.28 | 2 | 19.53 | 0.0001 |
| G-1 vs. G-3 | 8.86 | 32 | 0.0020 | ||
| G-2 vs. G-3 | 2.42 | 34 | 0.0499 | ||
| 0.0001 | |||||
| len. Intr | G-1 vs. G-2 | 1.72 | 2 | 0.0030 | |
| G-1 vs. G-3 | 2.43 | 32 | 15.02 | 0.0001 | |
| G-2 vs. G-3 | 0.71 | 34 | 0.1666 | ||
| 0.0001 | |||||
| ser.pr | G-1 vs. G-2 | 2 | 0.4178 | ||
| G-1 vs. G-3 | 32 | 3.92 | 0.0239 | ||
| G-2 vs. G-3 | 34 | 0.0023 | |||
| 0.0298 | |||||
| ser.sc | G-1 vs. G-2 | 0.740 | 2 | 0.0757 | |
| G-1 vs. G-3 | 0.109 | 32 | 3.69 | 0.9313 | |
| G-2 vs. G-3 | 0.631 | 34 | 0.0552 | ||
| 0.0361 | |||||
| htm.pl | G-1 vs. G-2 | 1.509 | 2 | 0.7040 | |
| G-1 vs. G-3 | 0.348 | 32 | 0.414 | 0.9786 | |
| G-2 vs. G-3 | 1.161 | 34 | 0.7286 | ||
| 0.6642 | |||||
| lfl.shp | G-1 vs. G-2 | 2 | 0.0003 | ||
| G-1 vs. G-3 | 0.026 | 32 | 0.026 | 0.0006 | |
| G-2 vs. G-3 | 34 | 0.7739 | |||
| 0.0002 | |||||
| den.lf | G-1 vs. G-2 | 1.605 | 2 | 0.735 | |
| G-1 vs. G-3 | 0.238 | 32 | 0.4057 | 0.992 | |
| G-2 vs. G-3 | 1.366 | 34 | 0.706 | ||
| 0.6699 | |||||
| len.pet | G-1 vs. G-2 | 2.154 | 2 | < 0.0001 | |
| G-1 vs. G-3 | 3.371 | 32 | 14.86 | 0.0020 | |
| G-2 vs. G-3 | 1.218 | 34 | 0.0499 | ||
| < 0.0001 |
Legends: df: degree of freedom, F: F-statistic (a value used in statistical tests like ANOVA), p-value: Probability value
Using Spearman’s correlation coefficient, a highly positive correlation was found between high average temperature and the five morpho-agronomic traits such as plant height female, plant height male, stem diameter, number of leaves, and number of branches but these traits were highly negatively correlated with low average temperature, high altitude, and high relative humidity (Fig. 5).
Fig. 5.
Pearson Plot of morpho-agronomical, climatic, and geographical correlation coefficients for sixteen agronomic traits of 35 locations of wild hemp across the Lesser Himalayas and Potohar Plateau. Legend: Lat: Latitude, long: Longitude, Alt: Altitude, rh: Relative Humidity, tmax: Maximum Temperature, tmin: Minimum Temperature, tavg: Average Temperature, prec: Precipitation, htf.pl: Plant height female, htm.pl: Plant height male dia.st: stem diameter, nm.lf: number of leaves, nm.br: Number of branches, len.cl: Central leaflet length, wid.cl: Central leaflet width, ara. lf: leaf area, len.pet: Petiole Length, ser.pr: Primary serration, ser.sc: Secondary Serrattion, len. Intr: Internode length, lfl. shp: leaflet shape, den.lf: leaf density
Correlations between morpho-agronomic traits and geomorphic features
There was a significant correlation between the morphological traits of wild hemp and environmental variables (Fig. 5). Latitude had a negative correlation with a plant’s height female, and number of branches. However, it had a positive correlation with internode length, petiole length, central leaflet length & width, number of leaves, leaf area, leaflet shape, primary serration, secondary serration, leaf density, and petiole length. Stem diameter and plant height in males were not affected by latitude. There was a positive correlation of longitude with the plant height female, number of leaves, leaflet shape, and leaf density. Nevertheless, a moderate negative correlation of longitude was observed with central leaflet length, central leaflet width, and leaf area. Longitude did not correlate with number of branches, primary and secondary serration, petiole length, plant height male, and internode length (Fig. 5).
Altitude had a negative correlation with plant height female, plant height male, number of branches, number of leaves, and stem diameter. On the other hand, it has a positive correlation with internode length, petiole length, central leaflet width, leaf area, leaflet shape, and leaf density (Fig. 5). Central leaflet length and primary and secondary serration were not affected by altitude.
Correlations between morpho-agronomic traits and climatic conditions
The relative humidity had a negative correlation with the number of branches. In contrast, a positive correlation was observed with central leaflet length, central leaflet width, leaf area, leaflet shape, primary serration, secondary serration, leaf density, and petiole length. In addition, the relative humidity had no correlation with the number of leaves, plant height female, plant height male, and stem diameter. The maximum temperature has a positive correlation with plant height female, plant height male, number of branches, and stem diameter. In contrast, a negative correlation was observed with internode length, central leaflet width, leaf area, leaflet shape, primary serration, leaf density, and petiole length. The maximum temperature was no correlated with the number of leaves, stem diameter, and central leaflet length. The minimum temperature had a positive correlation with plant height female, plant height male, number of leaves, and number of branches. On the other hand, minimum temperature had a negative correlation with internode length, petiole length, central leaflet width, leaf area, leaflet shape, and leaf density. The minimum temperature showed no correlation with stem diameter, central leaflet length, primary serration, and secondary serration. Furthermore, average temperature has a positive correlation with plant height female, plant height male, and number of branches. On the other hand, negative correlation was investigated with internode length, petiole length, central leaflet length, central leaflet width, leaf area, leaflet shape, primary serration, and leaf density. The average temperature had no correlation with the number of leaves, stem diameter, and secondary serration. Most of the morpho-agronomic traits of wild hemp were not directly affected by precipitation such as plant height male, stem diameter, central leaflet length, central leaflet width, leaf area, leaflet shape, and leaf density. However, a slightly positive correlation was observed with plant height female, number of leaves, number of branches, petiole length, primary and secondary serration, and internode length was negatively correlated with precipitation (Fig. 5).
The simple mantel test identified non-significant correlations between the morphological and geographic distance matrices, but established significant correlations between morphological and climatic distance matrices (Table 4). The Correlations between the morphological and climatic matrices were at r = 0.3081, p = 0.001.
Table 4.
Correlations between the morphological, geographic and climatic parameters
| Comparison | r | p-value | Significance |
|---|---|---|---|
| Morphological, Geographic | -0.0057 | 0.4936 | ns |
| Morphological, Climatic | 0.3081 | 0.001 | ** |
Significance: ns means not significant, ** p ≤ 0.001
Multivariate analysis of associated traits of wild hemp
Principal component analysis (PCA) was carried out to determine the main significant variables in the morphological characters recorded. The results are presented in Fig. 6; Table 5 First two components explained 58.48% of the total variation among the tested traits. It describes 41.12% and 17.36% of the total variation in PC1 and PC2, respectively. In PC1, the agro-morphological traits such as central leaflet length, central leaflet width, leaf area, petiole length, and leaflet shape and internode length were significantly associated with each other (Fig. 6). It was positively correlated with all other traits. The PC2 was mainly contributed and associated with many traits, including plant height in both females and males, number of leaves, number of branches, stem diameter, primary and secondary serration, and PC2 exhibited a positive correlation with all morpho-agronomic traits except internode length.
Fig. 6.
Principal component analysis of morphological parameters of wild hemp. Legends: htf.pl: Plant height female, htm.pl: Plant height male, dia.st: stem diameter, nm.lf: number of leaves, nm.br: Number of branches, len.cl: Central leaflet length, wid.cl: Central leaflet width, ara. lf: leaf area, num.lfl: No of leaflets, len.pet: Petiole Length, ser.pr: Primary serration, ser.sc: Secondary Serrattion, len. Intr: Internode length, lfl. shp: leaflet shape, den.lf: leaf density
Table 5.
Principal component analysis loadings of the morpho-agronomic traits of wild hemp
| Morpho-agronomic traits | Coefficients of PC1 | Coefficients of PC2 | Eigenvalue | Percentage of Variance | Cumulative |
|---|---|---|---|---|---|
| htf.pl | -0.29102 | 0.35145 | 5.82078 | 38.81% | 38.81% |
| htm.pl | -0.2467 | 0.33557 | 2.71142 | 18.08% | 56.88% |
| nm.lf | -0.33167 | 0.09106 | 1.9911 | 13.27% | 70.16% |
| nm.br | -0.35842 | 0.21644 | 1.55863 | 10.39% | 80.55% |
| len. Intr | 0.31962 | -0.21462 | 0.98524 | 6.57% | 87.11% |
| dia.st | -0.28852 | 0.12434 | 0.60354 | 4.02% | 91.14% |
| len.pet | 0.25429 | 0.34974 | 0.50904 | 3.39% | 94.53% |
| len.cl | 0.22679 | 0.31647 | 0.28906 | 1.93% | 96.46% |
| wid.cl | 0.32947 | 0.33886 | 0.19581 | 1.31% | 97.76% |
| ara.lf | 0.31053 | 0.30849 | 0.13504 | 0.90% | 98.66% |
| lfl.shp | 0.28897 | 0.18458 | 0.11284 | 0.75% | 99.42% |
| ser.pr | 0.01629 | -0.15204 | 0.06189 | 0.41% | 99.83% |
| ser.sc | -0.09822 | -0.1469 | 0.01429 | 0.10% | 99.97% |
| den.lf | -0.01676 | -0.19462 | 0.01058 | 0.07% | 100.00% |
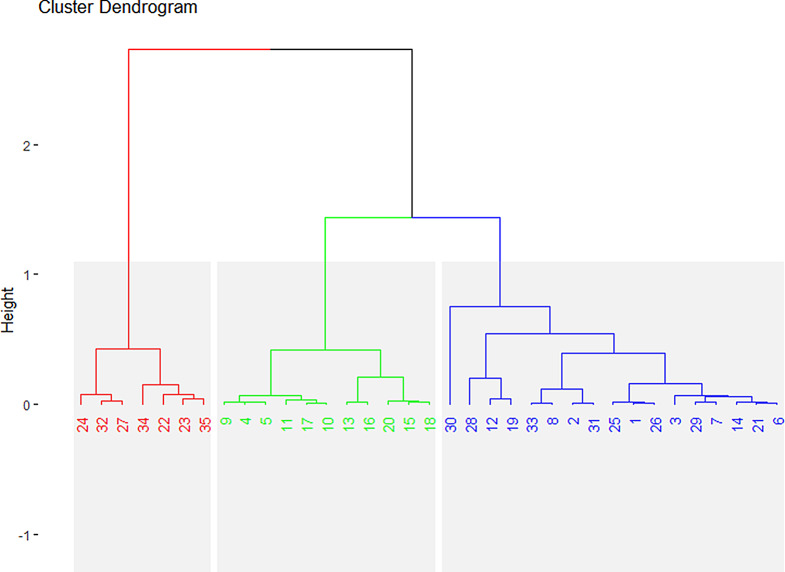
The cluster dendrogram of morpho-agronomic traits analysis was classified into three main groups (Fig. 7) Group 1 (n = 7), Group 2 (n = 11) and Group 3 (n = 17). Significant morphological differences were observed across all characters within the same groups (G-1, G-2, and G-3) and among different populations across these groups (Fig. 3). Group 1 from the Lesser Himalayas is represented by a red box plot in Fig. 3 and the red cluster in Fig. 7 encompassed high relative humidity (> 64%) and low average temperature (< 15 °C) that contained seven sites such as 22, 23, 24, 27, 32, 34, and 35. Contrary, morpho-agronomic traits of group 2 from the Potohar Plateau are exhibited by green box pot in Fig. 3 and green clusters in Fig. 7. This group, which comprises 11 sites such as 4, 5, 9, 10, 11, 13, 15, 16, 17, 18, and 20 exhibited intermediate average temperature (19–22 °C). The Potohar Plateau also made Group 3 but exhibited high average temperature (> 22 °C) encompassing 17 sites such as 1, 2, 3,6, 7, 8,12, 14, 19, 21, 25, 26, 28, 29,30 and site 33. The morpho-agronomic traits of this group, exhibited by different populations, are depicted by a blue box plot in Fig. 3 and a corresponding cluster in Fig. 7.
Fig. 7.
Cluster dendrogram analysis of morphological traits of wild Hemp and the number represents the study sites shown in Table 1
Novelty of research
This study addressed the first detailed assessment of the effect of explicit environmental variables, like altitude, topographical area, precipitation, relative humidity, most extreme, least, and normal temperature on the phenotypic diversity of wild hemp populations growing in the Potohar Plateau and Lesser Himalayas of Pakistan. Utilizing advanced statistical techniques like clustering analysis, Principal Component Analysis (PCA), analysis of variance (ANOVA), correlations analysis, and simple mantel test, the research uncovers distinct groupings and demonstrates how these environmental variables essentially impact the morpho-agronomic traits of populations of hemp. This novel approach gives crucial insights into the adaptive strategies of wild hemp germplasm to climatic circumstances, which is fundamental for conservation and breeding initiatives.
Discussion
Phenotypic plasticity is regarded as one of the significant means by which plants can adapt to environmental factor fluctuation [38]. We studied the general geographic trends in agro-morphology and their relationships with climatic variables. The correlation analyses explored the strong relationships among the phenotypic diversity of 16 agronomic traits of wild hemp under different climate conditions (Fig. 5). To the best of our knowledge, this is the first comprehensive study of morpho-agronomic of a wild hemp germplasm of the Potohar Plateau, and Lesser Himalayas, Pakistan.
According to the results, there were great levels of variation in morphology throughout their geographical range. The coefficient of variation of 16 phenotypes of hemp ranged from 11.14 to 43.10% supplementary (Table 1), and there was a wide phenotypic variation among the native germplasm. The highest coefficient of variation was observed in ten phenotypic traits such as number of leaves, number of branches, leaf area, petiole length, leaf density, internode length, stem diameter, secondary serration, central leaflet width, and plant height female (Fig. 3). This variation may be attributed to the climatic variables [1, 39]. Likewise, broad gene exchange inside dioecious plant populations can prompt a serious level of genetic heterozygosity, bringing about phenotypic variety inside populations [40, 41]. Furthermore, the traits with a superior quantity range show a higher coefficient of variation, indicating the existence of a higher range of selection for those characteristics [42]. Apart from the 10 traits with higher coefficients of variation, the remaining six traits exhibited lower variation ranging from 14.86 to 15.77%. Morphological traits with a lower coefficient of variation are more homogeneous and can be viewed as stable characters among accessions [43].
The morphological attributes of wild hemp such as plant height, stem diameter, number of leaves, number of branches, central leaflet length, central leaflet width, petiole length, primary and secondary serration, and stem and petiole color exhibited considerable variations. Morphological variation in plants is attributed to changes in ecological factors along latitudinal, longitudinal, and altitudinal angles at various geographic scales [44–49]. Changes in the local environment would prompt clear communications with plant genes, which could additionally prompt the improvement of plant adaptability [50]. Furthermore, genetic variation emphatically affects the drawn-out tirelessness of plant species by expanding the capacity of plant individuals to adjust to changing environmental conditions [51]. In our study, significant correlations were found between environmental factors and phenotypic characteristics of wild hemp germplasm. The present study was consistent with the previous study [52]. According to Li et al. [53] the phenotypic variation was connected with ecological factors such as average annual daylight hours, average temperature, and average annual rainfall. Besides, according to them, variation changed along slopes of longitude, latitude, and elevation. In addition, Nievas et al. [54] reported that elevation is an essential factor in determining the phenotypic diversity of plants. Naturalized plants are restricted to lowland environments because of the harsh temperature and resource limitations at higher altitudes [55].
In our study a positive correlation of Longitude with the plant height female, number of leaves, leaflet shape, and leaf density while a moderate negative correlation of longitude was observed with central leaflet length, central leaflet width, and leaf area. Moreover, the altitudinal gradient was also found responsible for changing the microclimatic conditions, like temperature, humidity, and precipitation [56, 57], which could be ascribed to particular vegetation [58–61].
Testing the Mantel analysis showed that the morphological variation.
was positively correlated with climatic distance matrices as compared to geographical distance matrices (Table 4). Our findings aligned with the study by Alcantara-Ayala et al. [62] who reported that climate had a higher impact rather than geography. Similarly, Khan et al. [63] and Qi et al. [64] stated that environmental factors co-drive the phenotypic diversity of plants. On the contrary, Sun et al. [36] observed that the phenotypic variation was significantly correlated with geographical matrices but had a very low correlation with climate matrices. Our study found that almost all of the studied traits are significantly correlated with climatic factors. Cluster analysis of the 35 wild hemp germplasm exhibited geographically close populations in the same group. Population numbers 22, 23, 24, 27, 32, 34, and 35 clustered in group 1, representing a population from the Lesser Himalayas (relative humidity > 64%) and low average temperature < 15 °C). Meanwhile, the populations of the Potohar Plateau were placed in group 2. These two groups (intermediate average temperature 19–22 °C and high average temperature > 22 °C) indicated a regional and continuous pattern of geographic variation. However, some populations with long geographic distances, such as populations no 1 and 25, 26 from group 3 (blue cluster) were found to be clustered in the same group, demonstrating a random variation pattern (Fig. 7). These results indicated that the phenotypic diversity of the wild hemp population in the Pakistani Himalayas and Potohar Plateau represents three geographical variation patterns (viz., continuous variation, regional variation, and nominal variation). Such contrasts in the phenotypic diversity were also found in different species that occur in various biogeographical locales with different climates [65–69]. Furthermore, the observed hierarchical inconsistency of trait variations may reflect intraspecific genetic variation [70].
Conclusion
We examined the influence of ecological factors on the morpho-agronomic characteristics of the wild hemp population in the Lesser Himalayas and Potohar Plateau of Pakistan. Our study showed that the most persuasive environmental variables for variation in the phenotypic diversity of wild hemp germplasm were temperature regimes, elevation and relative humidity. Besides, clustering and Principal Component Analysis (PCA) uncovered distinct groupings among the investigated populations in light of their climatic variables and morpho-agronomic characters. Interestingly, we noticed a convergence in morpho-agronomic traits between the Lesser Himalayas and the Potohar Plateau which seems to be an equal response to comparative climatic factors rather than to geological nearness. Thus, our findings highlight the significant role of the environment in the morphological dissimilarity between wild hemp populations at the local level. These results could help to anticipate the adaptative strategy of wild hemp germplasm to climatic variables.
Acknowledgements
This paper was extracted from the Ph. D thesis of the first author, Muhammad Younas. The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R154), King Saud University, Riyadh, Saudi Arabia.
Author contributions
R.Q. visualized, designed, supervised the study and edited the article, Z.U.R.M worked as supervisory committee member; M.Y performed the experiment; M.Y, Z.S and R.V.V statistically analyzed the data; M.Y, A, A and S. R, M.A.F, M.A.A wrote the first draft and rewrite the final paper. All authors have read and approved the published version of the manuscript.
Funding
There is no funding received for this research work.
Data availability
All the data and materials available in this manuscript.
Declarations
Ethics approval and consent to participate
All the authors declare and certify that all the work done in this research is the author’s original work and has not been submitted to any other journal for publication and that the paper is not considered for publication elsewhere. All the data in this paper are original and reflect the active contribution of the author and coauthor leading to the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Younas, Email: muhammadyounas872@gmail.com.
Rahmatullah Qureshi, Email: rahmatullahq@yahoo.com.
References
- 1.Pavlovic R, Panseri S, Giupponi L, Leoni V, Citti C. Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) grown in a mountain environment of Italian Alps. Front Plant Sci. 2019;10:470737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small E. Classification of Cannabis sativa L. in relation to agricultural, biotechnological, medical and recreational utilization. Cannabis sativa L-Botany Biotechnol 2017:1–62.
- 3.Andre CM, Hausman J-F, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:174167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upton R, ElSohly M. Cannabis Inflorescence: Cannabis Spp.; Standards of Identity. Analysis, and QualityControl American Herbal Pharmacopoeia 2013.
- 5.Appendino G, Minassi A, Taglialatela-Scafati O. Recreational drug discovery: natural products as lead structures for the synthesis of smart drugs. Nat Prod Rep. 2014;31(7):880–904. [DOI] [PubMed] [Google Scholar]
- 6.Thomas BF, ElSohly MA. The analytical chemistry of cannabis: quality assessment, assurance, and regulation of medicinal marijuana and cannabinoid preparations. Elsevier; 2015.
- 7.Datwyler SL, Weiblen GD. Genetic variation in hemp and marijuana (Cannabis sativa L.) according to amplified fragment length polymorphisms. J Forensic Sci. 2006;51(2):371–5. [DOI] [PubMed] [Google Scholar]
- 8.Amaducci S, Scordia D, Liu F, Zhang Q, Guo H, Testa G, Cosentino S. Key cultivation techniques for hemp in Europe and China. Ind Crops Prod. 2015;68:2–16. [Google Scholar]
- 9.Bennici A, Mannucci C, Calapai F, Cardia L, Ammendolia I, Gangemi S, Calapai G, Griscti Soler D. Safety of medical cannabis in neuropathic chronic pain management. Molecules. 2021;26(20):6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultes RE. Hallucinogens of Plant Origin: interdisciplinary studies of plants sacred in primitive cultures yield results of academic and practical interest. Science. 1969;163(3864):245–54. [DOI] [PubMed] [Google Scholar]
- 11.Merlin MD. Archaeological evidence for the tradition of psychoactive plant use in the old world. Econ Bot. 2003;57(3):295–323. [Google Scholar]
- 12.Zargari A. Medicinal plants. Tehran University of Medical Sciences; 1997.
- 13.Scientific Co, Research I. The Wealth of India: a dictionary of Indian raw materials and industrial products, vol. 9; 1972.
- 14.Anwar F, Latif S, Ashraf M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J Am Oil Chem Soc. 2006;83(4):323–9. [Google Scholar]
- 15.Sharma G. Significance of eco-chemical studies in cannabis [hemp (drug plant), USA]. Sci Cult 1979, 45.
- 16.McPartland JM. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018;3(1):203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPartland JM, Hegman W, Long T. Cannabis in Asia: its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg History Archaeobotany. 2019;28:691–702. [Google Scholar]
- 18.Clarke RC. Marijuana botany: an advanced study: the propagation and breeding of distinctive cannabis. Ronin publishing; 1981. [DOI] [PubMed]
- 19.Small E, Jui PY, Lefkovitch LP. A numerical taxonomic analysis of Cannabis with special reference to species delimitation. Syst Bot 1976:67–84.
- 20.Clarke R, Merlin M. Cannabis. Evolution and Ethnobotany, University of Cali. In.: fornia Press, Berkeley and Los Angeles; 2013.
- 21.Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966–75. [DOI] [PubMed] [Google Scholar]
- 22.Jehangir S, Khan SM, Ahmad Z, Ejaz U, Ain QU, Lho LH, Han H, Raposo A. Distribution of the Cannabis sativa L. in the Western Himalayas: a tale of the ecological factors behind its continuous invasiveness. Global Ecol Conserv. 2024;49:e02779. [Google Scholar]
- 23.Fiorentino N, Formisano C, Delfine S, Chianese G. Environmental and agronomic factors affecting the chemical composition and biological activity of cannabis extracts. Volume 15. Frontiers Media SA; 2024. p. 1407262. [DOI] [PMC free article] [PubMed]
- 24.Garcia Tejero I, Duran Zuazo V, Pérez-Álvarez R, Hernández A, Casano S, Morón M, Muriel-Fernández J. Impact of plant density and irrigation on yield of hemp (Cannabis sativa L.) in a Mediterranean semi-arid environment. J Agricultural Sci Technol. 2014;16(4):887–95. [Google Scholar]
- 25.Akram NA, Shafiq F, Ashraf M, Iqbal M, Ahmad P. Advances in salt tolerance of some major fiber crops through classical and advanced biotechnological tools: a review. J Plant Growth Regul. 2021;40:891–905. [Google Scholar]
- 26.Formisano C, Fiorentino N, Di Mola I, Iaccarino N, Gargiulo E, Chianese G. Effect of saline irrigation and plant-based biostimulant application on fiber hemp (Cannabis sativa L.) growth and phytocannabinoid composition. Front Plant Sci. 2024;15:1293184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massuela DC, Munz S, Hartung J, Nkebiwe PM, Graeff-Hönninger S. Cannabis Hunger games: nutrient stress induction in flowering stage–impact of organic and mineral fertilizer levels on biomass, cannabidiol (CBD) yield and nutrient use efficiency. Front Plant Sci. 2023;14:1233232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamayun M, Shinwari ZK. Folk methodology of charas (hashish) production and its marketing at Afridi Tirah, federally administered tribal areas (FATA), Pakistan. J Industrial Hemp. 2004;9(2):41–50. [Google Scholar]
- 29.Tayyab M, Shahwar D. GCMS analysis of Cannabis sativa L. from four different areas of Pakistan. Egypt J Forensic Sci. 2015;5(3):114–25. [Google Scholar]
- 30.Hussain F, Ilahi I. Ecology and vegetation of lesser Himalayas Pakistan. Department of Botany, University of Peshawar; 1991. p. 187.
- 31.Abbasi AM, Shah MH, Khan MA, Abbasi AM, Shah MH, Khan MA. Pakistan and Pakistani Himalayas. Wild Edible Vegetables of Lesser Himalayas: Ethnobotanical and Nutraceutical Aspects, Volume 1 2015:1–18.
- 32.Khan MA, Khan MA, Hussain M, Ghulam GM. An ethnobotanical inventory of himalayan region poonch valley azad kashmir (Pakistan). Ethnobotany Res Appl. 2010;8:107–23. [Google Scholar]
- 33.Rashid K, Rasul G. Rainfall variability and maize production over the Potohar Plateau of Pakistan. Pakistan J Meteorol. 2011;8(15):63–74. [Google Scholar]
- 34.Schillaci MA, Schillaci ME. Estimating the population variance, standard deviation, and coefficient of variation: sample size and accuracy. J Hum Evol. 2022;171:103230. [DOI] [PubMed] [Google Scholar]
- 35.Ali S, Khan SM, Ahmad Z, Abdullah A, Kazi N, Nawaz I, Almutairi KF, Avila-Quezada GD, Abd_Allah EF. Relative humidity, soil phosphorus, and stand structure diversity determine aboveground biomass along the elevation gradient in various forest ecosystems of Pakistan. Sustainability. 2023;15(9):7523. [Google Scholar]
- 36.Sun J, Shi W, Wu Y, Ji J, Feng J, Zhao J, Shi X, Du C, Chen W, Liu J. Variations in acorn traits in two oak species: Quercus mongolica Fisch. Ex Ledeb Quercus variabilis Blume Forests. 2021;12(12):1755. [Google Scholar]
- 37.Poljak I, Idžojtić M, Šapić I, Korijan P, Vukelić J. Diversity and structure of Croatian continental and Alpine-Dinaric populations of grey alder (Alnus incana/L./Moench subsp. incana); isolation by distance and environment explains phenotypic divergence. Šumarski list. 2018;142(1–2):19–31. [Google Scholar]
- 38.Gratani L. Plant phenotypic plasticity in response to environmental factors. Advances in botany 2014, 2014.
- 39.Dempsey JM. Fibre crops; 1975.
- 40.Nagy ES. Selection for native characters in hybrids between two locally adapted plant subspecies. Evolution. 1997;51(5):1469–80. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Lin F, Zhou Y, Wang J, Sun S, Wang B, Zhang Z, Li G, Lin X, Wang X. Genomic mosaicism due to homoeologous exchange generates extensive phenotypic diversity in nascent allopolyploids. Natl Sci Rev. 2021;8(5):nwaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammadi SA, Prasanna B. Analysis of genetic diversity in crop plants—salient statistical tools and considerations. Crop Sci. 2003;43(4):1235–48. [Google Scholar]
- 43.Khadivi-Khub A, Etemadi-Khah A. Phenotypic diversity and relationships between morphological traits in selected almond (Prunus amygdalus) germplasm. Agroforest Syst. 2015;89:205–16. [Google Scholar]
- 44.Rico-Gray V, Palacios-Rios M. Leaf area variation in Rhizophora mangle L.(Rhizophoraceae) along a latitudinal gradient in Mexico. Global Ecol Biogeogr Lett 1996:30–5.
- 45.Niinemets Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82(2):453–69. [Google Scholar]
- 46.Chalcoff VR, Ezcurra C, Aizen MA. Uncoupled geographical variation between leaves and flowers in a South-Andean Proteaceae. Ann Botany. 2008;102(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uribe-Salas D, Sáenz-Romero C, González-Rodríguez A, Téllez-Valdéz O, Oyama K. Foliar morphological variation in the white oak Quercus rugosa Née (Fagaceae) along a latitudinal gradient in Mexico: potential implications for management and conservation. For Ecol Manag. 2008;256(12):2121–6. [Google Scholar]
- 48.De Frenne P, Graae BJ, Rodríguez-Sánchez F, Kolb A, Chabrerie O, Decocq G, De Kort H, De Schrijver A, Diekmann M, Eriksson O. Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J Ecol. 2013;101(3):784–95. [Google Scholar]
- 49.Moles AT, Perkins SE, Laffan SW, Flores-Moreno H, Awasthy M, Tindall ML, Sack L, Pitman A, Kattge J, Aarssen LW. Which is a better predictor of plant traits: temperature or precipitation? J Veg Sci. 2014;25(5):1167–80. [Google Scholar]
- 50.Li Z, Zhang H. Diversity in fruit morphology and nutritional composition of Juglans mandshurica Maxim in northeast China. Front Plant Sci. 2022;13:820457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khaleghi A, Khadivi A. Morphological characterizations of wild nitre-bush (Nitraria Schoberi L.) specimens. Genet Resour Crop Evol. 2024;71(1):413–26. [Google Scholar]
- 52.SHE, C-q. FANG S-z, YANG W-x: geographical variation of morphologic characteristics of Cyclocarya paliurus seeds. J NANJING FORESTRY Univ. 2008;51(04):63. [Google Scholar]
- 53.Li Y, Li S, Lu X, Wang Q, Han H, Zhang X, Ma Y, Gan X. Leaf phenotypic variation of endangered plant Tetracentron Sinense Oliv. And influence of geographical and climatic factors. J Forestry Res. 2021;32(2):623–36. [Google Scholar]
- 54.Nievas RP, Calderon MR, Moglia MM. Environmental factors affecting the success of exotic plant invasion in a wildland-urban ecotone in temperate South America. Neotropical Biology Conserv. 2019;14(2):257–74. [Google Scholar]
- 55.Pauchard A, Alaback P. La Amenaza De Plantas Invasoras. Chile Forestal. 2002;289:13–5. [Google Scholar]
- 56.Vittoz P, Bayfield N, Brooker R, Elston DA, Duff EI, Theurillat JP, Guisan A. Reproducibility of species lists, visual cover estimates and frequency methods for recording high-mountain vegetation. J Veg Sci. 2010;21(6):1035–47. [Google Scholar]
- 57.Zhang J, Shao D. Attributes of forest diversity in the Yunmeng mountain national forest park in Beijing, China. Appl Ecol Environ Res. 2015;13(3):769–82. [Google Scholar]
- 58.Champion SH, Seth SK, Khattak GM. Forest types of Pakistan. 1965.
- 59.Khan N, Ahmed M, Shaukat SS, Wahab M, Siddiqui MF. Structure, diversity, and regeneration potential of Monotheca Buxifolia (Falc.) A. DC. Dominated forests of Lower Dir District, Pakistan. Front Agric China. 2011;5:106–21. [Google Scholar]
- 60.Khan N, Shaukat SS, Ahmed M, Siddiqui MF. Vegetation-environment relationships in the forests of Chitral district Hindukush range of Pakistan. J Forestry Res. 2013;24(2):205–16. [Google Scholar]
- 61.Nasrullah K, Fayaz A, Kishwar A, Shahid S. Composition, structure and regeneration dynamics of Olea ferruginea Royle forests from Hindukush range of Pakistan. J Mt Sci. 2015;12:647–58. [Google Scholar]
- 62.Alcántara-Ayala O, Oyama K, Ríos-Muñoz CA, Rivas G, Ramirez-Barahona S, Luna-Vega I. Morphological variation of leaf traits in the Ternstroemia lineata species complex (Ericales: Penthaphylacaceae) in response to geographic and climatic variation. PeerJ. 2020;8:e8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan N, Ullah R, Okla MK, Abdel-Maksoud MA, Saleh IA, Abu-Harirah HA, AlRamadneh TN, AbdElgawad H. Climate and soil factors co-derive the functional traits variations in naturalized downy thorn apple (Datura innoxia Mill.) Along the altitudinal gradient in the semi-arid environment. Heliyon 2024, 10(6). [DOI] [PMC free article] [PubMed]
- 64.Qi H, Sun X, Wang C, Chen X, Yan W, Chen J, Xia T, Ye H, Yu J, Dai J. Geographic isolation causes low genetic diversity and significant pedigree differentiation in populations of Camellia drupifera, a woody oil plant native to China. Ind Crops Prod. 2023;192:116026. [Google Scholar]
- 65.Škvorc Ž, Franjić J, Idžojtić M. Population structure of Quercus pubescens Willd.(Fagaceae) in Croatia according to morphology of leaves. Acta Bot Hungarica. 2005;47(1–2):183–96. [Google Scholar]
- 66.Temunović M, Franjić J, Satovic Z, Grgurev M, Frascaria-Lacoste N, Fernández-Manjarrés JF. Environmental heterogeneity explains the genetic structure of continental and Mediterranean populations of Fraxinus angustifolia Vahl. 2012. [DOI] [PMC free article] [PubMed]
- 67.Poljak I, Kajba D, Ljubic I, Idzojtic M. Morphological variability of leaves of Sorbus domestica L. in Croatia. Acta Soc Bot Pol 2015, 84(2).
- 68.Zebec M, Idžojtić M, Šatović Z, Poljak I, Liber Z. Alive and kicking, or, living on borrowed time?–Microsatellite diversity in natural populations of the endangered Ulmus minor Mill. Sensu Latissimo from Croatia. Acta Bot Croatica. 2016;75(1):53–9. [Google Scholar]
- 69.Gao S, Wang B, Liu F, Zhao J, Yuan J, Xiao S, Masabni J, Zou F, Yuan D. Variation in fruit morphology and seed oil fatty acid composition of Camellia Oleifera collected from diverse regions in southern China. Horticulturae. 2022;8(9):818. [Google Scholar]
- 70.Kwapata K, Mwase WF, Bokosi J, Kwapata M, Munyenyembe P. Genetic diversity of Annona senegalensis Pers. Populations as revealed by simple sequence repeats (SSRs). Afr J Biotechnol 2007, 6(10).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and materials available in this manuscript.