Abstract
Tryptophan (Trp) metabolism involves three primary pathways: the kynurenine (Kyn) pathway (KP), the 5-hydroxytryptamine (serotonin, 5-HT) pathway, and the indole pathway. Under normal physiological conditions, Trp metabolism plays crucial roles in regulating inflammation, immunity, and neuronal function. Key rate-limiting enzymes such as indoleamine-2,3-dioxygenase (IDO), Trp-2,3-dioxygenase (TDO), and kynurenine monooxygenase (KMO) drive these metabolic processes. Imbalances in Trp metabolism are linked to various cancers and often correlate with poor prognosis and adverse clinical characteristics. Dysregulated Trp metabolism fosters tumor growth and immune evasion primarily by creating an immunosuppressive tumor microenvironment (TME). Activation of the KP results in the production of immunosuppressive metabolites like Kyn, which modulate immune responses and promote oncogenesis mainly through interaction with the aryl hydrocarbon receptor (AHR). Targeting Trp metabolism therapeutically has shown significant potential, especially with the development of small-molecule inhibitors for IDO1, TDO, and other key enzymes. These inhibitors disrupt the immunosuppressive signals within the TME, potentially restoring effective anti-tumor immune responses. Recently, IDO1 inhibitors have been tested in clinical trials, showing the potential to enhance the effects of existing cancer therapies. However, mixed results in later-stage trials underscore the need for a deeper understanding of Trp metabolism and its complex role in cancer. Recent advancements have also explored combining Trp metabolism inhibitors with other treatments, such as immune checkpoint inhibitors, chemotherapy, and radiotherapy, to enhance therapeutic efficacy and overcome resistance mechanisms. This review summarizes the current understanding of Trp metabolism and signaling in cancer, detailing the oncogenic mechanisms and clinical significance of dysregulated Trp metabolism. Additionally, it provides insights into the challenges in developing Trp-targeted therapies and future research directions aimed at optimizing these therapeutic strategies and improving patient outcomes.
Keywords: Tryptophan metabolism, Expression changes, Clinical characteristics, Cancer, Targeted therapies
Introduction
Trp, an essential amino acid not synthesized by the human body, must be obtained through diet [1–5]. It is a fundamental component of protein synthesis and a precursor for many crucial biomolecules, influencing various metabolic pathways [6–10]. Although a small fraction of free Trp contributes to protein synthesis and the production of neurotransmitters like serotonin and neuromodulators such as tryptamine, over 95% is utilized in the kynurenine (Kyn) pathway (KP) of Trp degradation. This pathway generates several metabolites with distinct biological activities in immune responses and neurotransmission [11–14].
Trp and its metabolites are crucial in various physiological processes, including biomass production, cellular energy, and cell growth [15–18]. They play a significant role in coordinating organismal responses to environmental changes, acting as key elements in both metabolic and signaling pathways [19–21]. The primary metabolic pathways for Trp include the synthesis of serotonin and the KP. Serotonin significantly influences the central nervous system and plays a critical role in regulating intestinal motility, emesis, vasoconstriction, platelet aggregation, and wound healing [22–26]. It also serves as a precursor to melatonin, which regulates sleep and circadian rhythms in diurnal animals. The KP produces a series of bioactive metabolites such as picolinic acid, quinolinic acid (QA), kynurenic acid (KynA), cinnabarinic acid (CA), xanthurenic acid (XA), and Kyn. These metabolites regulate the immune system by modulating the infiltration and activity of immune cells in the TME [27–29]. Another significant product of this pathway is nicotinamide adenine dinucleotide (NAD+), vital for cellular homeostasis [30–32]. Furthermore, Trp metabolites influence the gut microbiota’s composition and functionality, affecting the gut microbiome balance and the gut-brain axis, which can alter the immune response and inflammation levels within the gastrointestinal tract [33–37].
Trp enzymes and metabolites are widely distributed across various cells and tissues, with their expression finely regulated [38–40]. Disruptions in Trp and its metabolites’ levels have been linked to several diseasea, especially cancer [41–45]. Research indicates that key enzymes such as IDO1 and TDO2 are upregulated in various cancer types, including brain, digestive system, breast, and lung cancers [46–49]. This upregulation enhances Trp catabolism in tumors, creating immunosuppression, impairing multiple barriers, and promoting tumor growth and metastasis [41, 50–53]. Consequently, IDO1 has become a focal point for cancer therapy, with inhibitors currently being tested in various clinical trials to restore immune surveillance and enhance the efficacy of treatments like chemotherapy and immunotherapy [35, 52, 54–56]. However, results from later-stage trials have been mixed, highlighting the complexity of targeting metabolic pathways within the TME.
This review offers a comprehensive overview of the main metabolic pathways, abnormal expression features, and primary roles of Trp metabolism and signaling in cancer. Emphasis is placed on the molecular mechanisms by which dysregulated Trp metabolism and signaling contribute to oncogenesis across various tumor types. Furthermore, the latest advancements in anticancer therapies targeting Trp metabolism and signaling are discussed, alongside the current challenges and future prospects of these therapeutic strategies. This review highlights the critical importance of Trp metabolism and signaling in cancer biology and therapy, emphasizing its potential as a significant area for ongoing research and clinical development.
Physiological Properties of Trp and Its Metabolites
Trp: Dietary Sources, Absorption, and Degradation
Trp, an essential amino acid, is vital for human health due to its complex metabolic pathways and physiological effects. As humans cannot synthesize Trp, it must be obtained from dietary sources such as turkey, chicken, eggs, cheese, fish, and plant-based proteins like pumpkin seeds, soy products, and tofu [1, 57–59]. These sources provide the necessary intake to maintain adequate Trp levels essential for various biological functions [60–62]. Upon ingestion, Trp is absorbed in the intestines and transported through the blood to various tissues [63–66]. Key transporters, such as solute carrier family 1 member 5 (SLC1A5) and solute carrier family 7 member 5 (SLC7A5), facilitate the cellular uptake of Trp and its distribution to organs including the brain, heart, and muscles, where it undergoes further metabolism [67–70]. Beyond its role in protein synthesis, Trp is a precursor to several bioactive compounds [71–73]. Trp metabolism is orchestrated through three principal pathways, facilitated by distinct enzymatic reactions within barrier organs such as the intestines, lungs, and skin, largely influenced by resident microbiota [74–77]. The gut microbiota, which outnumbers human cells significantly, profoundly influences Trp metabolism [78–81]. The interaction between dietary Trp intake, bacterial utilization, and local turnover in the gastrointestinal tract has crucial implications for maintaining physiological balance and influencing disease states [82–84]. Recent evidence highlights the critical role of gut microbiota-mediated Trp metabolism in modulating immune responses and contributing to the pathogenesis of gastrointestinal cancers. Certain gut-resident microbes can metabolize Trp into bioactive compounds, such as indole and its derivatives, including indole-3-propionic acid (IPA), indole-3-aldehyde (IAld), indole-3-carboxaldehyde (ICAld), and indole-3-acetaldehyde (IAAld) [43, 85–87]. These metabolites serve as key signaling molecules that interact with the aryl hydrocarbon receptor (AHR), which regulates genes crucial for maintaining intestinal barrier integrity, modulating immune cell differentiation, and promoting anti-inflammatory responses [88–91]. Notably, the gut-cancer axis has gained considerable interest, as dysbiosis-an imbalance in gut microbiota composition-has been linked to the progression of gastrointestinal cancers [92–94]. Gut microbes capable of converting Trp into indole derivatives may influence tumor growth by modulating local immune environments and epithelial cell proliferation. For example, IPA has demonstrated anti-inflammatory, antioxidant, and immunoregulatory properties, potentially reducing the risk of carcinogenesis [95, 96]. Conversely, the accumulation of certain metabolites, such as Kyn, through the KP, can promote immune escape mechanisms and tumor progression by activating immunosuppressive pathways such as AHR-mediated CD8+ T-cell exhaustion [97, 98]. Besides gut microbes, Trp metabolism is also intricately affected by several factors, including genetic alterations, diet, stress, exercise, and aging, which further modulate enzymatic activity and determine the dominance of specific metabolic pathways [99–103].
The three primary metabolic pathways for Trp involve its conversion into serotonin, Kyn, and indole-3-pyruvate (I3P) and its derivatives (Fig. 1). Approximately 1% of dietary Trp is converted into serotonin, a crucial neurotransmitter [6, 104, 105]. The conversion process begins with Trp hydroxylase (TPH) converting Trp to 5-hydroxytryptophan (5-HTP), which is then decarboxylated by aromatic amino acid decarboxylase (AADC) to produce serotonin [106–109]. Serotonin is subsequently metabolized into several compounds, including 5-hydroxyindoleacetic acid (5-HIAA) by monoamine oxidase (MAO), N-acetylserotonin (NAS) by arylalkylamine N-acetyltransferase (AANAT), and ultimately melatonin by N-acetylserotonin O-methyltransferase (ASMT). The KP is the primary catabolic route for Trp, initiated by either IDO or TDO, which are pivotal in neuroprotection, neurotoxicity, immune modulation, and homeostatic balance within different cellular environments [110–114]. These heme-containing enzymes convert Trp to N-formylkynurenine (NFK). NFK is then metabolized to Kyn by arylformamidase (AFMID), which serves as a precursor for several bioactive metabolites. KMO and kynureninase (KYNU) further process Kyn into 3-hydroxykynurenine (3-HK) and anthranilic acid (AA), respectively. Kynurenine aminotransferase (KAT) also transforms Kyn into KynA and 3-HK into XA. Subsequently, 3-hydroxyanthranilic acid (3-HAA), derived from 3-HK by KYNU, is converted into QA and ultimately contributes to the synthesis of nicotinamide and NAD + by quinolinate phosphoribosyl transferase (QPRT) [115, 116]. Notably, Kyn serves as an endogenous ligand for the AHR, part of a cytoplasmic complex that dissociates upon ligand binding [14, 80, 117]. This dissociation allows AHR to bind with the aryl hydrocarbon receptor nuclear translocator (ARNT) and activate genes crucial for cytoprotection, including those encoding cytochrome P450 enzymes such as CYP1A1 and CYP1B1 [84, 85, 118, 119]. Metabolites like KynA are known for their neuroprotective properties, whereas others like 3-HK, 3-HAA, and QA have neurotoxic effects [120–123]. As the end product of the KP, NAD + is a crucial cofactor in cellular reactions vital for energy metabolism, influencing pathways like glycolysis, β-oxidation, and oxidative phosphorylation [118, 124–127].
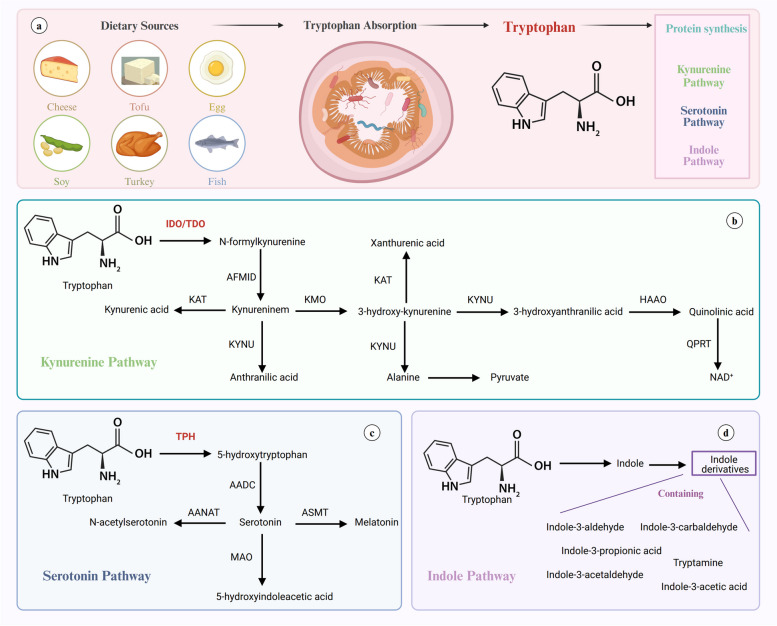
Fig. 1.
Dietary Sources of Tryptophan and Main Pathways of Tryptophan Degradation (a) Tryptophan, an essential amino acid, is commonly acquired from dietary sources such as turkey, eggs, cheese, tofu, seeds, and fish. After ingestion, tryptophan is absorbed in the gut and enters the bloodstream for use in various metabolic processes. Besides protein synthesis, tryptophan undergoes three main catabolic pathways: the serotonin pathway, the kynurenine pathway, and the indole pathway. b In the kynurenine pathway, tryptophan is first converted into N-formyl-L-kynurenine by the enzymes indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO), which is then broken down into several metabolites, including kynurenine, leading to the production of nicotinamide adenine dinucleotide (NAD+). c In the serotonin pathway, tryptophan is converted into serotonin via the enzyme tryptophan hydroxylase (TPH), followed by conversion to 5-hydroxytryptophan (5-HTP) and then to serotonin. Serotonin can further be converted into melatonin. d In the indole pathway, intestinal microbiota metabolizes tryptophan into various indole derivatives such as indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), and indole-3-aldehyde (IAld)
Quantification Techniques of Trp Metabolism
Quantifying Trp and its metabolites in biological fluids such as plasma, urine, tissue samples, and cerebrospinal fluid is essential for identifying potential biomarkers for various diseases [128–132]. Trp’s natural fluorescence facilitates the development of fluorometric detection methods [133–136]. Conventional techniques like liquid chromatography and gas chromatography, paired with UV detection, fluorescence, or mass spectrometry (MS), enhance the sensitivity and specificity of Trp metabolite detection [137–141]. These methods are foundational for assessing Trp metabolic profiles, providing insights into their roles in both physiological and pathological states. Additionally, refined Enzyme-Linked Immunosorbent Assay (ELISA) techniques quantify specific Trp metabolites within predefined detection limits, making them particularly useful for focused studies on individual metabolites, such as Kyn [142–145]. Immunohistochemistry, utilizing antibodies specifically targeting Trp metabolites, enables visualization and quantification within tissue samples, linking metabolic alterations to pathological states (Fig. 2) [146–148].
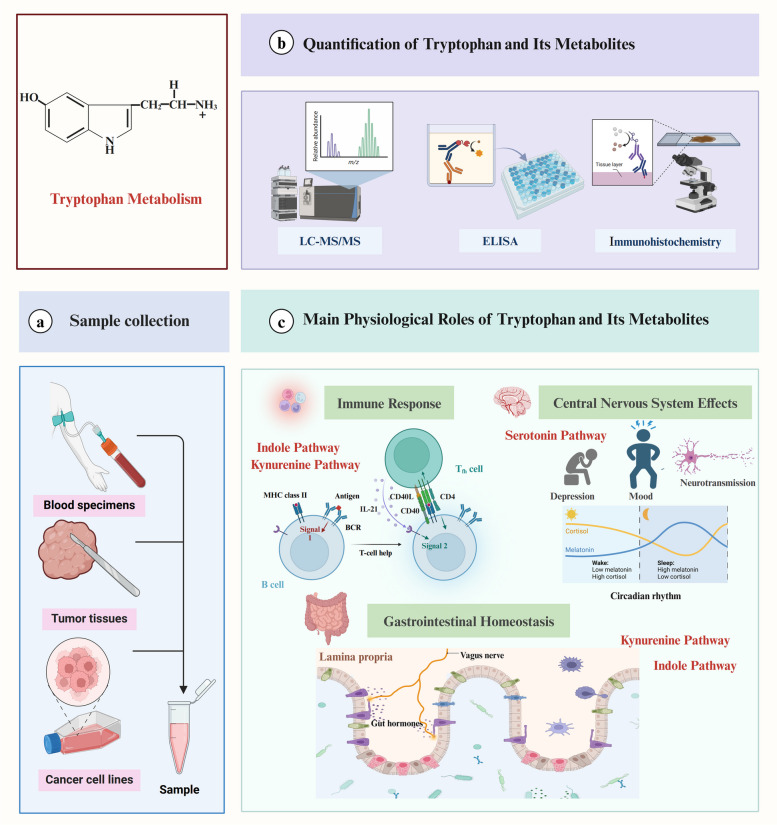
Fig. 2.
Tryptophan Sample Collection, Detection Methods, and Biological Functions Samples for tryptophan detection are typically obtained from blood specimens, cancer tissues, and cancer cell lines. Detection methods for tryptophan and its metabolites include enzyme-linked immunosorbent assay (ELISA), liquid chromatography-tandem mass spectrometry (LC-MS/MS), and immunohistochemistry. Tryptophan plays crucial roles in various physiological processes. In the immune system, metabolites from the kynurenine pathway, such as kynurenine (Kyn), modulate immune responses by regulating immune cell development, activation, and infiltration, thereby contributing to immune suppression and tumor immune evasion. Additionally, indole derivatives significantly also impact the immune modulation and immune homeostasis, particularly through the activation of Aryl Hydrocarbon Receptor (AHR). In the central nervous system, it serves as a precursor for serotonin, which influences mood, depression, and circadian rhythms, while its derivative, melatonin, regulates sleep-wake cycles. In the gastrointestinal tract, tryptophan is metabolized by the gut microbiota into indole derivatives that help maintain gut health and microbial balance, promoting intestinal barrier integrity and mucosal immunity. Moreover, the kynurenine pathway also significantly impacts the gastrointestinal system, particularly in maintaining immune homeostasis, regulating inflammation, and shaping the gut microenvironment
An advanced detection method, liquid chromatography-mass spectrometry (LC-MS), particularly ultra-high-performance LC-electrospray ionization-tandem MS (UHPLC-ESI-MS/MS), is robust for quantifying Trp and its metabolites, including Kyn [149, 150]. This approach offers comprehensive coverage of the Trp metabolic pathway, mapping intricate relationships between Trp and its derivatives. It is highly sensitive and specific, ideal for analyzing complex biological samples like blood and peritoneal fluid. Moreover, capillary electrochromatography-mass spectrometry (CEC-MS), using novel stationary phases like 4-vinylphenylboronic acid (4-VPBA) columns, enables Trp and Kyn quantification in plasma [151, 152]. This method combines the high-resolution capabilities of capillary electrophoresis with the sensitivity of MS, offering a simple, fast, and repeatable approach for Trp metabolite analysis.
Advancements in high-technology methods have significantly improved the accuracy and efficiency of analyzing Trp and its metabolites, providing vital insights into the biological and pathological effects of Trp metabolism and supporting the development of therapeutic strategies targeting Trp metabolism in diseases [153–157].
Expression Changes of Trp Metabolism in Cancer
Increased Trp uptake and upregulation of Trp-metabolizing enzymes in various tumor types correlate with poor disease prognosis (Table 1) [158–162]. Among these enzymes, abnormal IDO1 levels are common in diverse cancers and are studied as a factor to enhance sensitivity to cancer therapy [51, 163, 164]. Conversely, TDO expression in cancers is less characterized due to the lack of validated bioassay systems for detecting TDO and identifying TDO-expressing cells [165, 166]. Recent advancements in TDO-specific monoclonal antibodies have shown prevalent TDO expression in many human cancers, including hepatocarcinoma (HCC), glioblastomas, and kidney cancer. This understanding underscores the significance of Trp metabolism in cancer and highlights the potential of changes in Trp metabolism expression for prognosis prediction (Fig. 3). This section discusses the abnormal expression of Trp, key metabolic enzymes, and their products in various tumors, along with their potential clinical implications and prognostic value.
Table 1.
Expression changes and molecular mechanisms of tryptophan metabolism and signaling in cancers
| Cancer type | Molecule in Tryptophan Metabolism | Expression changes | Downstream targets | Mechanisms | Functions | Cell lines | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| Glioma | IDO | Increased | CFH and FHL-1 | IDO/CFH and FHL-1 | Induce immunosuppression | human U87 cells and mouse GBM cells | 2021 | [167] |
| Glioma | IDO | Increased | GITR | IDO/GITR | Recruit Tregs to induce immunosuppression | GL261 cells | 2012 | [168] |
| Glioma | IDO | Increased | / | / | Inhibit T cell population and induce immunosuppression | Murine glioma GL261 cells and human glioma U87 cells | 2016 | [169] |
| Glioma | IDO | Increased | Trp | IFN-γ/IDO/Trp | Induce inactivation of the T cells and immune resistance | LN229, U251, T98G, and U87 cells | 2009 | [170] |
| Glioma | IDO | Increased | / | / | / | / | 2018 | [171] |
| Glioma | IDO | Increased | Kyn | IFN-γ/IDO/Kyn/AHR | Increase MDSC and Treg populations | GL261, and GSC-005 cells | 2022 | [172] |
| Glioma | IDO | Increased | / | IFN-γ/IDO | Induce immunosuppression | GL261 cells | 2015 | [173] |
| Glioma | IDO | Increased | / | / | Induce immunosuppression | / | 2024 | [174] |
| Glioma | IDO | Increased | / | / | / | / | 2013 | [175] |
| Glioma | IDO1 and TDO | Increased | Kyn | IDO1/TDO/Kyn/AHR/AQP4 | Promote cell migration and invasion | U87MG, U251, A172, and GL261 cells | 2020 | [176] |
| Glioma | TDO | Increased | / | FKBP52/TDO | Suppress T-cell immunity | U87MG and LN-18 cells | 2015 | [177] |
| Glioma | TDO | Increased | / | OCT4 and SOX2/TDO | Inhibit the function and infiltration of CD8+ T cells and expand immune-suppressive M2 macrophages and Foxp3+ Tregs | GBM neurosphere lines (GBM1A and GBM1B), GL261 cells | 2021 | [178] |
| Glioma | TDO2 | Increased | / | / | / | / | 2015 | [179] |
| Glioma | TDO2 | Increased | Kyn | TDO2/Kyn/AHR | Promote cell viability | neuroblastoma cell lines (SK-N-AS, CHP-212, SK-N-BE (2), LA-N-5, NB-69, and SK-N-SH) | 2024 | [180] |
| CRC | SERT | Increased | mTORC1 | SERT/mTORC1 | Promote cancer growth | SW480 and HCT116 cells | 2021 | [45] |
| CRC | IDO | Increased | / | / | Induce immune inhibition | CRC CT26 cells | 2022 | [181] |
| CRC | IDO | Increased | / | / | / | / | 2022 | [182] |
| CRC | IDO1 | Increased | CDC20 | IDO1/Kyn/CDC20 | Promote cell proliferation | HCT116 and HT29 cells | 2018 | [183] |
| CRC | SLC7A5, SLC1A5, and AFMID | Increased | Kyn | MYC/SLC7A5, SLC1A5, and AFMID/Kyn/AHR | Promote cell growth | DLD1, HT29, HCT116, HCT15, RKO, LoVo cells | 2019 | [184] |
| PC | IDO1 | Increased | nucleotide synthesis | IFNγ/JAK-STAT/IDO1/serine and purine nucleotide biosynthesis | Contribute one-carbon units to serine and purine nucleotide biosynthesis and support cancer cell proliferation | Human PC cell lines (BxPC-3, CFPAC-1, HPAF-II, Panc 10.05, AsPC-1, SW 1990, and SW 1990), and mouse PC cell lines (ImPSC and KPC) | 2021 | [185] |
| PC | 3-IAA | Decreased | ROS | MPO/3-IAA/ROS/autophagy | Increase ROS accumulation and reduce autophagic activity and cancer cell proliferation | KPC cells | 2023 | [186] |
| PC | IDO1 | Increased | / | / | Increase cellular proliferation and macropinocytic ability, decrease immunogenicity, lower IDO-1 activity, and accelerate distant metastasis | KPIC cells | 2023 | [187] |
| PC | IDO1 and TDO/ | Increased | Kyn | IDO1 and TDO/Kyn/AHR/Cyp1a1 | Promote migration and invasion | KPIC, PANC1, and Pan02 cells | 2021 | [188] |
| HCC | TDO | Increased | Kyn | TDO/Kyn/AHR | Inhibit CD4+ and CD8+ T cell proliferation | HepG2 cells | 2021 | [189] |
| HCC | TDO2 | Increased | Kyn | TDO2/Kyn/AhR/IL-6/STAT3/NF-kB signals | Strengthen cell proliferation and cancer growth | SMC-7721 and HepG2 cells | 2021 | [190] |
| HCC | KMO | Decreased | 3-HAA | KMO/3-HAA/ | Induce cell apoptosis, inhibit cancer growth, and prolong survival | 2022 | [191] | |
| HCC | IAA, ICAld, and IPA | Decreased | SREBP2 | Gut Flora Disequilibrium/IAA, ICAld, and IPA/AhR/SREBP2 | Prevent liver cancer initiation | Hep1-6 cells | 2022 | [115] |
| GC | IDO | Increased | / | / | / | / | 2019 | [192] |
| GC | IDO | Increased | / | / | / | / | 2016 | [193] |
| GC | Kyn | Increased | NK cells | IDO/Kyn/NK cells | Promote ferroptosis and NK cell loss | NK-92 cells, GC SGC-7901, and MGC-803 cells | 2023 | [194] |
| Melanoma | TPH1/2, IDO1, TDO2, and LAT1 | Increased | / | / | Lead to defects in T cell effector function | SK-MEL-2 and MeWo cells | 2023 | [195] |
| Melanoma | AHR | Increased | / | IDO/TDO/Kyn/AHR | Induce immunosuppression | melanoma B16F10 cells | 2020 | [196] |
| Melanoma | IDO1 | Increased | / | / | / | / | 2019 | [197] |
| Melanoma | TDO | Increased | CSC markers | dexamethasone/TDO/CSC markers | Promote melanospheres and stemness | SK-Mel-28 and A375 cells | 2022 | [198] |
| BC | IL4I1, IDO1, KMO, and KYNU | Increased | / | / | Promote macrophage M1 polarization | M1 macrophages | 2023 | [199] |
| BC and Melanoma | IAN | Increased | / | Carbidopa/IAN | Promote cell proliferation | BC MCF-7 and melanoma A375 cells | 2019 | [200] |
| BC and Lymphoma | IDO | Increased | / | macrophages/FcγR signaling/AIM2/PD-L1 and IDO | Induce immunosuppression | BC HER2 + SKBR3 and BT-474 cells, and CD20 + Raji lymphoma cells | 2018 | [201] |
| BC, Melanoma, and CRC | Kyn | Increased | / | / | Inhibit the cancer infiltration and proliferation of polyfunctional CD8+ cells | mouse B16-F10 melanoma (CRL-6475), 4T1 metastatic BC (CRL-2539) and CT26 CRC (CRL-2638) cell lines | 2018 | [202] |
| BC | IDO | Increased | / | / | / | / | 2018 | [203] |
| BC | IDO1 | Increased | caspase-8 and caspase-9 pathways/MDR1, MRP 1/2, and BCRP | Thiosemicarbazide derivatives 1–3/IDO 1 and topoisomerase IIα/Caspase-8 and Caspase-9 pathways/MDR1, MRP 1/2, and BCRP | Inhibit apoptosis and cell cycle arrest | MCF-7 and MDA-MB-231 | 2023 | [204] |
| BC | IDO1 and TDO2 | Increased | / | PDPN + CAFs/IDO1 and TDO2/NK cells | Suppress ADCC of NK cells and induce cell resistance to trastuzumab | Human trastuzumab-resistant HER2 + breast cancer cell JIMT-1, and CAFs | 2023 | [205] |
| BC | KMO | Increased | β-catenin | KMO /β-catenin/pluripotent genes | Increase cell growth, colony, mammosphere formation, migration, invasion, and stemness | MDA-MB-231 and MDA-MB-468 | 2020 | [206] |
| OC | IDO1, IDO2, TDO2 and IL4I1 | Increased | / | / | / | / | 2023 | [207] |
| LC | IDO1, IDO2, and TDO | Increased | Kyn | IDO1, IDO2, and TDO/Kyn | Inhibit the accumulation and infiltration of T cells | LC Lewis cells | 2019 | [208] |
| LC | IDO | Increased | Kyn | / | / | / | 2018 | [209] |
| LC | TDO | Increased | Kyn | TDO/Kyn/AHR/AKT/ERK | Promote cell proliferation, cancer growth, and development of EGFR TKI resistance | Human LC cells A549, and murine LC cells Lewis | 2022 | [210] |
| HCC, Glioma, PC and CRC | TDO | Increased | Kyn | TDO/Kyn | Induce immunosuppression | CRC MC38 and CT26, glioblastoma A172 cells | 2020 | [163] |
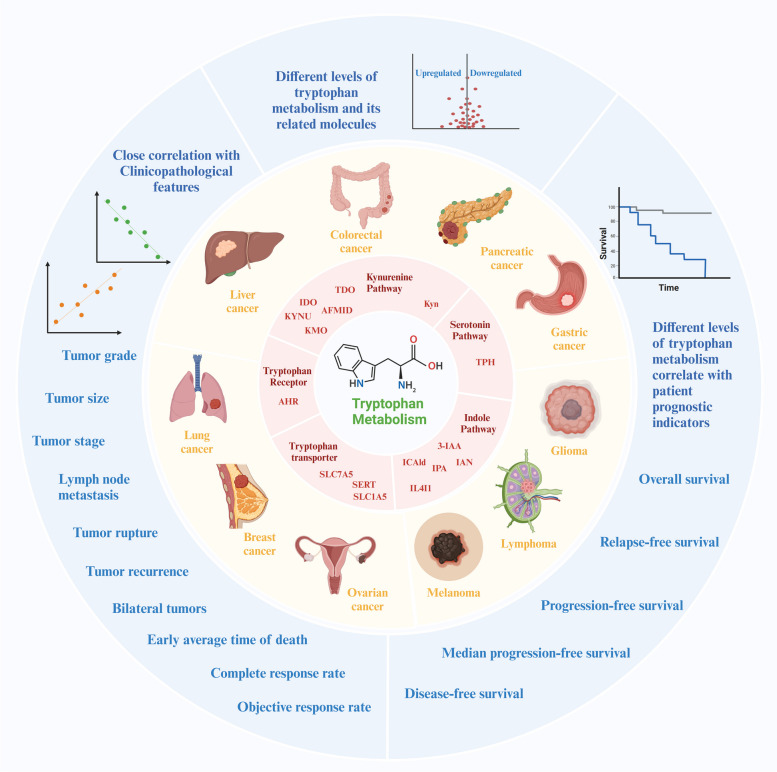
Fig. 3.
Abnormal Expression of Tryptophan Metabolism in Cancers and Its Correlation with Clinicopathological Features and Prognosis Differentially abnormal expression of tryptophan metabolism and related molecules involved in cancer progression and patient outcomes. Critical enzymes in the tryptophan metabolism pathway, such as indoleamine 2,3-dioxygenase (IDO), tryptophan 2,3-dioxygenase (TDO), kynurenine 3-monooxygenase (KMO), kynureninase (KYNU), tryptophan hydroxylase (TPH), play significant roles in regulating tryptophan breakdown. Transporters such as solute carrier family proteins (SLC1A5, SLC7A5) and the serotonin transporter (SERT) facilitate cellular uptake and signaling of tryptophan and its metabolites, while the aryl hydrocarbon receptor (AHR) mediates biological effects of tryptophan-derived metabolites. These enzymes, transporters, and receptors are frequently found to be upregulated or downregulated in various cancers such as glioma, melanoma, lymphoma, and cancers of the digestive system, breast, and lung. The altered expression levels of these molecules are closely associated with clinicopathological features, including tumor grade, stage, size, and lymph node metastasis. Elevated or reduced levels of tryptophan metabolism-related molecules reflect the imbalance in tryptophan metabolism that influence disease progression. Furthermore, abnormal tryptophan metabolism and its associated molecules are strongly correlated with patient prognosis, usually as demonstrated by Kaplan-Meier survival curves. These alterations in tryptophan metabolism show a significant relationship with key prognostic indicators such as overall survival, relapse-free survival, and progression-free survival, suggesting that dysregulated tryptophan metabolism could serve as a prognostic biomarker and therapeutic target in cancer
In glioma, analysis of TCGA data reveals that both IDO and complement factor H (CFH) mRNA levels increase with tumor grade, peaking in glioblastoma (GBM). IDO and CFH exhibit coordinated upregulation, with elevated CFH expression being inversely correlated with patient survival across all tumor grades [167]. Additionally, TCGA data highlights increased TPH-1 expression, which is associated with sustained glioma progression and poor overall survival [168]. Analysis of 343 glioma patients from the REpository of Molecular BRAin Neoplasia DaTa (REMBRANDT) confirms that upregulated IDO expression predicts significantly worse patient prognosis [169]. Immunohistochemical staining of 75 surgical specimens shows that stronger IDO expression is more prevalent in high-grade and secondary gliomas than in low-grade gliomas. Kaplan-Meier survival analysis demonstrated that patients with highly malignant gliomas and high IDO expression have worse prognoses compared to those with low IDO expression [170]. Furthermore, a positive correlation exists between IDO1 and TDO expression and glioma pathological grades. Both IDO1 and TDO expression are positively associated with overall survival (OS), and their co-expression represents independent prognostic values for OS of glioma patients [171]. AMT-PET, based on increased uptake of α-[11 C]-methyl-L-Trp (AMT) in glioma, shows high accuracy in distinguishing grade I from grade II/III gliomas. Additionally, TDO2 shows the highest immunostaining scores, particularly in grade I gliomas, followed by IDO2 and IDO1 [172]. Data from the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) database (phs000467), involving 249 pediatric patients, indicates that high expression of Trp transporters SLC1A5 and SLC7A5 predicts worse prognosis for neuroblastoma patients (Fig. 4) [173].
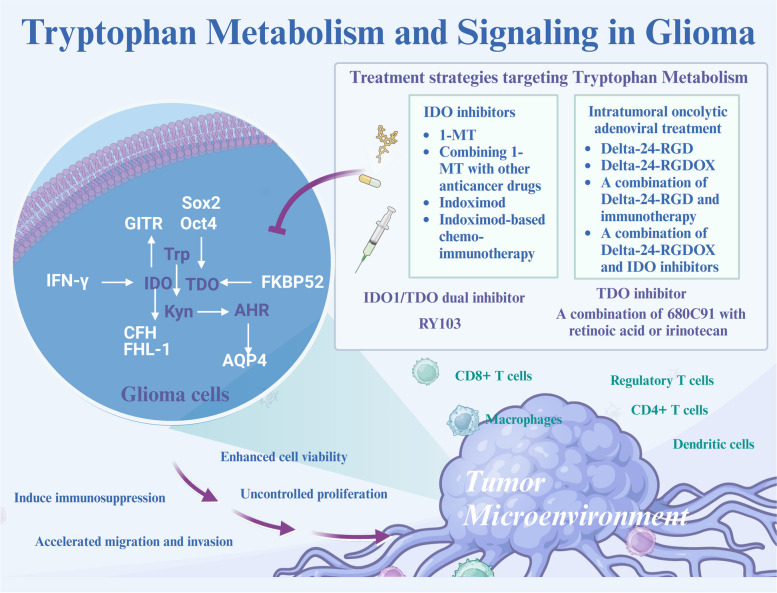
Fig. 4.
Molecular Mechanisms and Therapeutic Strategies of Tryptophan Metabolism in Glioma In glioma, tryptophan metabolism plays a crucial role in tumor progression and immune evasion through the upregulation of key enzymes like indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). These enzymes increase kynurenine production, activating the aryl hydrocarbon receptor (AHR), which promotes glioma cell proliferation, migration, and invasion while inducing immunosuppression by depleting tryptophan and accumulating immunosuppressive metabolites. Therapeutic strategies targeting tryptophan metabolism include IDO inhibitors such as 1-MT and indoximod, which reduce immunosuppression and enhance the efficacy of other anticancer drugs. The TDO inhibitor 680C91 and the dual IDO/TDO inhibitor RY103 also lower kynurenine levels, mitigating its effects on AHR signaling. Additionally, combining oncolytic adenoviral treatments, such as Delta-24-RGD and Delta-24-RGDOX, with immunotherapy or IDO inhibitors enhances therapeutic outcomes by reducing the immunosuppressive environment within the glioma
In colorectal cancer (CRC), several studies have demonstrated elevated levels of Trp transporters SLC7A5 and SLC1A5, along with Kyn, AHR, and key KP enzymes (TDO2, IDO1, and AFMID) [174–176]. In patients with locally advanced rectal cancer (LARC) receiving preoperative chemoradiotherapy (CRT), IDO expression has been identified as a significant prognostic marker. Patients with IDO-positive tumors exhibit a markedly poorer 5-year OS compared to those with IDO-negative tumors, and multivariate analysis identifies IDO expression as an independent prognostic indicator, highlighting its potential as a marker for individualizing treatment strategies in LARC [177]. In pancreatic cancer (PC), prevalent expression of IDO1 and TDO is negatively correlated with patient OS and relapse-free survival (RFS) [178, 179]. IDO1 expression is upregulated during tumor formation in immunocompetent settings, especially in the presence of IFN-γ or through JAK/STAT signaling [178]. TCGA data confirms a negative correlation between high IDO1 expression and patient survival in PC [178]. The co-expression of IDO1 and TDO, rather than individual expression, offers independent prognostic value for PC [179]. Interestingly, IDO1 expression increases in cells within PC ducts but decreases in PC cells, contributing to epithelial-mesenchymal transition (EMT) [180]. Additionally, an increase in microbiota-derived 3-IAA is observed in the serum of both patients and mice with PC who are susceptible to chemotherapy, correlating with improved progression-free survival (PFS) and OS in the PC Hamburg cohort [181]. In HCC, advanced-stage cancer tissues exhibit enhanced TDO2 expression, which is correlated with poor prognosis, as validated by the TCGA database [182]. However, both KMO and its substrate 3-HAA are reduced in HCC cells and clinical HCC tissues, and patients with high KMO expression show longer disease-free survival (DFS) [183]. Dysbiosis of the gut flora in HCC reduces the levels of AhR ligands derived from Trp metabolism, such as 3-IAA, ICAld, and IPA [115]. In gastric cancer (GC), SGC-7901 cells exhibit significantly higher levels of Kyn compared to GES-1 and MGC-803 cells [184]. IDO is a powerful prognostic biomarker for GC following gastrectomy and is closely associated with the immunosuppressive GC TME [185–187]. Immunohistochemical staining analysis of 99 GC cancer tissues from patients who received radical resection reveals that larger tumors, advanced T stages, and poorer prognosis are more positively associated with IDO expression. Additionally, IDO-positive patients possess higher levels of Foxp3+ Treg cells but lower levels of CD4/CD8+ T cells in the TME [185]. Another involving 357 GC patients shows that high intratumoral IDO expression is associated with poor OS, deeper tumor invasion, and increased lymph node metastasis [186].
Melanoma exhibits dysregulation in Trp metabolism, characterized by high intratumoral expression of TPH1/2, IDO1, TDO2, and the transporter SLC7A5. Notably, higher SLC7A5 expression in melanoma cells is associated with worse OS, and baseline Trp levels strongly predict clinical benefits from the PD1 inhibitor pembrolizumab [188]. The LCCC1531 trial in melanoma demonstrated that high Trp PET imaging correlates with shorter clinical benefits from pembrolizumab in PD1 inhibitor-naïve stage IIIB-IV melanoma patients. Additionally, the theragnostic value of baseline Trp metabolism effectively prolongs PFS, as shown by optimal cut-point post-hoc analysis [188]. In breast cancer (BC), analysis of single-cell transcriptome data indicates that elevated levels of Trp metabolic enzymes, such as IDO1, KMO, and KYNU, in macrophages are linked with a positive response to immunotherapy, suggesting that Trp metabolism could be a predictive marker for BC treatment [189]. Furthermore, the evaluation of 4 TMAs, containing 242 invasive primary BC and 39 metastatic BC cases, showed that IDO expression is prevalent in high-grade, triple-negative BC. Notably, 70% of PD-L1-positive BC cases also express IDO, contributing to poor outcomes with anti-PD-L1 treatment despite strong PD-L1 expression [190]. There is also a notable increase in indole-3-acetonitrile (IAN) levels over time in BC MCF-7 cells and melanoma A375 cells exposed to carbidopa, a DOPA decarboxylase inhibitor used in Parkinson’s disease (PD) treatment [191]. Moreover, the investigation based on 86 clinical canine mammary tumor (CMT) cases indicates the ability of KMO for discriminating malignant from benign CMTs and the strong correlation of KOM expression with overall survival rates in patients with malignant CMTs [192]. In ovarian cancer (OC), IDO1, IDO2, TDO2, and IL4I1 exhibit high positive expression rates in cancer specimens, with IDO1-positive patients being more resistant to platinum-based chemotherapy. Increased IDO1 expression is also associated with advanced cancer stages and lymph node metastasis. In contrast, TDO2 expression negatively correlates with the presence of bilateral tumors and endometriosis, while negative IL4I1 expression is commonly observed in cases of cancer rupture [193]. Finally, radiotherapy (RT) in lung cancer (LC) patients impacts systemic IDO-mediated anticancer immune activity, evidenced by changes in serum levels of IDO-mediated Kyn production and the Kyn(K) ratios before, during, and after RT. The Kratio decreases during RT but returns to baseline levels post-RT. Notably, these changes in IDO-associated molecules correlate with clinical outcomes in RT-treated LC patients. Greater Kyn levels post-RT significantly indicate worse OS and PFS [194]. Additionally, an enriched distribution of cancer-associated fibroblasts (CAFs) with activated TDO and elevated secretion of Kyn is observed in epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) resistant cancer tissues from LC patients [195].
Molecular Mechanisms of Tryptophan and Its Metabolites in Cancer
Involvement of KP in Carcinogenesis
Involvement of KP in Glioma
In glioma, cancer-derived IDO expression recruits immunosuppressive regulatory T cells (Tregs) and increases their glucocorticoid-induced TNFR-related protein (GITR) expression while decreasing CD8+ T cell frequency. This immune imbalance triggers immunosuppression and tumor growth, relying on coordinated actions of CD4+ and CD8+ T cells [169]. Additionally, IFN-γ robustly induces IDO expression, leading to increased Trp consumption and Kyn accumulation, creating a local immunosuppressive environment that inactivates T cells and promotes glioma cell proliferation [196]. As an oncolytic adenovirus, Delta-24-RGD, engineered to selectively replicate in and destroy cancer cells, shows promising anti-glioma effects by enhancing the anticancer immune response [197–199]. Delta-24-RGD downregulates IDO expression in glioma cells and Foxp3 levels in Tregs, decreasing tumor-infiltrating CD4+ Foxp3+ Tregs and increasing IFN-γ-producing CD8+ T cells, significantly improving the TME and systemic tumor-antigen-specific T cell therapy in GBM [200]. Additionally, tumor-propagating stem-like cells in glioblastoma (GSCs) contribute to the immunosuppressive TME, driven by reprogramming transcription factors OCT4 and SOX2. Co-expression of OCT4 and SOX2 in GSCs upregulates multiple immunosuppressive checkpoints, including TDO, and immunosuppressive cytokines and chemokines, inhibiting CD8+ T cell function and infiltration while promoting the expansion of immunosuppressive M2 macrophages and Foxp3+ Tregs [201]. Furthermore, recent findings indicate nonenzymic IDO in GBM U87 cells increases CFH and FHL-1 expression, independent of Trp metabolism, further enhancing immune suppression by raising intratumoral Tregs and myeloid-derived suppressor cells [202]. Recent studies have also shown that the IDO1/TDO/Kyn/AHR/AQP4 signaling pathway is central to glioma progression, particularly in cell motility. IDO1 and TDO facilitate Kyn generation, which activates AHR and increases AQP4 expression, enhancing the migratory and invasive capabilities of U87MG glioma cells (Fig. 4) [203].
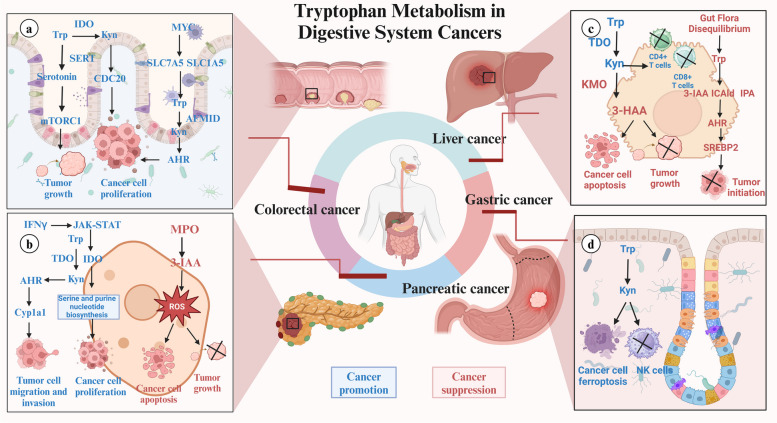
Involvement of KP in Digestive System Cancers
In CRC, IDO generates Kyn to activate CDC20 transcription, maintaining HCT-116 and HT-29 cell proliferation and resisting cell cycle arrest-mediated apoptosis (Fig. 5a) [204]. Additionally, KMO knockdown suppresses the expression of cancer stem cells markers including Nanog and CD44 in CRC, thereby repressing CRC cell stemness, migration, and invasion [205]. In PC cancer with increased IDO1 expression, Trp serves as a viable one-carbon source for the tetrahydrofolate (THF) cycle, supporting PC cell proliferation and tumor growth. Liquid chromatography-mass spectrometry analysis confirms that Trp-derived one-carbon units integrate into serine and purine nucleotides in PC cells, offering an alternative to serine, particularly when serine availability is restricted. Pancreatic stellate cells also uptake and utilize Trp-derived formate released by PC cells for nucleotide biosynthesis in an IDO1-dependent manner [178]. However, recent studies show conflicting roles for IDO1 in PC, with evidence suggesting both pro-tumorigenic and anti-metastatic effects, depending on the immune context. In immunocompetent mice, deleting IDO1 in PC KPIC cells reduces tumor-forming ability, cellular proliferation, and macropinocytic capability. Conversely, IFN-γ-induced IDO1 inhibition using INB24360 triggers liver metastasis of PC organoid cancer [180]. Additionally, Kyn-mediated AHR activation in PC further leads to the induction of Cyp1a1 transcription, enhancing the migration and invasion capabilities of KPIC cells (Fig. 5b) [179]. In HCC, TDO2 overexpression significantly increases Kyn expression, leading to IL-6 secretion and activation of the STAT3/NF-kB signaling pathway. This enhancement boosts colony formation and cell proliferation capabilities of HCC cells, demonstrating a key role for TDO in HCC pathogenesis [182]. Moreover, KMO knockdown has demonstrated a significantly inhibitory effect on HCC cancer progression, possibly through abnormal NAD concentration and subsequent destruction of NADH/NAD + redox homeostasis [183, 206]. While KMO overexpression are also confirmed to increase 3-HAA concentration, accelerating apoptosis in HCC SMMC7721 and HepG2 cells and impairing cancer growth (Fig. 5c) [183]. In GC, restoring the number of NK cells in the TME is crucial for effective treatment [207–209]. Kyn from GC cells induces ferroptosis in NK cells via an AHR-independent mechanism, leading to NK cell depletion and an immunosuppressive TME. Engineered NK cells with higher glutathione peroxidase 4 (GPX4) expression show resistance to Kyn-induced ferroptosis and therapeutic benefits in humanized GC cell-derived xenograft (CDX) cancer (Fig. 5d) [184].
Fig. 5.
Molecular Mechanisms of Tryptophan Metabolism in Digestive System Cancers (a) Tryptophan metabolism plays dual roles in digestive system cancers, promoting or suppressing tumor growth. In colorectal cancer, altered metabolism enhances tumor cell proliferation and survival through upregulated transporters like SLC1A5 and SLC7A5, increasing tryptophan uptake and metabolism. Kynurenine, via AHR signaling, supports cancer growth and immune evasion. b In liver cancer, gut microbiota-produced metabolites such as indole-3-acetic acid (IAA) and indole-3-aldehyde (IAld) inhibit tumor initiation and progression. c Pancreatic cancer progression is driven by the JAK-STAT signaling pathway, which increases IDO expression to support tumor growth and immune evasion. Conversely, the myeloperoxidase (MPO) pathway suppresses tumors by inducing oxidative stress and promoting cancer cell apoptosis. d In gastric cancer, kynurenine fosters cancer cell proliferation, migration, and NK cell loss, creating an immunosuppressive environment that facilitates tumor growth
Involvement of KP in Other Cancer
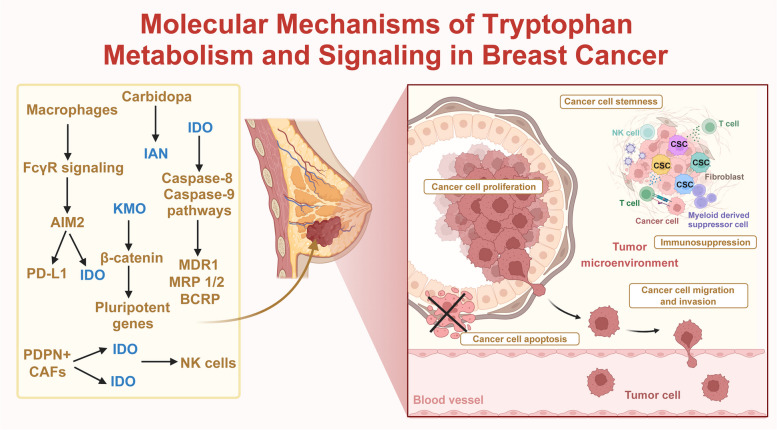
In BC, macrophages recruited to the TME via Fc gamma receptor (FcγR) signaling upregulate PD-L1, and IDO, leading to immunosuppression and cancer growth [210]. Furthermore, a novel population of podoplanin-positive (PDPN+) CAFs enriched in the BC TME secrete IDO1 and TDO2, leading to resistance to trastuzumab therapy [174]. Thiosemicarbazide derivatives (1–3), acting as dual inhibitors of topoisomerase IIα and IDO1, induce apoptosis in BC MCF-7 and MDA-MB-231 cells through caspase-8 and caspase-9 pathways. These derivatives also increase the proportion of BC cells in the G2/M phase and enhance sensitivity to anticancer treatments by inhibiting major ATP-binding cassette (ABC) transporters [211]. Overexpression of KMO functions as an oncogene in TNBC progression by preventing β-catenin degradation, upregulating pluripotent genes, leading to increased cell growth, colony and mammosphere formation, migration, invasion, and stemness in BC cells, and enhanced cancer metastasis and growth in vivo [212] (Fig. 6).
Fig. 6.
Molecular Mechanisms of Tryptophan Metabolism and Signaling in Breast Cancer In breast cancer, key enzymes and metabolites in tryptophan metabolism, including indoleamine 2,3-dioxygenase (IDO), kynurenine 3-monooxygenase (KMO), and indole-3-acetonitrile (IAN), significantly contribute to creating an immunosuppressive tumor microenvironment. These factors promote cancer cell migration and invasion and maintain cancer stem cell (CSC) properties
In melanoma, IFN-γ induces IDO1-mediated Trp depletion, diversifying the peptidome landscape at Trp residues. This altered peptidome is presented on HLA-I molecules, triggering peptide-specific T-cell responses crucial for immune recognition and melanoma therapy [213]. Melanoma cells exhibit a greater capacity for Trp uptake and metabolism within the competitive TME, depriving adjacent TILs of Trp and impairing their proliferation and survival. Additionally, Trp metabolism in melanoma cells produces Kyn and serotonin, which regulate TILs, leading to impaired T cell effector function [188]. TDO plays a crucial role in melanoma cancer stem cells (CSCs). Dexamethasone drives melanosphere formation and stemness in melanoma SK-Mel-28 and A375 cells in a TDO-dependent manner, resulting in a highly proliferative and metastatic phenotype [214]. In LC A549 and Lewis cells, CAFs produce Kyn and upregulate AHR expression to activate AKT and ERK signals, facilitating cell proliferation and resistance to EGFR TKIs [195].
Other Trp Metabolic Pathways and Signaling Mechanisms in Cancer
The serotonin and indole pathways, along with transport proteins and receptors involved in Trp signaling, play significant roles in carcinogenesis. Serotonin, as an important neurotransmitter, influences cancer growth and progression across various cancer types [215–219]. Previous research has shown that TPH1 overexpression increases serotonin production in prostate cancer, which activates the Axin 1/β-catenin signaling pathway. β-catenin then interacts with the transcription factor zinc finger binding protein (ZBP)-89 to further upregulate TPH1, forming a positive feedback loop (TPH1/5-HT/β-catenin/ZBP-89/TPH1), ultimately driving enhanced cell proliferation and migration [220]. In glioma, overexpressed TPH-1 facilitates serotonin generation to upregulate L1-cell adhesion molecule (L1CAM) and NF-κB signaling activation, subsequently promoting cell proliferative and migration ability [168]. Additionally, serotonin uptake via the serotonin transporter (SERT) is crucial for its recycling and degradation. In CRC, targeting SERT reduces mTORC1 serotonylation, leading to mTOR inactivation and increased Trp uptake. This process enhances Trp catabolism, boosting serotonin biosynthesis and accelerating cell proliferation and cancer growth in HCT116 and SW480 cells [45]. The serotonin receptor (5-HT(1D)R) is also promoted the activation of Axis Inhibition Protein 1(Axin1)/β-catenin/Matrix Metalloproteinase-7 (MMP-7) pathway, therefore enhancing cancer metastasis in an orthotopic CRC mouse model [221]. The intervention of a 5-HT(1D)R antagonist (GR127935) restrains CRC cancer invasion and migration activity. Furthermore, increased serotonin in BC interacts with 5-HTR2A/C to trigger ak1/STAT3 and ERK1/2 pathway, contributing to the upregulation of pyruvate kinase M2 (PKM2) and BC cell glycolysis. Administration of 5-HTR2A/C antagonist, ketanserin, significantly suppresses the glucose metabolism and cell growth rate in BC MCF-7 cells [222, 223].
It has also been demonstrated that metabolites from the indole pathway, primarily produced by gut microbiota, significantly impact systemic metabolism and the local TME [224–228]. Elevated levels of 3-IAA in PC cells increase reactive oxygen species (ROS) accumulation and reduce autophagic activity, contributing to cancer suppression [181]. Carbidopa, used to treat PD, alters Trp metabolism to increase production of the pro-proliferative metabolite IAN in BC MCF-7 cells and melanoma A375 cells, enhancing cell viability and cancer incidence [191]. Upregulation of specific transport proteins that facilitate Trp import into cancer cells is vital for maintaining the altered metabolism supporting cancer growth and immune evasion [67, 229–232]. Moreover, transporters like SLC1A5 and SLC7A5 maintain the influx of Trp, meeting the high metabolic demand of cancer cells. In CRC, the oncogene MYC overexpresses Trp transporters (SLC7A5 and SLC1A5) and KP enzymes (AFMID), leading to increased Trp uptake and Kyn generation. Elevated Kyn supports CRC cell proliferation through AHR activation, an effect reversed by IDO, TDO, and AHR inhibitors (Epacadostat, 680C91, and CH223191) [174]. Additionally, Trp signaling receptors, such as AHR, significantly influence cancer growth and immune evasion [60, 233–236]. Gut microbiota dysbiosis reduces levels of AHR ligands, including 3-IAA, ICAld, and IPA, impairing AHR activation and increasing sterol regulatory element-binding protein 2 (SREBP2) levels, promoting HCC initiation [237]. These pathways underscore the multifaceted role of Trp metabolism in cancer and highlight the potential for targeted therapies to disrupt these processes.
Targeting Trp Metabolism and Signaling in Cancers
Considering the multifaceted roles of Trp metabolism, a significant exploration into small-molecule inhibitors targeting Trp metabolism, particularly IDO and TDO, has yielded promising advancements in cancer therapy [238]. Preclinical studies indicate that IDO1 and TDO inhibitors can reduce cancer growth and enhance the efficacy of existing treatments, such as immune checkpoint inhibitors [47, 239]. Additionally, dual inhibitors targeting both IDO1 and TDO are being developed for a broader and more effective approach to cancer therapy [240]. Various combination strategies involving IDO1 and TDO inhibitors with immune checkpoint inhibitors, chemotherapy, or radiotherapy are under investigation to maximize synergistic therapeutic efficacy (Table 2) [82, 241]. Moreover, increasing evidence supports the therapeutic potential of targeting other key enzymes in the tryptophan metabolism pathway, such as KMO and TPH, which have shown promise in a range of disorders, including neurodegenerative diseases [242, 243]. This suggests that investigating these enzymes as potential therapeutic targets may also be crucial, broadening the scope of Trp metabolism as a therapeutic avenue.
Table 2.
Roles and clinical significance of tryptophan metabolism and signaling in cancers
| Cancer type | Molecule in Tryptophan Metabolism | Role | Clinical characteristics | Drug/Treatment | Clinical samples and In vivo model | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Glioma | IDO | Cancer promoter | tumor grade and OS | / | Peripheral blood from patients with GBM and aneurysm, tissues from patients with GBM, The Cancer Genome Atlas (TCGA), and syngeneic and humanized mouse GBM models database | 2021 | [167] |
| Glioma | IDO | Cancer promoter | OS, early average time of death, and tumor grade | / | The NCI Repository for Molecular Brain Neoplasia Data (REMBRANDT) database, the GL261 orthotopic mouse model, and the RasB8 transgenic mouse model | 2012 | [168] |
| Glioma | IDO | Cancer promoter | OS | 1-MT | A subcutaneous ectopic model, a syngeneic intracranial orthotopic model, and an allogenic intracranial orthotopic model. | 2016 | [169] |
| Glioma | IDO | cancer promoter | / | 1-MT | / | 2009 | [170] |
| Glioma | IDO | Cancer promoter | / | Delta-24-RGD | A phase I clinical trial involving 37 patients with recurrent malignant glioma | 2018 | [171] |
| Glioma | IDO | Cancer promoter | OS | a combination of Delta-24-RGDOX and IDO inhibitors | Orthotopic gliomas models | 2022 | [172] |
| Glioma | IDO | Cancer promoter | / | a combination of Delta-24-RGD and immunotherapy | A syngeneic GBM mouse model | 2015 | [173] |
| Glioma | IDO | Cancer promoter | / | Indoximod-based chemo-immunotherapy | A phase I clinical trial involving 81 children with recurrent brain cancer or newly DIPG | 2024 | [174] |
| Glioma | IDO | Cancer promoter | tumor grade and OS | / | 75 tissue specimens from 68 patients with glioma | 2013 | [175] |
| Glioma | IDO1 and TDO | Cancer promoter | OS | RY103 | 75 glioma tissues, 34 blood samples, and GL261 orthotopic glioma mouse model | 2020 | [176] |
| Glioma | TDO | Cancer promoter | / | FK506 | 25 diffuse glioma tissues, GEO dataset: GSE18015, GSE16237, GSE9834, GSE9835, and U87MG orthotopic glioma mouse model | 2015 | [177] |
| Glioma | TDO | Cancer promoter | / | Pan-BET inhibitors | GlioVis and TCGA datasets and GL261 orthotopic glioma mouse model | 2021 | [178] |
| Glioma | TDO2 | Cancer promoter | tumor grade | / | 47 glioma tissues | 2015 | [179] |
| Glioma | TDO2 | Cancer promoter | OS | a combination of 680C91 with retinoic acid or irinotecan | GSE89413 and TARGET database (phs000467) | 2024 | [180] |
| CRC | SERT | Cancer promoter | / | a combination of SERT inhibition and dietary Trp restriction or trametinib | 66 matched pairs of CRC tissues and adjacent tissues, alongside xenograft and primary CRC mouse model | 2021 | [45] |
| CRC | IDO | Cancer promoter | / | 1-MT@OMV-Mal | Animal models | 2022 | [181] |
| CRC | IDO | Cancer promoter | OS | / | 90 CRC tissue samples | 2022 | [182] |
| CRC | IDO1 | Cancer promoter | / | 1-MT and Epacadostat | Animal models | 2018 | [183] |
| CRC | SLC7A5, SLC1A5, and AFMID | Cancer promoter | / | TDO2 inhibitor (680C91), IDO inhibitor (Epacadostat), and AHR inhibitor (CH223191) | TCGA database and mouse organoids | 2019 | [184] |
| PC | IDO1 | Cancer promoter | survival | a combination of Epacadostat and serine restriction | TCGA-PAAD dataset and subcutaneous allograft mouse models | 2021 | [185] |
| PC | 3-IAA | Cancer suppressor | PFS or OS | FIRINOX/a high-tryptophan dietary intervention | Fecal and plasma samples of PC patients who do not respond and are responsive to the therapy and animal models | 2023 | [186] |
| PC | IDO1 | Cancer promoter | / | Epacadostat | 62 cancer samples from PC patients, KPIC organoids, and subcutaneous transplantation of organoids | 2023 | [187] |
| PC | IDO1 and TDO/ | Cancer promoter | OS and RFS | RY103 | TCGA database, KPIC orthotopic PC mice, and Pan02 cancer-bearing mice | 2021 | [188] |
| HCC | TDO | Cancer promoter | / | PVIS-Ir and PVIG-Ir | H22 BALB/c mice model | 2021 | [189] |
| HCC | TDO2 | Cancer promoter | OS and tumor grade | a combination of PDM2 and chemotherapy | TCGA database as well as 16 HCC cancer tissues and subcutaneous mice models | 2021 | [190] |
| HCC | KMO | Cancer suppressor | DFS | / | A series of HCC animal models | 2022 | [191] |
| HCC | IAA, ICAld, and IPA | Cancer suppressor | / | Lactobacillus Reuteri/Ficz | Fecal samples of HCC patients and healthy subjects, and animal models | 2022 | [115] |
| GC | IDO | Cancer promoter | tumor size, tumor stage, and OS | / | 99 GC cancer tissues and blood samples | 2019 | [192] |
| GC | IDO | Cancer promoter | OS, tumor invasion, and lymph node metastasis | / | 357 GC cancer tissues from patients with GAC undergoing gastrectomy | 2016 | [193] |
| Melanoma | TPH1/2, IDO1, TDO2, and LAT1 | Cancer promoter | OS | a combination of Telotristat and Pembrolizumab | The Biomax Normal Skin/Benign Nevus/Primary Melanoma TMA, the UNC-CH 09–1737 Metastatic Melanoma TMA, and the LCCC1531 trial | 2023 | [195] |
| Melanoma | AHR | Cancer promoter | / | KYN-101/CH-223,191 | Animal models | 2020 | [196] |
| Melanoma | IDO1 | Cancer promoter | / | Epacadostat | 706 patients with unresectable stage III or IV melanoma previously untreated with PD-1 or PD-L1 checkpoint inhibitors | 2019 | [197] |
| Melanoma | TDO | Cancer promoter | / | 680C91 | Melanospheres were generated from A375 and SK-Mel-28 human melanoma cells | 2022 | [198] |
| BC | IL4I1, IDO1, KMO, and KYNU | Cancer promoter | serve as a biomarker for predicting the efficacy of immunotherapy | / | GEO database (GSE75688, GSE176078, GSE210616), XENA database, and cBioPortal database | 2023 | [199] |
| BC and Melanoma | IAN | Cancer promoter | / | Carbidopa | / | 2019 | [200] |
| BC and Lymphoma | IDO | Cancer promoter | poor trastuzumab response | a combination of trastuzumab or rituximab with inhibitors of PD-L1 and IDO | 307 paired biopsy samples and surgically resected samples from invasive HER2 + BC patients before and after neoadjuvant treatments and animal models | 2018 | [201] |
| BC, Melanoma, and CRC | Kyn | Cancer promoter | / | a combination of PEG-KYNase with approved checkpoint inhibitors or with a cancer vaccine | Animal models | 2018 | [202] |
| BC | IDO | Cancer promoter | tumor grade | / | 4 TMA containing BC tissues from 242 invasive primary breast carcinomas, 20 nodal metastases, and 19 distant metastases | 2018 | [203] |
| BC | IDO1 | Cancer promoter | / | Thiosemicarbazide derivatives 1–3 | / | 2023 | [204] |
| BC | IDO1 and TDO2 | Cancer promoter | / | IDO/TDO-IN-3 | 70 BC tissues and orthotopic BC mouse model | 2023 | [205] |
| BC | KMO | Cancer promoter | tumor metastasis, recurrence, RFS, and DFS | UPF 648 and Ro 61–8048 | BC cancer tissues and paired mammary epithelial samples, TCGA database, and animal models | 2020 | [206] |
| OC | IDO1, IDO2, TDO2 and IL4I1 | Cancer promoter | late tumor stage, lymph node metastasis, bilateral tumors, endometriosis, and tumor rupture | Platinum | 127 OC tissue samples | 2023 | [207] |
| LC | IDO1, IDO2, and TDO | Cancer promoter | / | F04 | Immunocompetent C57BL6 mice and lung metastasis of Lewis cells model | 2019 | [208] |
| LC | IDO | Cancer promoter | OS and PFS | / | 110 serum samples from patients with stage III inoperable/unresectable NSCLC | 2018 | [209] |
| LC | TDO | Cancer promoter | / | a combination of AhR inhibitor DMF and EGFR TKIs | 20 LC cancer samples, and a xenograft mouse model using A549 or Lewis cells | 2022 | [210] |
| HCC, Glioma, PC and CRC | TDO | Cancer promoter | / | PF06845102/EOS200809 | CT26 cancer and TDO-KO mice bearing MC38 cancer | 2020 | [163] |
Targeting Trp Metabolism in Glioma
In glioma, elevated IDO expression plays a significant role in promoting immunosuppression and cancer progression. Preclinical studies have shown that the IDO inhibitor 1-methyl-tryptophan (1-MT) significantly suppresses cancer growth in a subcutaneous glioma model, especially when used in combination with temozolomide (TMZ), an established chemotherapeutic agent. Mice with intracranially inoculated IDO knockdown glioma cells exhibit longer survival compared to control mice [244]. The combination of 1-MT with other chemotherapeutic agents (e.g., TMZ, bischloroethylnitrosourea, etoposide, cisplatin) in glioma cell lines has further demonstrated enhanced IDO inhibition, reversing immune resistance and impairing glioma cell proliferation [196]. Clinical trials are currently exploring the efficacy of IDO inhibitors in glioma treatment. A phase I trial (NCT02502708) of the oral IDO inhibitor indoximod in children with recurrent brain cancer, including diffuse intrinsic pontine glioma (DIPG), showed promising early results, such as reduced disease burden and extended periods of disease control [245]. Building on these findings, a phase II trial (NCT04049669) has been initiated, combining indoximod with chemo-immunotherapy, and a phase I salvage trial (NCT05106296) is testing its combination with ibrutinib to counteract immune evasion. Another promising approach involves oncolytic viruses, which have shown potent anti-immunosuppressive effects in glioma by lysing cancer cells and stimulating a stronger immune response [246–250]. In a phase I study, the oncolytic virus Delta-24-RGD (DNX-2401, AdCMVdelta24) led to complete cancer regression in 20% of patients with recurrent glioblastoma [251]. The third-generation adenovirus Delta-24-RGDOX (DNX-2440) demonstrated even more effective T-cell-mediated anticancer responses in preclinical models. When combined with IDO inhibitors, Delta-24-RGDOX increased CD8+ T cells and decreased immunosuppressive cells like MDSCs and Tregs, leading to the complete eradication of glioma in murine models [202]. Clinical trials for Delta-24-RGDOX are ongoing in patients with malignant gliomas (NCT03714334) and liver metastases (NCT04714983). Targeting both IDO1 and TDO simultaneously has emerged as a promising strategy for overcoming the limitations of single-enzyme inhibition. The IDO1/TDO dual inhibitor RY103 demonstrated potent anti-glioma effects by disrupting the IDO1/TDO/Kyn/AHR/AQP4 signaling axis, reducing tumor size and extending survival in orthotopic glioma models [171]. Additionally, novel therapeutic approaches targeting TDO have shown promise. The interaction between FKBP52 and the glucocorticoid receptor (GR) has been identified as a key regulator of TDO expression in gliomas. Treatment with FK506, an immunosuppressant that binds FKBP52, increases TDO expression and Kyn production, suggesting that modulating GR signaling could be a potential avenue for controlling TDO expression in gliomas [252]. The TDO2 inhibitor 680C91, when combined with chemotherapeutic agents such as retinoic acid or irinotecan, has demonstrated synergistic anticancer effects in neuroblastoma cells by inhibiting the Kyn/AHR pathway [173].
Targeting Trp Metabolism in Digestive System Cancers
In CRC therapy, combining the SERT inhibitor sertraline with dietary Trp restriction or the MEK inhibitor trametinib significantly weakens Trp uptake and degradation, leading to decreased CRC cell viability and cancer growth [45]. Additionally, local photothermal therapy (PTT) further induces cancer cells to release antigens, activating immune responses against residual lesions and distant metastases [253–255]. However, the immunosuppressive microenvironment often limits anticancer immunity by reducing the recognition efficiency of cancer antigens [256–260]. Recent advancements in in situ vaccines, such as outer membrane vesicles (OMVs) loaded with the IDO inhibitor 1-MT (1-MT@OMV-Mal), have shown promise in facilitating immune-mediated cancer clearance after PTT. This approach enhances the recognition and capture of cancer antigens by dendritic cells, leading to improved cancer-specific cytotoxic T cell (CTL) activation. In situ administration of 1-MT@OMV-Mal has demonstrated significant inhibition of both primary and distant CRC tumors [203]. In addition to vaccines, IDO1 inhibitors such as 1-MT and Epacadostat have been shown to reduce CRC cell viability by suppressing IDO expression and inhibiting Kyn-induced CDC20 transcription. A 1-MT-supplemented diet al.so prevents the development of sporadic colon cancer in mice induced by azoxymethane (AOM) and dextran sodium sulfate (DSS), suggesting its potential use in chemoprevention for colitis-associated CRC [204]. Moreover, PEGylated kynureninase (PEG-KYNase), a pharmacologically optimized enzyme, degrades Kyn into immunologically inactive metabolites. This enhances CD8+ T cell proliferation and infiltration in the TME, impairing tumor growth. Notably, PEG-KYNase has demonstrated enhanced therapeutic efficacy when combined with checkpoint inhibitors or cancer vaccines, showing promising results in breast cancer, melanoma, and CRC treatment [261]. In PC, the IDO1 inhibitor Epacadostat, combined with serine starvation, effectively reduces the proliferation of IDO1-expressing cells, thus inhibiting cancer growth [178]. However, the dual functions of IDO1 in both cancerogenesis and metastasis complicate its application, contributing to setbacks in clinical trials [180]. In orthotopic PC mouse models, the dual IDO1/TDO inhibitor RY103 inhibits KPIC cell migration and invasion, reducing cancer metastasis by blocking the Kyn/AHR signaling pathway. Additionally, RY103 improves the immunosuppressive state by decreasing PMN-MDSCs and M-MDSCs in Pan02 cancer-bearing mice [179]. In a separate approach, a high-Trp diet in PC gnotobiotic mice elevates serum 3-indoleacetic acid (3-IAA) levels, which resulted in reduced cancer weight and enhanced responsiveness to FIRINOX treatment. Repeated cycles of 3-IAA combined with FIRINOX extends survival times in these orthotopic PC models [181]. In HCC, administering Lactobacillus reuteri, which produces Trp metabolites, or the AHR agonist 6-formylindolo(3,2-b) carbazole (Ficz), suppresses SREBP2 expression and inhibits cancer growth in mice with imbalanced gut flora [115]. Additionally, TDO-targeted conjugates, which combine the TDO inhibitor PVI with irinotecan (Ir), improves CD4+ and CD8+ T cell proliferation by inhibiting TDO expression and blocking Kyn production in the HCC TME. These conjugates also induce cell cycle arrest in the G2 phase and triggered apoptosis in HepG2 cells by releasing irinotecan, thus demonstrating the synergistic effects of combining immunotherapy and chemotherapy in HCC treatment [262]. Moreover, the combination of the AHR inhibitor PDM2 with chemotherapy agents such as Doxorubicin or 5-Fluorouracil enhances cancer-suppressive effects and prolongs OS duration in TDO2 overexpressing SMC-7721 bearing HCC mice by inhibiting AHR/IL-6/STAT3/NF-kB signaling [182]. The TDO inhibitor PF06845102/EOS200809 has also shown promise for treating TDO-expressing cancer, including HCC, glioblastomas, PC, and CRC, especially when used in combination with checkpoint inhibitors. Notably, TDO inhibitors increases Trp levels and enhances the efficacy of immunotherapy by overcoming IDO1-mediated immunosuppression, even in cancers without TDO expression at the tumor site [163]. Furthermore, in various HCC mouse models, overexpression of KMO or treatment with its substrate 3-HAA significantly enhances the efficacy of the IDO1 inhibitor Epacadostat, resulting in reduced cancer numbers and prolonged survival [183].
Targeting Trp Metabolism in BC
Antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) are critical for the effectiveness of anticancer therapeutic antibodies. However, recent studies have highlighted the detrimental role of ADCP macrophages in cancer immunosuppression. In HER2 + BC patients receiving neoadjuvant trastuzumab therapy, cancer-associated macrophages (TAMs) significantly upregulate PD-L1 and IDO, creating an immunosuppressive TME and contributing to poor treatment responses to trastuzumab. Combining anti-PD-L1 and IDO inhibitors with therapeutic antibodies, such as trastuzumab or rituximab for BC and lymphoma treatment, has been shown to synergistically enhance therapeutic efficacy by boosting anticancer immunity [210]. The dual inhibitor IDO/TDO-IN-3 also restores NK cell-mediated cytotoxicity and enhances trastuzumab efficacy, effectively inhibiting cancer progression in orthotopic BC mouse models [263]. Thiosemicarbazide derivatives (1–3), as double inhibitors of topoisomerase IIα and IDO1, induce apoptosis, cause cell cycle arrest, and increase drug sensitivity in BC MCF-7 and MDA-MB-231 cells in a dose-dependent manner. Their pro-apoptotic efficacy is significantly higher than that of etoposide and they exhibit beneficial ADME-Tox properties [211]. Additionally, the novel pan IDO1/IDO2/TDO inhibitor F04 increases the accumulation and infiltration of T cells in the TME, suppressing cancer progression dose-dependently in immunocompetent C57BL6 mice and a lung metastasis model of Lewis cells. Notably, F04 demonstrates a more potent effect in reducing the Kyn/Trp ratio compared to Epacadostat, further emphasizing its therapeutic potential [264].
Targeting Trp Metabolism in Other Cancers
In metastatic melanoma, the phase I/II MM1636 trial (NCT03047928) involving thirty anti-PD1 therapy-naive patients showed encouraging results for an immune-modulatory vaccine (IO102/IO103) targeting IDO/PD-L1 combined with adjuvant Montanide and nivolumab. The trial achieved an objective response rate (ORR) of 80%, a complete response rate (CR) of 43%, and a median PFS (mPFS) of 26 months. Vaccine-specific T cells from vaccinated patients recognized cancer cells in a target- and HLA-restricted manner and polarized myeloid cells to a cancer-associated phenotype, enhancing vaccine-specific responses [265]. However, the phase III ECHO-301/KEYNOTE-252 trial (NCT02752074) with 706 patients with unresectable or metastatic melanoma, who were randomly assigned to receive the IDO1 selective inhibitor Epacadostat plus the PD-1 inhibitor pembrolizumab (n = 354) or placebo plus pembrolizumab (n = 352), did not show additional benefits in PFS or OS over the placebo group [266]. The AHR pathway exhibits selective activity in cancer overexpressing IDO/TDO and is associated with resistance to immune checkpoint inhibitors. The AHR pathway drives T cell dysfunction by promoting a suppressive axis between Tregs and macrophages within the melanoma TME. Selective AHR blockade with CH-223,191 reverses IDO-Kyn-AHR-mediated immunosuppression and delays melanoma progression. Additionally, using the AHR antagonist KYN-101 in IDO/TDO-expressing cancer improves the limitations of targeting IDO or TDO alone and sensitizes cancer to anti-PD-1 therapy in melanoma [267]. IFN-γ prompts endogenous frameshifting events at Trp residues, leading to their presentation on HLA-I molecules and triggering peptide-specific T-cell responses in melanoma MD55A3 cells. This process diversifies the peptidome landscape, driving IDO1-mediated Trp depletion, and plays a crucial role in enhancing immune recognition in anti-melanoma treatments [213]. In LC xenograft mouse models, the combined administration of the AHR inhibitor DMF and TKIs also significantly inhibits cancer growth, reverses resistance to TKIs, and prolongs survival time [193]. Furthermore, a Phase I trial (NCT01219348) is currently underway to evaluate a novel immunotherapeutic strategy for patients with locally advanced or metastatic LC. This strategy involves IDO peptide vaccination in combination with the immune-stimulating agent Aldara and the adjuvant Montanide to enhance the immune response. Additionally, targeting the serotonin pathway in carcinogenesis has emerged as a promising approach in various cancers, showing anticancer effects in some preclinical trials. In prostate cancer, the TPH1 inhibitor 4-chloro-dl-phenylalanine (PCPA) disrupts the TPH1/5-HT/β-catenin/ZBP-89/TPH1 feedback loop, significantly enhancing the anticancer effects of paclitaxel and suppressing lung metastasis in prostate cancer-bearing mice [220].
Current Status and Future Prospects of Trp Metabolism in Cancer
Trp metabolism plays a crucial role in cancer progression and immune modulation, with research primarily focusing on the KP, which generates multiple bioactive compounds with immunosuppressive properties. However, despite significant attention on KP, the serotonin and indole pathways are less frequently explored in cancer [53, 64, 268]. Recent insights suggest that a more nuanced understanding of these alternative pathways is necessary to broaden the scope of therapeutic applications targeting Trp metabolism.
Current Status of IDO1 and TDO inhibitors
Early research efforts were largely concentrated on developing IDO1 inhibitors, as IDO1 is a key enzyme that suppresses anticancer immunity by driving the conversion of Trp into Kyn [269–273]. While preclinical studies yielded promising results, translating these findings into clinical success has been more challenging. A notable example is the ECHO-301 phase III trial, which evaluated the selective IDO1 inhibitor Epacadostat (INCB24360) in combination with pembrolizumab (anti-PD-1 antibody) in patients with advanced melanoma. Unfortunately, the trial did not show significant improvement in PFS or OS compared to the placebo group, prompting a reevaluation of the therapeutic potential of IDO1 inhibition alone [274–277]. This result has shifted the focus toward understanding the broader role of Trp metabolism in cancer and exploring more effective combination therapies. One key limitation of these trials is the absence of reliable biomarkers to assess IDO1 levels and activity before and during treatment. Given that the efficacy of IDO1 inhibitors hinges on the presence and functionality of the enzyme, the development of robust clinical tools to stratify patients based on IDO1 expression and to monitor enzyme activity in real time is crucial [278]. Additionally, the lack of standardized methods for measuring metabolite and drug concentrations at target sites complicates the evaluation of treatment efficacy [279, 280].
In response to these challenges, recent research has expanded beyond IDO1 to include dual inhibitors that target both IDO1 and TDO. Preclinical data suggest that dual IDO1/TDO inhibitors may offer a more comprehensive blockade of Trp metabolism, potentially overcoming the limitations of selective IDO1 inhibition [51, 55, 281]. In addition, an open-label, Phase I multicenter study (NCT03208959) is currently underway to assess the preliminary efficacy and safety of a novel orally administered small-molecule IDO1/TDO dual inhibitor, HTI-1090, in patients with advanced solid tumors.
Expanding the Scope: KMO and TPH Inhibitors
Since KMO is overexpressed in several cancer types and plays a role in cancer development, the development of KMO inhibitors represents a novel strategy for cancer treatment [282–284]. In recent years, research into KMO inhibitors has shown potential as a promising therapeutic approach for various diseases. However, the majority of KMO inhibitors currently under investigation are focused on neurodegenerative diseases [243, 285, 286]. Due to the relatively poor efficacy of these inhibitors and limited preclinical trials, few have successfully completed clinical trials in cancer treatment. Expanding the focus of KMO inhibitors to cancer may open new therapeutic avenues, particularly by targeting KMO’s immunosuppressive and pro-tumorigenic effects [242]. Similarly, the TPH-serotonin signaling pathway has gained attention as a contributor to cancer progression. Several TPH inhibitors and serotonin receptor antagonists have shown the anticancer effects in animal models [287, 288]. Notably, TPH inhibitors have shown promising results in the treatment of diverse disorders, such as neuropsychiatric conditions, gastrointestinal dysfunction, osteoporosis, and bone homeostasis. Additionally, the excessive secretion of serotonin by cancer cells can lead to the typical symptoms of carcinoid syndrome and in 2017, TPH inhibitors were approved by the United States Food and Drug Administration (FDA) for managing gastrointestinal symptoms associated with this condition [289–291]. Additionally, excessive serotonin secretion by cancer cells can cause the typical symptoms of carcinoid syndrome. In 2017, TPH inhibitors were approved by the United States Food and Drug Administration (FDA) for managing gastrointestinal symptoms associated with this condition. A pilot clinical trial (NCT03453489) is also investigating TPH levels in neuroendocrine cancer to assess the efficacy of Etiprate treatment. Despite these findings concerning the secretion pathway, its specific role in cancer progression and the therapeutic efficacy of targeting serotonin, TPH, and its receptors in cancer treatment remains limited.
Overcoming Translational Challenges
Despite significant advancements, the development of selective, potent, and safe inhibitors for Trp-metabolizing enzymes remains a challenge. Inhibitors targeting IDO1, TDO, and other enzymes within the Trp metabolic pathway must balance efficacy with safety, as Trp metabolism is essential for normal physiological processes [292]. Off-target effects and toxicity continue to be concerns, underscoring the need for improved detection tools to monitor tissue-specific Trp concentrations and metabolite levels throughout treatment.
Combination Therapies and Future Directions
Given the challenges encountered with IDO1 inhibitors, there is growing interest in combining Trp metabolism inhibitors with immune checkpoint inhibitors, chemotherapy, or radiotherapy to enhance anticancer immune responses and improve clinical outcomes [293]. Recent clinical trials (NCT03291054, NCT01961115, NCT02785250, NCT03006302, NCT03516708, NCT03661320, NCT02077881 and NCT02835729) have tested novel combinations, such as combining IDO1 inhibitors with immunotherapies, or with radiotherapy and/or chemotherapy [82]. While some trials have demonstrated improvements in PFS and OS for specific patient populations, broader success remains elusive. Understanding the optimal sequencing and timing of combination therapies is critical, as Trp metabolism modulation may need to occur in specific stages of the immune response to maximize therapeutic benefit [294–296].
Furthermore, researchers are increasingly focused on uncovering downstream effector mechanisms in Trp metabolism that may be relevant to cancer progression [180, 297]. While the immunosuppressive effects of kynurenine are well-established, emerging studies suggest that indole derivatives may also promote cancer through activation of the AHR [88–91]. AHR regulates genes involved in immune suppression, inflammation, and cell proliferation, making it a promising target for future therapies.
Addressing these challenges necessitates uncovering additional oncogenic mechanisms of Trp metabolism, identifying the relevance of Trp-related molecules, and evaluating the roles and therapeutic significance of other enzymes within Trp metabolism [298–300]. The integration of cutting-edge technologies such as multi-omics, CRISPR gene editing, and single-cell sequencing could help identify new therapeutic targets within Trp metabolism [269, 294, 301–303]. Moreover, the strategic combination of Trp metabolism inhibitors with other immunotherapies, guided by improved biomarker detection and patient stratification, represents a forward-looking approach that could enhance treatment outcomes in cancer.
Conclusion
Trp, an essential amino acid, influences various physiological and pathological processes through its metabolism into serotonin, Kyn, and indole derivatives. Dysregulated Trp metabolismis observed in many cancers and is strongly linked to clinical features such as tumor stage, size, and lymph node metastasis. Additionally, Trp metabolite levels correlate with patient prognosis, serving as robust predictive markers. Aberrant Trp metabolism affects multiple malignant processes in cancer, including cell proliferation, migration, invasion, and immune evasion, primarily through interactions with various cancer-related molecules and signaling pathways. Consequently, targeting Trp metabolism has emerged as a promising avenue in cancer therapy. While numerous preclinical trials have demonstrated the anticancer effects of inhibiting Trp metabolism, translating these findings into clinical success remains a challenge. The failure of IDO1 inhibitors in clinical trials highlights the complexity of the TME, the compensatory activation of alternative immune-suppressive pathways, and the heterogeneity of Trp metabolism across different cancer types and patient populations. To overcome these clinical challenges, future research should prioritize a deeper exploration of the underlying mechanisms driving resistance, as well as utilizing multi-omics approaches to identify novel biomarkers and therapeutic targets. Another critical area for future research is the design of combination therapies that address the limitations of current Trp metabolism inhibitors. Given the compensatory activation of alternative immune-suppressive pathways, combining Trp-targeted therapies with immune checkpoint inhibitors, targeted therapies, or even next-generation cancer vaccines to simultaneously target multiple metabolic pathways may enhance therapeutic efficacy while minimizing resistance.
In summary, although significant progress has been made in understanding the role of Trp metabolism in cancer, addressing these research gaps is essential for clinical translation. The continued investigation of Trp metabolism, coupled with advanced technologies and innovative combination strategies, holds substantial promise for advancing cancer therapy and ultimately improving patient outcomes.
Acknowledgements
Not applicable.
Abbreviations
- Trp
Tryptophan
- KP
kynurenine pathway
- IDO
indoleamine-2,3-dioxygenase
- TDO
Trp-2,3-dioxygenase
- KMO
kynurenine monooxygenase
- TME
tumor microenvironment
- Kyn
kynurenine
- AHR
aryl hydrocarbon receptor
- KynA
kynurenic acid
- QA
quinolinic acid
- CA
cinnabarinic acid
- XA
xanthurenic acid
- NAD+
nicotinamide adenine dinucleotide
- SLC1A5
solute carrier family 1 member 5
- SLC7A5
solute carrier family 7 member 5
- I3P
indole-3-pyruvate
- TPH
Trp hydroxylase
- 5-HTP
5-hydroxytryptophan
- AADC
aromatic amino acid decarboxylase
- 5-HIAA
5-hydroxyindoleacetic acid
- MAO
monoamine oxidase
- NAS
N-acetylserotonin
- AANAT
arylalkylamine N-acetyltransferase
- ASMT
N-acetylserotonin O-methyltransferase
- AFMID
arylformamidase
- KYNU
kynureninase
- 3-HK
3-hydroxykynurenine
- AA
anthranilic acid
- KAT
Kynurenine aminotransferase
- 3-HAA
3-hydroxyanthranilic acid
- QPRT
quinolinate phosphoribosyl transferase
- ARNT
aryl hydrocarbon receptor nuclear translocator
- ELISA
Enzyme-Linked Immunosorbent Assay
- LC-MS
liquid chromatography-mass spectrometry
- UHPLC-ESI-MS/MS
ultra-high-performance LC-electrospray ionization-tandem MS
- GBM
glioblastoma
- OS
overall survival
- PC
pancreatic cancer
- RFS
relapse-free survival
- PFS
progression-free survival
- DFS
disease-free survival
- TILs
tumor-infiltrating lymphocytes
- PD
Parkinson's disease
- CAFs
cancer-associated fibroblasts
- Tregs
regulatory T cells
- GSCs
stem-like cells in glioblastoma
- HCC
hepatocellular carcinoma
- CRC
colorectal cancer
- GC
gastric cancer
- BC
breast cancer
- OC
ovarian cancer
- LC
lung cancer
- ROS
reactive oxygen species
- ADCC
antibody-dependent cellular cytotoxicity
- TKIs
tyrosine kinase inhibitors
- TMZ
temozolomide
Authors' contributions
Dongming Yan, Hongjiang Li, Lixia Xu, and Jingliang Cheng designed the structure of the review. Jing Yan, Di Chen, and Zi Ye wrote the article. Xuqiang Zhu, Xueyuan Li, and Henan Jiao draw the figures. Mengjiao Duan, and Chaoli Zhang completed the tables. Jing Yan, Jingliang Cheng, and Dongming Yan helped with the final revision of the review. All authors reviewed the manuscript and approved the final manuscript.
Funding
This work was supported by the China National Natural Science Foundation (82102149, 82472069, and 82401623), the China Postdoctoral Science Foundation (2024M752952), the Excellent Youth Talent Cultivation Program of Innovation in Health Science and Technology of Henan Province (YXKC2022061), and the Henan Medical Science and Technology Joint Building Program (LHGJ20230239).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The corresponding author has received consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Yan, Di Chen and Zi Ye contributed equally to this work.
Contributor Information
Lixia Xu, Email: mslixiaxu@163.com.
Hongjiang Li, Email: hongjianglineuron@163.com.
Dongming Yan, Email: mrdmyan@163.com.
References
- 1.Sadok I, Jędruchniewicz K. Dietary kynurenine pathway metabolites-source, fate, and chromatographic determinations. Int J Mol Sci. 2023;24:24. 10.3390/ijms242216304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamshed L, Debnath A, Jamshed S, Wish JV, Raine JC, Tomy GT, et al. An emerging cross-species marker for organismal health: tryptophan-kynurenine pathway. Int J Mol Sci. 2022;23:23. 10.3390/ijms23116300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nongonierma AB, FitzGerald RJ. Milk proteins as a source of tryptophan-containing bioactive peptides. Food Funct. 2015;6:2115–27. 10.1039/c5fo00407a. [DOI] [PubMed] [Google Scholar]
- 4.Attenburrow MJ, Williams C, Odontiadis J, Powell J, Van de Ouderaa F, Williams M, et al. The effect of a nutritional source of tryptophan on dieting-induced changes in brain 5-HT function. Psychol Med. 2003;33:1381–6. 10.1017/s0033291703008547. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt M, Greathouse KL. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients. 2021;13. 10.3390/nu13041189. [DOI] [PMC free article] [PubMed]
- 6.Melhem NJ, Taleb S. Tryptophan: from diet to cardiovascular diseases. Int J Mol Sci. 2021;22. 10.3390/ijms22189904. [DOI] [PMC free article] [PubMed]
- 7.Tsuji A, Ikeda Y, Yoshikawa S, Taniguchi K, Sawamura H, Morikawa S, et al. The tryptophan and kynurenine pathway involved in the development of immune-related diseases. Int J Mol Sci. 2023;24:24. 10.3390/ijms24065742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41:1195–205. 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4:199–220. 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- 10.Hinkley JM, Yu GX, Standley RA, Distefano G, Tolstikov V, Narain NR, et al. Exercise and ageing impact the kynurenine/tryptophan pathway and acylcarnitine metabolite pools in skeletal muscle of older adults. J Physiol. 2023;601:2165–88. 10.1113/jp284142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badawy AA, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond). 2016;130:1327–33. 10.1042/cs20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai W, Huang Z, Li S, Li XG, Luo D. Kynurenine pathway metabolites modulated the comorbidity of IBD and depressive symptoms through the immune response. Int Immunopharmacol. 2023;117: 109840. 10.1016/j.intimp.2023.109840. [DOI] [PubMed] [Google Scholar]
- 13.Curzon G, Bridges PK. Tryptophan metabolism in depression. J Neurol Neurosurg Psychiatry. 1970;33:698–704. 10.1136/jnnp.33.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Goot AT, Nollen EA. Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends Mol Med. 2013;19:336–44. 10.1016/j.molmed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Anaya JM, Bollag WB, Hamrick MW, Isales CM. The role of tryptophan metabolites in musculoskeletal stem cell aging. Int J Mol Sci. 2020;21:21. 10.3390/ijms21186670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galligan JJ. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol Motil. 2018;30:30. 10.1111/nmo.13283. [DOI] [PubMed] [Google Scholar]
- 17.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–10. 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddison DC, Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol. 2015;40:134–41. 10.1016/j.semcdb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Gargaro M, Scalisi G, Manni G, Briseño CG, Bagadia P, Durai V, et al. Indoleamine 2,3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication. Immunity. 2022;55:1032–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koper JE, Troise AD, Loonen LM, Vitaglione P, Capuano E, Fogliano V, et al. Tryptophan supplementation increases the production of microbial-derived AhR agonists in an in vitro simulator of intestinal microbial ecosystem. J Agric Food Chem. 2022;70:3958–68. 10.1021/acs.jafc.1c04145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang X, Su T, Wu P, Dai Y, Chen Y, Wang Q, et al. Identification of paeoniflorin from Paeonia lactiflora pall. As an inhibitor of tryptophan 2,3-dioxygenase and assessment of its pharmacological effects on depressive mice. J Ethnopharmacol. 2023;317: 116714. 10.1016/j.jep.2023.116714. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan R, Anand NS, Wang G, Hong X, Pearson C, Zuckerman B, et al. Association between cord blood metabolites in tryptophan pathway and childhood risk of autism spectrum disorder and attention-deficit hyperactivity disorder. Transl Psychiatry. 2022;12:270. 10.1038/s41398-022-01992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutanto CN, Loh WW, Kim JE. The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutr Rev. 2022;80:306–16. 10.1093/nutrit/nuab027. [DOI] [PubMed] [Google Scholar]
- 24.Kennaway DJ. The mammalian gastro-intestinal tract is a NOT a major extra-pineal source of melatonin. J Pineal Res. 2023;75:e12906. 10.1111/jpi.12906. [DOI] [PubMed] [Google Scholar]
- 25.Liaqat H, Parveen A, Kim SY. Neuroprotective natural products’ regulatory effects on depression via gut-brain axis targeting tryptophan. Nutrients. 2022;14:14. 10.3390/nu14163270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Yin L, Liu Q, Guan Y, Nie S, Zhu Y, et al. Tryptophan metabolic pathway plays a key role in the stress-induced emotional eating. Curr Res Food Sci. 2024;8: 100754. 10.1016/j.crfs.2024.100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone TW, Williams RO. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol Sci. 2023;44:442–56. 10.1016/j.tips.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Gargaro M, Manni G, Scalisi G, Puccetti P, Fallarino F. Tryptophan metabolites at the crossroad of immune-cell interaction via the Aryl hydrocarbon receptor: implications for tumor immunotherapy. Int J Mol Sci. 2021;22:22. 10.3390/ijms22094644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Günther J, Fallarino F, Fuchs D, Wirthgen E, Editorial. Immunomodulatory roles of tryptophan metabolites in inflammation and cancer. Front Immunol. 2020;11: 1497. 10.3389/fimmu.2020.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak S, Nadar R, Kim S, Liu K, Govindarajulu M, Cook P, et al. The influence of kynurenine metabolites on neurodegenerative pathologies. Int J Mol Sci. 2024;25:25. 10.3390/ijms25020853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrawy MM, Westbrook R, King A, Khosravian N, Ochaney N, DeCarvalho T, et al. Dual treatment with kynurenine pathway inhibitors and NAD(+) precursors synergistically extends life span in Drosophila. Aging Cell. 2024;23: e14102. 10.1111/acel.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joisten N, Ruas JL, Braidy N, Guillemin GJ, Zimmer P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med. 2021;27:946–54. 10.1016/j.molmed.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Pereira MS, Kriegel MA. Translocating lactobacillus torments tumors via tryptophan catabolism. Cell. 2023;186:1821–3. 10.1016/j.cell.2023.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Wojciech L, Png CW, Koh EY, Kioh DYQ, Deng L, Wang Z, et al. A tryptophan metabolite made by a gut microbiome eukaryote induces pro-inflammatory T cells. Embo j. 2023;42: e112963. 10.15252/embj.2022112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71:734–45. 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, Yang Z, Li J, Zhang Y, Zhang W, Doherty M, et al. Association between gut microbiome-related metabolites and symptomatic hand osteoarthritis in two independent cohorts. EBioMedicine. 2023;98: 104892. 10.1016/j.ebiom.2023.104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madella AM, Van Bergenhenegouwen J, Garssen J, Masereeuw R, Overbeek SA. Microbial-derived tryptophan catabolites, kidney disease and gut inflammation. Toxins (Basel). 2022;14. 10.3390/toxins14090645. [DOI] [PMC free article] [PubMed]
- 38.Ballesteros J, Rivas D, Duque G. The role of the kynurenine pathway in the pathophysiology of frailty, sarcopenia, and osteoporosis. Nutrients. 2023;15:15. 10.3390/nu15143132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meisel JW, Patel MB, Garrad E, Stanton RA, Gokel GW. Reversal of tetracycline resistance in Escherichia coli by Noncytotoxic bis(Tryptophan)s. J Am Chem Soc. 2016;138:10571–7. 10.1021/jacs.6b05578. [DOI] [PubMed] [Google Scholar]
- 40.Ahn YH, Oh SC, Zhou S, Kim TD. Tryptophanyl-tRNA synthetase as a potential therapeutic target. Int J Mol Sci. 2021;22:22. 10.3390/ijms22094523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435–40. 10.1158/0008-5472.Can-12-0569. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Wang N, Xu L, Liu Y, Huang L, Gu M, et al. Aryl hydrocarbon receptor dependent anti-inflammation and neuroprotective effects of tryptophan metabolites on retinal ischemia/reperfusion injury. Cell Death Dis. 2023;14:92. 10.1038/s41419-023-05616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeitler L, Murray PJ. IL4i1 and IDO1: oxidases that control a tryptophan metabolic nexus in cancer. J Biol Chem. 2023;299: 104827. 10.1016/j.jbc.2023.104827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, Yao Q, Liu P, Zhang H, Liu Y, Li S, et al. Critical roles and clinical perspectives of RNA methylation in cancer. MedComm (2020). 2024;5:e559. 10.1002/mco2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye D, Xu H, Xia H, Zhang C, Tang Q, Bi F. Targeting SERT promotes tryptophan metabolism: mechanisms and implications in colon cancer treatment. J Exp Clin Cancer Res. 2021;40:173. 10.1186/s13046-021-01971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Lu J, Du W. Tryptophan metabolism in digestive system tumors: unraveling the pathways and implications. Cell Commun Signal. 2024;22:174. 10.1186/s12964-024-01552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacheco JHL, Elizondo G. Interplay between Estrogen, Kynurenine, and AHR pathways: an immunosuppressive axis with therapeutic potential for breast cancer treatment. Biochem Pharmacol. 2023;217:115804. 10.1016/j.bcp.2023.115804. [DOI] [PubMed] [Google Scholar]
- 48.Mandarano M, Orecchini E, Bellezza G, Vannucci J, Ludovini V, Baglivo S, et al. Kynurenine/Tryptophan ratio as a potential blood-based biomarker in non-small cell lung cancer. Int J Mol Sci. 2021;22:22. 10.3390/ijms22094403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F, Zhao Z, Zhang Z, Zhang Y, Guan W. Tryptophan metabolism induced by TDO2 promotes prostatic cancer chemotherapy resistance in a AhR/c-Myc dependent manner. BMC Cancer. 2021;21:1112. 10.1186/s12885-021-08855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanser L, Kink P, Egger EM, Willenbacher W, Fuchs D, Weiss G, et al. Inflammation-induced tryptophan breakdown is related with anemia, fatigue, and depression in cancer. Front Immunol. 2020;11: 249. 10.3389/fimmu.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M, Tomek P, Tryptophan. A rheostat of cancer immune escape mediated by immunosuppressive enzymes IDO1 and TDO. Front Immunol. 2021;12: 636081. 10.3389/fimmu.2021.636081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21:5427–33. 10.1158/1078-0432.Ccr-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20:347. 10.1186/s12967-022-03554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peyraud F, Guegan JP, Bodet D, Cousin S, Bessede A, Italiano A. Targeting tryptophan catabolism in cancer immunotherapy era: challenges and perspectives. Front Immunol. 2022;13: 807271. 10.3389/fimmu.2022.807271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Opitz CA, Somarribas Patterson LF, Mohapatra SR, Dewi DL, Sadik A, Platten M, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. 2020;122:30–44. 10.1038/s41416-019-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology. 2015;4: e982382. 10.4161/2162402x.2014.982382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonia Álvarez-Fernández M, Fernández-Cruz E, Valero E, Troncoso AM, Carmen García-Parrilla M. Efficiency of three intracellular extraction methods in the determination of metabolites related to tryptophan and tyrosine in winemaking yeast’s metabolism by LC-HRMS. Food Chem. 2019;297:124924. 10.1016/j.foodchem.2019.05.198. [DOI] [PubMed] [Google Scholar]
- 58.Meier AH, Wilson JM. Tryptophan feeding adversely influences pregnancy. Life Sci. 1983;32:1193–6. 10.1016/0024-3205(83)90187-x. [DOI] [PubMed] [Google Scholar]
- 59.Shabbir F, Patel A, Mattison C, Bose S, Krishnamohan R, Sweeney E, et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem Int. 2013;62:324–9. 10.1016/j.neuint.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Seo SK, Kwon B. Immune regulation through tryptophan metabolism. Exp Mol Med. 2023;55:1371–9. 10.1038/s12276-023-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–43. 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Grifka-Walk HM, Jenkins BR, Kominsky DJ. Amino acid trp: the far out impacts of host and commensal tryptophan metabolism. Front Immunol. 2021;12: 653208. 10.3389/fimmu.2021.653208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granados JC, Richelle A, Gutierrez JM, Zhang P, Zhang X, Bhatnagar V, et al. Coordinate regulation of systemic and kidney tryptophan metabolism by the drug transporters OAT1 and OAT3. J Biol Chem. 2021;296: 100575. 10.1016/j.jbc.2021.100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Platten M, Friedrich M, Wainwright DA, Panitz V, Opitz CA. Tryptophan metabolism in brain tumors - IDO and beyond. Curr Opin Immunol. 2021;70:57–66. 10.1016/j.coi.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Zhang ZH, Zabed HM, Yun J, Zhang G, Qi X. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol Nutr Food Res. 2021;65: e2000461. 10.1002/mnfr.202000461. [DOI] [PubMed] [Google Scholar]
- 66.Babitzke P. Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol Microbiol. 1997;26:1–9. 10.1046/j.1365-2958.1997.5541915.x. [DOI] [PubMed] [Google Scholar]
- 67.Fiore A, Zeitler L, Russier M, Groß A, Hiller MK, Parker JL, et al. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell. 2022;82:920–32. 10.1016/j.molcel.2022.02.007. .e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z, Schoones T, Wells JM, Fogliano V, Capuano E. Substrate-driven differences in tryptophan catabolism by gut microbiota and aryl hydrocarbon receptor activation. Mol Nutr Food Res. 2021;65: e2100092. 10.1002/mnfr.202100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lv Z, Shi W, Zhang Q. Role of essential amino acids in age-induced bone loss. Int J Mol Sci. 2022;23:23. 10.3390/ijms231911281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. 2021;22:22. 10.3390/ijms22062973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuka B, Körtési T, Nánási N, Tömösi F, Janáky T, Veréb D, et al. Cluster headache and kynurenines. J Headache Pain. 2023;24:35. 10.1186/s10194-023-01570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidson M, Rashidi N, Nurgali K, Apostolopoulos V. The role of tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci. 2022;23:23. 10.3390/ijms23179968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang F, Wang GZ, Wang Y, Yang YN, Wen ZS, Chen DN, et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct Target Ther. 2022;7:311. 10.1038/s41392-022-01127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, et al. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. 2017;54:4432–51. 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- 75.Mohapatra SR, Sadik A, Sharma S, Poschet G, Gegner HM, Lanz TV, et al. Hypoxia routes tryptophan homeostasis towards increased tryptamine production. Front Immunol. 2021;12: 590532. 10.3389/fimmu.2021.590532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Więdłocha M, Marcinowicz P, Janoska-Jaździk M, Szulc A. Gut microbiota, kynurenine pathway and mental disorders - Review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106: 110145. 10.1016/j.pnpbp.2020.110145. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Zelante T, Puccetti M, Giovagnoli S, Romani L. Regulation of host physiology and immunity by microbial indole-3-aldehyde. Curr Opin Immunol. 2021;70:27–32. 10.1016/j.coi.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 79.He Z, Guo J, Zhang H, Yu J, Zhou Y, Wang Y, et al. Atractylodes macrocephala Koidz polysaccharide improves glycolipid metabolism disorders through activation of aryl hydrocarbon receptor by gut flora-produced tryptophan metabolites. Int J Biol Macromol. 2023;253: 126987. 10.1016/j.ijbiomac.2023.126987. [DOI] [PubMed] [Google Scholar]
- 80.Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373–88. 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Zhu S, Ma N, Johnston LJ, Wu C, Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med Res Rev. 2021;41:1061–88. 10.1002/med.21752. [DOI] [PubMed] [Google Scholar]
- 82.Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401. 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 83.Zhang FL, Chen XW, Wang YF, Hu Z, Zhang WJ, Zhou BW, et al. Microbiota-derived tryptophan metabolites indole-3-lactic acid is associated with intestinal ischemia/reperfusion injury via positive regulation of YAP and Nrf2. J Transl Med. 2023;21:264. 10.1186/s12967-023-04109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357. 10.1126/science.aaf9794. [DOI] [PubMed]
- 85.Pilla R, Suchodolski JS. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front Vet Sci. 2019;6: 498. 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Gan M, Li J, Li H, Su M, Tan D, et al. Endogenous indole pyruvate pathway for tryptophan metabolism mediated by IL4I1. J Agric Food Chem. 2020;68:10678–84. 10.1021/acs.jafc.0c03735. [DOI] [PubMed] [Google Scholar]
- 87.Luo JM, Zhang TT, He YY, Luo HN, Hong YQ, Yang ZM. Human chorionic gonadotropin-stimulated Interleukin-4-Induced-1 (IL4I1) promotes human decidualization via Aryl Hydrocarbon Receptor. Int J Mol Sci. 2023;24:24. 10.3390/ijms24043163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Bostick JW, Ye J, Qiu J, Zhang B, Urban JF Jr., et al. Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity. 2018;49:915–28. 10.1016/j.immuni.2018.09.015. .e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–52. 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang ZB, Hu Z, Lu CX, Luo SD, Chen Y, Zhou ZP, et al. Gut microbiota-derived indole 3-propionic acid partially activates aryl hydrocarbon receptor to promote macrophage phagocytosis and attenuate septic injury. Front Cell Infect Microbiol. 2022;12: 1015386. 10.3389/fcimb.2022.1015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang Z, Pan T, Li L, Wang H, Zhu J, Zhang H, et al. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes. 2022;14: 2044723. 10.1080/19490976.2022.2044723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429–52. 10.1038/s41571-023-00766-x. [DOI] [PubMed] [Google Scholar]
- 93.Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22:703–22. 10.1038/s41568-022-00513-x. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–74. 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jia D, Wang Q, Qi Y, Jiang Y, He J, Lin Y, et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell. 2024;187:1651–e6521. 10.1016/j.cell.2024.02.022. [DOI] [PubMed] [Google Scholar]
- 96.Jia D, Kuang Z, Wang L. The role of microbial indole metabolites in tumor. Gut Microbes. 2024;16:2409209. 10.1080/19490976.2024.2409209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021;22:358–69. 10.1038/s41590-020-00850-9. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018;33:480–94. 10.1016/j.ccell.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Boros FA, Bohár Z, Vécsei L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat Res Rev Mutat Res. 2018;776:32–45. 10.1016/j.mrrev.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Ulvik A, Theofylaktopoulou D, Midttun Ø, Nygård O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–40. 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 101.Calabrese CM, Valentini A, Calabrese G. Gut microbiota and type 1 diabetes mellitus: the effect of Mediterranean diet. Front Nutr. 2020;7: 612773. 10.3389/fnut.2020.612773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.el-Sewedy SM, Abdel-Tawab GA, el-Zoghby SM, Zeitoun R, Mostafa MH, Shalaby SM. Studies with tryptophan metabolites in vitro. Effect of zinc, manganese, copper and cobalt ions on kynurenine hydrolase and kynurenine aminotransferase in normal mouse liver. Biochem Pharmacol. 1974;23:2557–65. 10.1016/0006-2952(74)90178-6. [DOI] [PubMed] [Google Scholar]
- 103.Fujigaki H, Yamamoto Y, Saito K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology. 2017;112:264–74. 10.1016/j.neuropharm.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Hu N, Yang D, Oxenkrug G, Yang Q. Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. Febs j. 2017;284:948–66. 10.1111/febs.14026. [DOI] [PubMed] [Google Scholar]
- 105.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1–27. 10.1017/s1462399406000068. [DOI] [PubMed] [Google Scholar]
- 106.Wu KK. Cytoguardin: a tryptophan metabolite against cancer growth and metastasis. Int J Mol Sci. 2021;22:4490. 10.3390/ijms22094490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 108.Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–8. 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- 109.Chen GL, Miller GM. Tryptophan hydroxylase-2: an emerging therapeutic target for stress disorders. Biochem Pharmacol. 2013;85:1227–33. 10.1016/j.bcp.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9:1777625. 10.1080/2162402x.2020.1777625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sultana S, Elengickal A, Bensreti H, Belin de Chantemèle E, McGee-Lawrence ME, Hamrick MW. The kynurenine pathway in HIV, frailty and inflammaging. Front Immunol. 2023;14:1244622. 10.3389/fimmu.2023.1244622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67:361–5. 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Ghiboub M, Verburgt CM, Sovran B, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy to modulate tryptophan metabolism and aryl hydrocarbon-receptor signaling activation in human diseases. Nutrients. 2020;12. 10.3390/nu12092846. [DOI] [PMC free article] [PubMed]
- 114.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W, Wen L, Bao Y, Tang Z, Zhao J, Zhang X, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci U S A. 2022;119:e2203894119. 10.1073/pnas.2203894119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu D, Liang CH, Huang B, Zhuang X, Cui W, Yang L, et al. Tryptophan Metabolism Acts as a New Anti-Ferroptotic Pathway to Mediate Tumor Growth. Adv Sci (Weinh). 2023;10:e2204006. 10.1002/advs.202204006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams S, Braidy N, Bessede A, Brew BJ, Grant R, Teo C, et al. The kynurenine pathway in brain tumor pathogenesis. Cancer Res. 2012;72:5649–57. 10.1158/0008-5472.Can-12-0549. [DOI] [PubMed] [Google Scholar]
- 118.Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131–47. 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends Pharmacol Sci. 2018;39:307–25. 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 120.Launay JM, Delorme R, Pagan C, Callebert J, Leboyer M, Vodovar N. Impact of IDO activation and alterations in the kynurenine pathway on hyperserotonemia, NAD(+) production, and AhR activation in autism spectrum disorder. Transl Psychiatry. 2023;13:380. 10.1038/s41398-023-02687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fathi M, Vakili K, Yaghoobpoor S, Tavasol A, Jazi K, Hajibeygi R, et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front Immunol. 2022;13:997240. 10.3389/fimmu.2022.997240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biernacki T, Sandi D, Bencsik K, Vécsei L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells. 2020;9. doi: 10.3390/cells9061564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P, Huang J, Gou M, Zhou Y, Tong J, Fan F, et al. Kynurenine metabolism and metabolic syndrome in patients with schizophrenia. J Psychiatr Res. 2021;139:54–61. 10.1016/j.jpsychires.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 124.Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20:50–63. 10.1038/s41590-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu B, Zhang P, Tang X, Wang S, Shen J, Zheng Y, et al. Metabolic Rewiring of Kynurenine Pathway during Hepatic Ischemia-Reperfusion Injury Exacerbates Liver Damage by Impairing NAD Homeostasis. Adv Sci (Weinh). 2022;9:e2204697. 10.1002/advs.202204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wan J, Cheng C, Hu J, Huang H, Han Q, Jie Z, et al. De novo NAD(+) synthesis contributes to CD8(+) T cell metabolic fitness and antitumor function. Cell Rep. 2023;42:113518. 10.1016/j.celrep.2023.113518. [DOI] [PubMed] [Google Scholar]
- 127.Dehhaghi M, Panahi HKS, Kavyani B, Heng B, Tan V, Braidy N, et al. The Role of Kynurenine Pathway and NAD(+) Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging Dis. 2022;13:698–711. 10.14336/ad.2021.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao J, Yang T, Song B, Ma X, Ma Y, Lin X, et al. Abnormal tryptophan catabolism in diabetes mellitus and its complications: Opportunities and challenges. Biomed Pharmacother. 2023;166:115395. 10.1016/j.biopha.2023.115395. [DOI] [PubMed] [Google Scholar]
- 129.Cheng Y, Li Y, Benkowitz P, Lamina C, Köttgen A, Sekula P. The relationship between blood metabolites of the tryptophan pathway and kidney function: a bidirectional Mendelian randomization analysis. Sci Rep. 2020;10:12675. 10.1038/s41598-020-69559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J, Xi K, Zhang L, Han M, Wang Q, Liu X. Tryptophan metabolites and gut microbiota play an important role in pediatric migraine diagnosis. J Headache Pain. 2024;25:2. 10.1186/s10194-023-01708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu E, Papandreou C, Ruiz-Canela M, Guasch-Ferre M, Clish CB, Dennis C, et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case-Cohort Study. Clin Chem. 2018;64:1211–20. 10.1373/clinchem.2018.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gáspár R, Halmi D, Demján V, Berkecz R, Pipicz M, Csont T. Kynurenine Pathway Metabolites as Potential Clinical Biomarkers in Coronary Artery Disease. Front Immunol. 2021;12:768560. 10.3389/fimmu.2021.768560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haldar T, Chatterjee S, Alam MN, Maity P, Bagchi S. Blue Fluorescence of Cyano-tryptophan Predicts Local Electrostatics and Hydrogen Bonding in Biomolecules. J Phys Chem B. 2022;126:10732–40. 10.1021/acs.jpcb.2c05848. [DOI] [PubMed] [Google Scholar]
- 134.Vaughn MB, Biren C, Li Q, Ragupathi A, Dyer RB. Site-Specific Tryptophan Labels Reveal Local Microsecond-Millisecond Motions of Dihydrofolate Reductase. Molecules. 2020;25. doi: 10.3390/molecules25173819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pal S, Bose D, Chakrabarti A, Chattopadhyay A. Comparative Analysis of Tryptophan Dynamics in Spectrin and Its Constituent Domains: Insights from Fluorescence. J Phys Chem B. 2022;126:1045–53. 10.1021/acs.jpcb.1c08600. [DOI] [PubMed] [Google Scholar]
- 136.Roy P, Claude JB, Tiwari S, Barulin A, Wenger J. Ultraviolet Nanophotonics Enables Autofluorescence Correlation Spectroscopy on Label-Free Proteins with a Single Tryptophan. Nano Lett. 2023;23:497–504. 10.1021/acs.nanolett.2c03797. [DOI] [PubMed] [Google Scholar]
- 137.van Zundert SKM, Griffioen PH, van Rossem L, Willemsen SP, de Rijke YB, van Schaik RHN, et al. Simultaneous quantification of tryptophan metabolites by liquid chromatography tandem mass spectrometry during early human pregnancy. Clin Chem Lab Med. 2023;61:442–51. 10.1515/cclm-2022-0790. [DOI] [PubMed] [Google Scholar]
- 138.Wang LS, Zhang MD, Tao X, Zhou YF, Liu XM, Pan RL, et al. LC-MS/MS-based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1112:24–32. 10.1016/j.jchromb.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 139.Dai M, Wang Q, Kou S, Li X, Jiang Z, Sun L, et al. A sensitive UPLC-MS/MS method for the simultaneous determination of the metabolites in the tryptophan pathway in rat plasma. J Pharm Biomed Anal. 2022;219: 114979. 10.1016/j.jpba.2022.114979. [DOI] [PubMed] [Google Scholar]
- 140.Pedraz-Petrozzi B, Marszalek-Grabska M, Kozub A, Szalaj K, Trzpil A, Stachniuk A, et al. LC-MS/MS-based quantification of tryptophan, kynurenine, and kynurenic acid in human placental, fetal membranes, and umbilical cord samples. Sci Rep. 2023;13:12554. 10.1038/s41598-023-39774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su M, Cheng Y, Zhang C, Zhu D, Jia M, Zhang Q, et al. Determination of the levels of tryptophan and 12 metabolites in milk by liquid chromatography-tandem mass spectrometry with the QuEChERS method. J Dairy Sci. 2020;103:9851–9. 10.3168/jds.2020-18260. [DOI] [PubMed] [Google Scholar]
- 142.Hu D, Liu J, Yu W, Li C, Huang L, Mao W, et al. Tryptophan intake, not always the more the better. Front Nutr. 2023;10: 1140054. 10.3389/fnut.2023.1140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang X, Gao H, Xiao J, Han S, He J, Yuan R, et al. Abrine, an IDO1 inhibitor, suppresses the immune escape and enhances the immunotherapy of anti-PD-1 antibody in hepatocellular carcinoma. Front Immunol. 2023;14: 1185985. 10.3389/fimmu.2023.1185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang T, Song Y, Ai Z, Liu Y, Li H, Xu W, et al. Pulsatilla chinensis saponins ameliorated murine depression by inhibiting intestinal inflammation mediated IDO1 overexpression and rebalancing tryptophan metabolism. Phytomedicine. 2023;116: 154852. 10.1016/j.phymed.2023.154852. [DOI] [PubMed] [Google Scholar]
- 145.Luo Y, Möhn N, Skripuletz T, Senel M, Tumani H, Peßler F, et al. Differentiation of viral and autoimmune central nervous system inflammation by kynurenine pathway. Ann Clin Transl Neurol. 2021;8:2228–34. 10.1002/acn3.51383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo D, Wang Y, Wu X, Gao Y, Wang A, Zhang Z, et al. Expression of tryptophan metabolism enzymes in patients with diffuse large B-cell lymphoma and NK/T-cell lymphoma. Cancer Med. 2023;12:12139–48. 10.1002/cam4.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Juhász C, Nahleh Z, Zitron I, Chugani DC, Janabi MZ, Bandyopadhyay S, et al. Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies. Nucl Med Biol. 2012;39:926–32. 10.1016/j.nucmedbio.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vavrincova-Yaghi D, Seelen MA, Kema IP, Deelman LE, van der Heuvel MC, Breukelman H, et al. Early posttransplant tryptophan metabolism predicts long-term outcome of human kidney transplantation. Transplantation. 2015;99:e97–104. 10.1097/tp.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 149.Rubio VY, Cagmat JG, Wang GP, Yost RA, Garrett TJ. Analysis of tryptophan metabolites in serum using wide-isolation strategies for UHPLC-HRMS/MS. Anal Chem. 2020;92:2550–7. 10.1021/acs.analchem.9b04210. [DOI] [PubMed] [Google Scholar]
- 150.Lefèvre A, Mavel S, Nadal-Desbarats L, Galineau L, Attucci S, Dufour D, et al. Validation of a global quantitative analysis methodology of tryptophan metabolites in mice using LC-MS. Talanta. 2019;195:593–8. 10.1016/j.talanta.2018.11.094. [DOI] [PubMed] [Google Scholar]
- 151.Chawdhury A, Shamsi SA, Miller A, Liu A. Capillary electrochromatography-mass spectrometry of kynurenine pathway metabolites. J Chromatogr A. 2021;1651: 462294. 10.1016/j.chroma.2021.462294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Patel VD, Shamsi SA, Miller A, Liu A. Quantitation of tryptophan and kynurenine in human plasma using 4-vinylphenylboronic acid column by capillary electrochromatography coupled with mass spectrometry. Electrophoresis. 2023;44:529–39. 10.1002/elps.202200251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pautova A, Khesina Z, Getsina M, Sobolev P, Revelsky A, Beloborodova N. Determination of tryptophan metabolites in serum and cerebrospinal fluid samples using microextraction by packed sorbent, Silylation and GC-MS detection. Molecules. 2020;25:25. 10.3390/molecules25143258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo H, Jiao Y, Wang X, Lu T, Zhang Z, Xu F. Twins labeling-liquid chromatography/mass spectrometry based metabolomics for absolute quantification of tryptophan and its key metabolites. J Chromatogr A. 2017;1504:83–90. 10.1016/j.chroma.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 155.Flieger J, Święch-Zubilewicz A, Śniegocki T, Dolar-Szczasny J, Pizoń M. Determination of tryptophan and its major metabolites in fluid from the anterior chamber of the eye in diabetic patients with cataract by liquid chromotography mass spectrometry (LC-MS/MS). Molecules. 2018;23:3012. 10.3390/molecules23113012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galla Z, Rajda C, Rácz G, Grecsó N, Baráth Á, Vécsei L, et al. Simultaneous determination of 30 neurologically and metabolically important molecules: a sensitive and selective way to measure tyrosine and tryptophan pathway metabolites and other biomarkers in human serum and cerebrospinal fluid. J Chromatogr A. 2021;1635:461775. 10.1016/j.chroma.2020.461775. [DOI] [PubMed] [Google Scholar]
- 157.Oh JS, Seo HS, Kim KH, Pyo H, Chung BC, Lee J. Urinary profiling of tryptophan and its related metabolites in patients with metabolic syndrome by liquid chromatography-electrospray ionization/mass spectrometry. Anal Bioanal Chem. 2017;409:5501–12. 10.1007/s00216-017-0486-4. [DOI] [PubMed] [Google Scholar]
- 158.Dolusić E, Larrieu P, Moineaux L, Stroobant V, Pilotte L, Colau D, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem. 2011;54:5320–34. 10.1021/jm2006782. [DOI] [PubMed] [Google Scholar]
- 159.Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ. Inflammatory reprogramming with IDO1 inhibitors: turning immunologically unresponsive ‘Cold’ Tumors ‘Hot.’ Trends Cancer. 2018;4:38–58. 10.1016/j.trecan.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sadok I, Rachwał K, Jonik I, Staniszewska M. Reliable chromatographic assay for measuring of indoleamine 2,3-dioxygenase 1 (IDO1) activity in human cancer cells. J Enzyme Inhib Med Chem. 2021;36:581–92. 10.1080/14756366.2021.1882451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abou El-Nour KM, El-Sherbiny IM, Khairy GM, Abbas AM, Salem EH. Investigation of thymine as a potential cancer biomarker employing tryptophan with nanomaterials as a biosensor. Spectrochim Acta Mol Biomol Spectrosc. 2023;301:122928. 10.1016/j.saa.2023.122928. [DOI] [PubMed] [Google Scholar]
- 162.Jankovskaja S, Engblom J, Rezeli M, Marko-Varga G, Ruzgas T, Björklund S. Non-invasive skin sampling of tryptophan/kynurenine ratio in vitro towards a skin cancer biomarker. Sci Rep. 2021;11:678. 10.1038/s41598-020-79903-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schramme F, Crosignani S, Frederix K, Hoffmann D, Pilotte L, Stroobant V, et al. Inhibition of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol Res. 2020;8:32–45. 10.1158/2326-6066.Cir-19-0041. [DOI] [PubMed] [Google Scholar]
- 164.Huo C, Luo Z, Ning X, Kang X, Yan Q, Guo Y, et al. 4,6-disubstituted-1H-Indazole-4-Amine derivatives with immune-chemotherapy effect and in vivo antitumor activity. Eur J Med Chem. 2022;241: 114625. 10.1016/j.ejmech.2022.114625. [DOI] [PubMed] [Google Scholar]
- 165.Zhang S, Qi F, Fang X, Yang D, Hu H, Huang Q, et al. Tryptophan 2,3-dioxygenase inhibitory activities of tryptanthrin derivatives. Eur J Med Chem. 2018;160:133–45. 10.1016/j.ejmech.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 166.Hoffmann D, Dvorakova T, Stroobant V, Bouzin C, Daumerie A, Solvay M, et al. Tryptophan 2,3-dioxygenase expression identified in human hepatocellular carcinoma cells and in intratumoral pericytes of most cancers. Cancer Immunol Res. 2020;8:19–31. 10.1158/2326-6066.Cir-19-0040. [DOI] [PubMed] [Google Scholar]
- 167.Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Nguyen B, et al. Tumor Cell IDO enhances immune suppression and decreases survival independent of tryptophan metabolism in glioblastoma. Clin Cancer Res. 2021;27:6514–28. 10.1158/1078-0432.Ccr-21-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang J, Guo Z, Xie Q, Zhong C, Gao X, Yang Q. Tryptophan hydroxylase 1 drives glioma progression by modulating the serotonin/L1CAM/NF-κB signaling pathway. BMC Cancer. 2022;22:457. 10.1186/s12885-022-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–21. 10.1158/1078-0432.Ccr-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72:1031–8. 10.1227/NEU.0b013e31828cf945. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 171.Du L, Xing Z, Tao B, Li T, Yang D, Li W, et al. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway. Signal Transduct Target Ther. 2020;5:10. 10.1038/s41392-019-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bosnyák E, Kamson DO, Guastella AR, Varadarajan K, Robinette NL, Kupsky WJ, et al. Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol. 2015;17:1284–92. 10.1093/neuonc/nov098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dos Santos IL, Mitchell M, Nogueira PAS, Lafita-Navarro MC, Perez-Castro L, Eriom J, et al. Targeting of neuroblastoma cells through Kynurenine-AHR pathway inhibition. Febs J. 2024;291:2172–90. 10.1111/febs.17109. [DOI] [PubMed] [Google Scholar]
- 174.Venkateswaran N, Lafita-Navarro MC, Hao YH, Kilgore JA, Perez-Castro L, Braverman J, et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33:1236–51. 10.1101/gad.327056.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang F, Zhao Y, Zhao J, Wu S, Jiang Y, Ma H, et al. Upregulated SLC1A5 promotes cell growth and survival in colorectal cancer. Int J Clin Exp Pathol. 2014;7:6006–14. [PMC free article] [PubMed] [Google Scholar]
- 176.Toda K, Nishikawa G, Iwamoto M, Itatani Y, Takahashi R, Sakai Y, et al. Clinical role of ASCT2 (SLC1A5) in KRAS-Mutated colorectal cancer. Int J Mol Sci. 2017;18:18. 10.3390/ijms18081632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takasu C, Nishi M, Yoshikawa K, Tokunaga T, Nakao T, Kashihara H, et al. Role of IDO expression in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. BMC Cancer. 2022;22:1263. 10.1186/s12885-022-10357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Newman AC, Falcone M, Huerta Uribe A, Zhang T, Athineos D, Pietzke M, et al. Immune-regulated IDO1-dependent tryptophan metabolism is source of one-carbon units for pancreatic cancer and stellate cells. Mol Cell. 2021;81:2290–302. 10.1016/j.molcel.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang H, Li T, Fang X, Xing Z, Zhang S, Shi L, et al. IDO1/TDO dual inhibitor RY103 targets Kyn-AhR pathway and exhibits preclinical efficacy on pancreatic cancer. Cancer Lett. 2021;522:32–43. 10.1016/j.canlet.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 180.Buhe H, Ma JX, Ye FZ, Song CY, Chen XY, Liu Y, et al. IDO-1 inhibitor INCB24360 elicits distant metastasis of basal extruded cancer cells in pancreatic ductal adenocarcinoma. Acta Pharmacol Sin. 2023;44:1277–89. 10.1038/s41401-022-01035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tintelnot J, Xu Y, Lesker TR, Schönlein M, Konczalla L, Giannou AD, et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615:168–74. 10.1038/s41586-023-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu Z, Yan L, Lin J, Ke K, Yang W. Constitutive TDO2 expression promotes liver cancer progression by an autocrine IL-6 signaling pathway. Cancer Cell Int. 2021;21:538. 10.1186/s12935-021-02228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi Z, Gan G, Gao X, Chen F, Mi J. Kynurenine catabolic enzyme KMO regulates HCC growth. Clin Transl Med. 2022;12: e697. 10.1002/ctm2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cui JX, Xu XH, He T, Liu JJ, Xie TY, Tian W, et al. L-kynurenine induces NK cell loss in gastric cancer microenvironment via promoting ferroptosis. J Exp Clin Cancer Res. 2023;42:52. 10.1186/s13046-023-02629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li F, Sun Y, Huang J, Xu W, Liu J, Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8:7330–44. 10.1002/cam4.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu H, Shen Z, Wang Z, Wang X, Zhang H, Qin J, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep. 2016;6: 21319. 10.1038/srep21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sørensen RB, Køllgaard T, Andersen RS, van den Berg JH, Svane IM, Straten P, et al. Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011;71:2038–44. 10.1158/0008-5472.Can-10-3403. [DOI] [PubMed] [Google Scholar]
- 188.Oldan JD, Giglio BC, Smith E, Zhao W, Bouchard DM, Ivanovic M, et al. Increased tryptophan, but not increased glucose metabolism, predict resistance of pembrolizumab in stage III/IV melanoma. Oncoimmunology. 2023;12: 2204753. 10.1080/2162402x.2023.2204753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xue L, Wang C, Qian Y, Zhu W, Liu L, Yang X, et al. Tryptophan metabolism regulates inflammatory macrophage polarization as a predictive factor for breast cancer immunotherapy. Int Immunopharmacol. 2023;125: 111196. 10.1016/j.intimp.2023.111196. [DOI] [PubMed] [Google Scholar]
- 190.Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol. 2018;31:1513–22. 10.1038/s41379-018-0061-3. [DOI] [PubMed] [Google Scholar]
- 191.Duarte D, Amaro F, Silva I, Silva D, Fresco P, Oliveira JC, et al. Carbidopa Alters tryptophan metabolism in breast cancer and melanoma cells leading to the formation of Indole-3-Acetonitrile, a pro-proliferative metabolite. Biomolecules. 2019;9: 9. 10.3390/biom9090409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chiu YH, Lei HJ, Huang KC, Chiang YL, Lin CS. Overexpression of kynurenine 3-monooxygenase correlates with cancer malignancy and predicts poor prognosis in canine mammary gland tumors. J Oncol. 2019;2019: 6201764. 10.1155/2019/6201764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang S, Gao Y, Wang P, Wang S, Wang Y, Li M, et al. Tryptophan metabolism enzymes are potential targets in ovarian clear cell carcinoma. Cancer Med. 2023;12:21996–2005. 10.1002/cam4.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang W, Huang L, Jin JY, Jolly S, Zang Y, Wu H, et al. IDO immune status after chemoradiation may predict survival in lung cancer patients. Cancer Res. 2018;78:809–16. 10.1158/0008-5472.Can-17-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feng H, Cao B, Peng X, Wei Q. Cancer-associated fibroblasts strengthen cell proliferation and EGFR TKIs resistance through aryl hydrocarbon receptor dependent signals in non-small cell lung cancer. BMC Cancer. 2022;22:764. 10.1186/s12885-022-09877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, et al. Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg. 2009;111:230–7. 10.3171/2008.10.Jns081141. [DOI] [PubMed] [Google Scholar]
- 197.Koch MS, Zdioruk M, Nowicki MO, Griffith AM, Aguilar E, Aguilar LK, et al. Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models. J Immunother Cancer. 2022;10. 10.1136/jitc-2021-003368. [DOI] [PMC free article] [PubMed]
- 198.Koske I, Rössler A, Pipperger L, Petersson M, Barnstorf I, Kimpel J, et al. Oncolytic virotherapy enhances the efficacy of a cancer vaccine by modulating the tumor microenvironment. Int J Cancer. 2019;145:1958–69. 10.1002/ijc.32325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Desai R, Suryadevara CM, Batich KA, Farber SH, Sanchez-Perez L, Sampson JH. Emerging immunotherapies for glioblastoma. Expert Opin Emerg Drugs. 2016;21:133–45. 10.1080/14728214.2016.1186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qiao J, Dey M, Chang AL, Kim JW, Miska J, Ling A, et al. Intratumoral oncolytic adenoviral treatment modulates the glioma microenvironment and facilitates systemic tumor-antigen-specific T cell therapy. Oncoimmunology. 2015;4:e1022302. 10.1080/2162402x.2015.1022302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ma T, Hu C, Lal B, Zhou W, Ma Y, Ying M, et al. Reprogramming transcription factors Oct4 and Sox2 induce a BRD-dependent immunosuppressive transcriptome in GBM-propagating cells. Cancer Res. 2021;81:2457–69. 10.1158/0008-5472.Can-20-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nguyen TT, Shin DH, Sohoni S, Singh SK, Rivera-Molina Y, Jiang H, et al. Reshaping the tumor microenvironment with oncolytic viruses, positive regulation of the immune synapse, and blockade of the immunosuppressive oncometabolic circuitry. J Immunother Cancer. 2022;10. 10.1136/jitc-2022-004935. [DOI] [PMC free article] [PubMed]
- 203.Li Y, Zhang K, Wu Y, Yue Y, Cheng K, Feng Q, et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small. 2022;18: e2107461. 10.1002/smll.202107461. [DOI] [PubMed] [Google Scholar]
- 204.Liu X, Zhou W, Zhang X, Ding Y, Du Q, Hu R. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. Int J Cancer. 2018;143:1516–29. 10.1002/ijc.31417. [DOI] [PubMed] [Google Scholar]
- 205.Ala M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int Rev Immunol. 2022;41:326–45. 10.1080/08830185.2021.1954638. [DOI] [PubMed] [Google Scholar]
- 206.Jin H, Zhang Y, You H, Tao X, Wang C, Jin G, et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep. 2015;5: 10466. 10.1038/srep10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li B, Jiang Y, Li G, Fisher GA Jr., Li R. Natural killer cell and stroma abundance are independently prognostic and predict gastric cancer chemotherapy benefit. JCI Insight. 2020;5. 10.1172/jci.insight.136570. [DOI] [PMC free article] [PubMed]
- 208.Li T, Zhang Q, Jiang Y, Yu J, Hu Y, Mou T, et al. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology. 2016;5: e1069936. 10.1080/2162402x.2015.1069936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–75. 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175:442–57. 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 211.Kaproń B, Czarnomysy R, Radomska D, Bielawski K, Plech T. Thiosemicarbazide derivatives targeting human TopoIIα and IDO-1 as small-molecule drug candidates for breast cancer treatment. Int J Mol Sci. 2023;24:24. 10.3390/ijms24065812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang TT, Tseng LM, Chen JL, Chu PY, Lee CH, Huang CT, et al. Kynurenine 3-monooxygenase upregulates pluripotent genes through β-catenin and promotes triple-negative breast cancer progression. EBioMedicine. 2020;54:102717. 10.1016/j.ebiom.2020.102717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bartok O, Pataskar A, Nagel R, Laos M, Goldfarb E, Hayoun D, et al. Anti-tumour immunity induces aberrant peptide presentation in melanoma. Nature. 2021;590:332–7. 10.1038/s41586-020-03054-1. [DOI] [PubMed] [Google Scholar]
- 214.Cecchi M, Mannini A, Lapucci A, Silvano A, Lulli M, Luceri C, et al. Dexamethasone promotes a stem-like phenotype in human melanoma cells via tryptophan 2,3 dioxygenase. Front Pharmacol. 2022;13: 911019. 10.3389/fphar.2022.911019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Balakrishna P, George S, Hatoum H, Mukherjee S. Serotonin Pathway in Cancer. Int J Mol Sci. 2021;22. 10.3390/ijms22031268. [DOI] [PMC free article] [PubMed]
- 216.Karmakar S, Lal G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics. 2021;11:5296–312. 10.7150/thno.55986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kannen V, Bader M, Sakita JY, Uyemura SA, Squire JA. The dual role of serotonin in colorectal cancer. Trends Endocrinol Metab. 2020;31:611–25. 10.1016/j.tem.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 218.Peters MA, Walenkamp AM, Kema IP, Meijer C, de Vries EG, Oosting SF. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resist Updat. 2014;17:96–104. 10.1016/j.drup.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 219.Ye D, Xu H, Tang Q, Xia H, Zhang C, Bi F. The role of 5-HT metabolism in cancer. Biochim Biophys Acta Rev Cancer. 2021;1876: 188618. 10.1016/j.bbcan.2021.188618. [DOI] [PubMed] [Google Scholar]
- 220.Ge C, Yan J, Yuan X, Xu G. A positive feedback loop between tryptophan hydroxylase 1 and β-Catenin/ZBP-89 signaling promotes prostate cancer progression. Front Oncol. 2022;12: 923307. 10.3389/fonc.2022.923307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sui H, Xu H, Ji Q, Liu X, Zhou L, Song H, et al. 5-hydroxytryptamine receptor (5-HT1DR) promotes colorectal cancer metastasis by regulating Axin1/β-catenin/MMP-7 signaling pathway. Oncotarget. 2015;6:25975–87. 10.18632/oncotarget.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sola-Penna M, Paixão LP, Branco JR, Ochioni AC, Albanese JM, Mundim DM, et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122:194–208. 10.1038/s41416-019-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sonier B, Arseneault M, Lavigne C, Ouellette RJ, Vaillancourt C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem Biophys Res Commun. 2006;343:1053–9. 10.1016/j.bbrc.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 224.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–24. 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 225.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8: 13. 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wan Y, Li Y, Yan C, Yan M, Tang Z, Indole. A privileged scaffold for the design of anti-cancer agents. Eur J Med Chem. 2019;183: 111691. 10.1016/j.ejmech.2019.111691. [DOI] [PubMed] [Google Scholar]
- 227.Liu Y, Pei Z, Pan T, Wang H, Chen W, Lu W. Indole metabolites and colorectal cancer: Gut microbial tryptophan metabolism, host gut microbiome biomarkers, and potential intervention mechanisms. Microbiol Res. 2023;272: 127392. 10.1016/j.micres.2023.127392. [DOI] [PubMed] [Google Scholar]
- 228.Fiore A, Murray PJ. Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol. 2021;70:7–14. 10.1016/j.coi.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 229.Nachef M, Ali AK, Almutairi SM, Lee SH. Targeting SLC1A5 and SLC3A2/SLC7A5 as a potential strategy to strengthen anti-tumor immunity in the tumor microenvironment. Front Immunol. 2021;12: 624324. 10.3389/fimmu.2021.624324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu X, Qin H, Li Z, Lv Y, Feng S, Zhuang W, et al. Inspiratory hyperoxia suppresses lung cancer metastasis through a MYC/SLC1A5-dependent metabolic pathway. Eur Respir J. 2022;60:60. 10.1183/13993003.00062-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Amaya ML, Inguva A, Pei S, Jones C, Krug A, Ye H, et al. The STAT3-MYC axis promotes survival of leukemia stem cells by regulating SLC1A5 and oxidative phosphorylation. Blood. 2022;139:584–96. 10.1182/blood.2021013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kanai Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol Ther. 2022;230: 107964. 10.1016/j.pharmthera.2021.107964. [DOI] [PubMed] [Google Scholar]
- 233.Sun M, Ma N, He T, Johnston LJ, Ma X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 2020;60:1760–8. 10.1080/10408398.2019.1598334. [DOI] [PubMed] [Google Scholar]
- 234.Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. 2020;182:1252–70.e34. [DOI] [PubMed] [Google Scholar]
- 235.Ma Z, Li Z, Mao Y, Ye J, Liu Z, Wang Y, et al. AhR diminishes the efficacy of chemotherapy via suppressing STING dependent type-I interferon in bladder cancer. Nat Commun. 2023;14:5415. 10.1038/s41467-023-41218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85. 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 237.Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–8. 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 238.Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018;15:447–57. 10.1038/cmi.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Trézéguet V, Fatrouni H, Merched AJ. Immuno-Metabolic Modulation of Liver Oncogenesis by the Tryptophan Metabolism. Cells. 2021;10. 10.3390/cells10123469. [DOI] [PMC free article] [PubMed]
- 240.Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the Tryptophan-Kynurenine-Aryl hydrocarbon axis. Clin Cancer Res. 2019;25:1462–71. 10.1158/1078-0432.Ccr-18-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xue C, Li G, Zheng Q, Gu X, Shi Q, Su Y, et al. Tryptophan metabolism in health and disease. Cell Metab. 2023;35:1304–26. 10.1016/j.cmet.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 242.Chen Y, Zhang J, Yang Y, Xiang K, Li H, Sun D, et al. Kynurenine-3-monooxygenase (KMO): From its biological functions to therapeutic effect in diseases progression. J Cell Physiol. 2022;237:4339–55. 10.1002/jcp.30876. [DOI] [PubMed] [Google Scholar]
- 243.Hughes TD, Güner OF, Iradukunda EC, Phillips RS, Bowen JP. The kynurenine pathway and kynurenine 3-monooxygenase inhibitors. Molecules. 2022;27:27. 10.3390/molecules27010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hanihara M, Kawataki T, Oh-Oka K, Mitsuka K, Nakao A, Kinouchi H. Synergistic antitumor effect with indoleamine 2,3-dioxygenase inhibition and temozolomide in a murine glioma model. J Neurosurg. 2016;124:1594–601. 10.3171/2015.5.Jns141901. [DOI] [PubMed] [Google Scholar]
- 245.Johnson TS, MacDonald TJ, Pacholczyk R, Aguilera D, Al-Basheer A, Bajaj M, et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: a first-in-children phase I trial. Neuro Oncol. 2024;26:348–61. 10.1093/neuonc/noad174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77:3894–907. 10.1158/0008-5472.Can-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jiang H, Fueyo J. Healing after death: antitumor immunity induced by oncolytic adenoviral therapy. Oncoimmunology. 2014;3: e947872. 10.4161/21624011.2014.947872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hu M, Liao X, Tao Y, Chen Y. Advances in oncolytic herpes simplex virus and adenovirus therapy for recurrent glioma. Front Immunol. 2023;14: 1285113. 10.3389/fimmu.2023.1285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Muthukutty P, Yoo SY. Oncolytic virus engineering and utilizations: cancer immunotherapy perspective. Viruses. 2023;15:15. 10.3390/v15081645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dong T, Shah JR, Phung AT, Larson C, Sanchez AB, Aisagbonhi O, et al. A local and abscopal effect observed with liposomal encapsulation of intratumorally injected oncolytic adenoviral therapy. Cancers (Basel). 2023;15:15. 10.3390/cancers15123157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36:1419–27. 10.1200/jco.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ott M, Litzenburger UM, Rauschenbach KJ, Bunse L, Ochs K, Sahm F, et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63:78–90. 10.1002/glia.22734. [DOI] [PubMed] [Google Scholar]
- 253.Liu J, Zheng J, Nie H, Zhang D, Cao D, Xing Z, et al. Molybdenum disulfide-based hyaluronic acid-guided multifunctional theranostic nanoplatform for magnetic resonance imaging and synergetic chemo-photothermal therapy. J Colloid Interface Sci. 2019;548:131–44. 10.1016/j.jcis.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 254.Wang C, Yan J, Yin P, Gui L, Ji L, Ma B, et al. β-Catenin inhibition shapes tumor immunity and synergizes with immunotherapy in colorectal cancer. Oncoimmunology. 2020;9: 1809947. 10.1080/2162402x.2020.1809947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhi D, Yang T, O’Hagan J, Zhang S, Donnelly RF. Photothermal therapy. J Control Release. 2020;325:52–71. 10.1016/j.jconrel.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 256.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7: 13193. 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463–74. 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 258.Chen Q, Chen J, Yang Z, Xu J, Xu L, Liang C, et al. Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv Mater. 2019;31:e1802228. 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 259.Ge R, Liu C, Zhang X, Wang W, Li B, Liu J, et al. Photothermal-activatable Fe(3)O(4) Superparticle Nanodrug Carriers with PD-L1 Immune Checkpoint Blockade for Anti-metastatic Cancer Immunotherapy. ACS Appl Mater Interfaces. 2018;10:20342–55. 10.1021/acsami.8b05876. [DOI] [PubMed] [Google Scholar]
- 260.Ji L, Qian W, Gui L, Ji Z, Yin P, Lin GN, et al. Blockade of β-Catenin-Induced CCL28 Suppresses Gastric Cancer Progression via Inhibition of Treg Cell Infiltration. Cancer Res. 2020;80:2004–16. 10.1158/0008-5472.Can-19-3074. [DOI] [PubMed] [Google Scholar]
- 261.Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36:758–64. 10.1038/nbt.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu Q, Hua S, Wang X, Chen F, Gou S. The introduction of immunosuppressor (TDO inhibitor) significantly improved the efficacy of irinotecan in treating hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:497–508. 10.1007/s00262-020-02697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Du R, Zhang X, Lu X, Ma X, Guo X, Shi C, et al. PDPN positive CAFs contribute to HER2 positive breast cancer resistance to trastuzumab by inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug Resist Updat. 2023;68: 100947. 10.1016/j.drup.2023.100947. [DOI] [PubMed] [Google Scholar]
- 264.Feng X, Shen P, Wang Y, Li Z, Bian J. Synthesis and in vivo antitumor evaluation of an orally active potent phosphonamidate derivative targeting IDO1/IDO2/TDO. Biochem Pharmacol. 2019;168:214–23. 10.1016/j.bcp.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 265.Kjeldsen JW, Lorentzen CL, Martinenaite E, Ellebaek E, Donia M, Holmstroem RB, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med. 2021;27:2212–23. 10.1038/s41591-021-01544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–97. 10.1016/s1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 267.Campesato LF, Budhu S, Tchaicha J, Weng CH, Gigoux M, Cohen IJ, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11:4011. 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Venkateswaran N, Garcia R, Lafita-Navarro MC, Hao YH, Perez-Castro L, Nogueira PAS, et al. Tryptophan fuels MYC-dependent liver tumorigenesis through indole 3-pyruvate synthesis. Nat Commun. 2024;15:4266. 10.1038/s41467-024-47868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110: 102461. 10.1016/j.ctrv.2022.102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang G, Xing J, Wang Y, Wang L, Ye Y, Lu D, et al. Discovery of novel inhibitors of indoleamine 2,3-Dioxygenase 1 through structure-based virtual screening. Front Pharmacol. 2018;9: 277. 10.3389/fphar.2018.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zou Y, Hu Y, Ge S, Zheng Y, Li Y, Liu W, et al. Effective virtual screening strategy toward heme-containing proteins: identification of novel IDO1 inhibitors. Eur J Med Chem. 2019;184: 111750. 10.1016/j.ejmech.2019.111750. [DOI] [PubMed] [Google Scholar]
- 272.Sun L. Advances in the discovery and development of selective heme-displacing IDO1 inhibitors. Expert Opin Drug Discov. 2020;15:1223–32. 10.1080/17460441.2020.1781811. [DOI] [PubMed] [Google Scholar]
- 273.Ogbechi J, Huang YS, Clanchy FIL, Pantazi E, Topping LM, Darlington LG, et al. Modulation of immune cell function, IDO expression and kynurenine production by the quorum sensor 2-heptyl-3-hydroxy-4-quinolone (PQS). Front Immunol. 2022;13: 1001956. 10.3389/fimmu.2022.1001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41–8. 10.1007/s00281-018-0702-0. [DOI] [PubMed] [Google Scholar]
- 275.Weng T, Qiu X, Wang J, Li Z, Bian J. Recent discovery of indoleamine-2,3-dioxygenase 1 inhibitors targeting cancer immunotherapy. Eur J Med Chem. 2018;143:656–69. 10.1016/j.ejmech.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 276.Yang C, Ng CT, Li D, Zhang L. Targeting indoleamine 2,3-Dioxygenase 1: Fighting cancers via dormancy regulation. Front Immunol. 2021;12: 725204. 10.3389/fimmu.2021.725204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ge S, Zhong H, Ma X, Zheng Y, Zou Y, Wang F, et al. Discovery of secondary sulphonamides as IDO1 inhibitors with potent antitumour effects in vivo. J Enzyme Inhib Med Chem. 2020;35:1240–57. 10.1080/14756366.2020.1765165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu XH, Zhai XY. Role of tryptophan metabolism in cancers and therapeutic implications. Biochimie. 2021;182:131–9. 10.1016/j.biochi.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 279.Tummala KS, Gomes AL, Yilmaz M, Graña O, Bakiri L, Ruppen I, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–39. 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 280.Günther J, Däbritz J, Wirthgen E. Limitations and off-target effects of tryptophan-related ido inhibitors in cancer treatment. Front Immunol. 2019;10:1801. 10.3389/fimmu.2019.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Peng X, Zhao Z, Liu L, Bai L, Tong R, Yang H, et al. Targeting indoleamine dioxygenase and tryptophan dioxygenase in cancer immunotherapy: clinical progress and challenges. Drug Des Devel Ther. 2022;16:2639–57. 10.2147/dddt.S373780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Liu IL, Chung TF, Huang WH, Hsu CH, Liu CC, Chiu YH, et al. Kynurenine 3-monooxygenase (KMO), and signal transducer and activator of transcription 3 (STAT3) expression is involved in tumour proliferation and predicts poor survival in canine melanoma. Vet Comp Oncol. 2021;19:79–91. 10.1111/vco.12641. [DOI] [PubMed] [Google Scholar]
- 283.Liu CY, Huang TT, Chen JL, Chu PY, Lee CH, Lee HC, et al. Significance of Kynurenine 3-Monooxygenase Expression in Colorectal Cancer. Front Oncol. 2021;11: 620361. 10.3389/fonc.2021.620361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sordillo LA, Sordillo PP. Suppression of kynurenine 3-Monooxygenase as a treatment for triple-negative breast carcinoma. Anticancer Res. 2023;43:5275–82. 10.21873/anticanres.16731. [DOI] [PubMed] [Google Scholar]
- 285.Smith JR, Jamie JF, Guillemin GJ. Kynurenine-3-monooxygenase: a review of structure, mechanism, and inhibitors. Drug Discov Today. 2016;21:315–24. 10.1016/j.drudis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 286.Zhang S, Collier MEW, Heyes DJ, Giorgini F, Scrutton NS. Advantages of brain penetrating inhibitors of kynurenine-3-monooxygenase for treatment of neurodegenerative diseases. Arch Biochem Biophys. 2021;697: 108702. 10.1016/j.abb.2020.108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Waløen K, Kleppe R, Martinez A, Haavik J. Tyrosine and tryptophan hydroxylases as therapeutic targets in human disease. Expert Opin Ther Targets. 2017;21:167–80. 10.1080/14728222.2017.1272581. [DOI] [PubMed] [Google Scholar]
- 288.Specker E, Matthes S, Wesolowski R, Schütz A, Grohmann M, Alenina N, et al. Structure-based design of xanthine-benzimidazole derivatives as novel and potent tryptophan hydroxylase inhibitors. J Med Chem. 2022;65:11126–49. 10.1021/acs.jmedchem.2c00598. [DOI] [PubMed] [Google Scholar]
- 289.Loughrey PB, Zhang D, Heaney AP. New treatments for the carcinoid syndrome. Endocrinol Metab Clin North Am. 2018;47:557–76. 10.1016/j.ecl.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 290.Mustala OO, Airaksinen MM. Excretion of histamine and metabolites of 5-hydroxytryptamine in a case of metastasized carcinoid tumour. Acta Med Scand. 1962;171:483–9. 10.1111/j.0954-6820.1962.tb04214.x. [DOI] [PubMed] [Google Scholar]
- 291.Mc IW, Page IH. New metabolites of serotonin in carcinoid urine. Science. 1958;128: 537. 10.1126/science.128.3323.537. [DOI] [PubMed] [Google Scholar]
- 292.Perez-Castro L, Garcia R, Venkateswaran N, Barnes S, Conacci-Sorrell M. Tryptophan and its metabolites in normal physiology and cancer etiology. Febs j. 2023;290:7–27. 10.1111/febs.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wyant GA, Moslehi J. Expanding the therapeutic world of tryptophan metabolism. Circulation. 2022;145:1799–802. 10.1161/circulationaha.122.059812. [DOI] [PubMed] [Google Scholar]
- 294.Guo Y, Liu Y, Wu W, Ling D, Zhang Q, Zhao P, et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials. 2021;276: 121018. 10.1016/j.biomaterials.2021.121018. [DOI] [PubMed] [Google Scholar]
- 295.Munn DH, Sharma MD, Johnson TS, Rodriguez P. IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment. Cancer Immunol Immunother. 2017;66:1049–58. 10.1007/s00262-017-2010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ye Z, Yue L, Shi J, Shao M, Wu T. Role of IDO and TDO in Cancers and related diseases and the therapeutic implications. J Cancer. 2019;10:2771–82. 10.7150/jca.31727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kober C, Roewe J, Schmees N, Roese L, Roehn U, Bader B, et al. Targeting the aryl hydrocarbon receptor (AhR) with BAY 2416964: a selective small molecule inhibitor for cancer immunotherapy. J Immunother Cancer. 2023;11:11. 10.1136/jitc-2023-007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Yentz S, Smith D. Indoleamine 2,3-Dioxygenase (IDO) inhibition as a strategy to augment cancer immunotherapy. BioDrugs. 2018;32:311–7. 10.1007/s40259-018-0291-4. [DOI] [PubMed] [Google Scholar]
- 299.Selvan SR, Dowling JP, Kelly WK, Lin J. Indoleamine 2,3-dioxygenase (IDO): biology and target in cancer immunotherapies. Curr Cancer Drug Targets. 2016;16:755–64. 10.2174/1568009615666151030102250. [DOI] [PubMed] [Google Scholar]
- 300.Kado SY, Bein K, Castaneda AR, Pouraryan AA, Garrity N, Ishihara Y, et al. Regulation of IDO2 by the Aryl Hydrocarbon Receptor (AhR) in breast cancer. Cells. 2023;12:12. 10.3390/cells12101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Seyfinejad B, Jouyban A. Importance of method validation in the analysis of biomarker. Curr Pharm Anal. 2022;18:567–9. 10.2174/1573412918666211213142638. [Google Scholar]
- 302.Xiang D, Han X, Li J, Zhang J, Xiao H, Li T, et al. Combination of IDO inhibitors and platinum(IV) prodrugs reverses low immune responses to enhance cancer chemotherapy and immunotherapy for osteosarcoma. Mater Today Bio. 2023;20: 100675. 10.1016/j.mtbio.2023.100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ahuja N, Hwaun E, Pungor JR, Rafiq R, Nemes S, Sakmar T, et al. Creation of an albino squid line by CRISPR-Cas9 and its application for in vivo functional imaging of neural activity. Curr Biol. 2023;33:2774–e2835. 10.1016/j.cub.2023.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.