Abstract
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition marked by difficulties in social interaction, communication, and repetitive behaviors or restricted interests. Although research on the link between ASD and parasitic diseases is limited, immune deficiency and inflammation may contribute to the development of parasitic infections.
Case report
We admitted a 14-year-old boy to the hospital who had a known history of ASD because he was presenting with respiratory symptoms, including cough and hemoptysis. During his time in the hospital, after conducting a series of tests and evaluations, we made a critical diagnosis of co-infection of lophomonas, tuberculosis, and hydatid cyst. In response to this diagnosis, we initiated a treatment plan that involved administering appropriate antibiotics as well as preparing for surgical intervention.
Conclusion
This case report highlights the complexities and challenges of managing such a rare combination of co-infections with TB, pulmonary hydatid disease and lophomonas in a pediatric patient with underlying developmental considerations such as ASD.
Keywords: Lophomonas blattarum, Parasite infection, Autism Spectrum Disorder, Tuberculosis, Hydatid cyst, Coinfection
Introduction
Lophomonas blattarum is a flagellated protozoan in the parabasalids group, primarily found in the intestines of insects like cockroaches. It aids in the digestion of cellulose and complex carbohydrates, contributing to the host’s gut microbiota and overall health as a symbiont. However, it can also cause infections or opportunistic diseases, particularly when the host’s immune system is weakened. Symptoms may include gastrointestinal disturbances, inflammation, and, in severe cases, systemic infections that threaten the host’s health. Clinical symptoms and radiographic findings are mostly non-specific. So, a microscopic examination of respiratory secretions is essential for diagnosing L. blattarum, as it mimics other infections. Bronchoscopy smears or BAL can confirm the diagnosis [1–3].
L. blattarum co-infection with Mycobacterium tuberculosis (TB) poses a serious public health risk, especially for individuals with weakened immune systems. In regions like Iran, where TB prevalence is high, and healthcare access in deprived areas is limited, co-infection rates are elevated. TB bacteria can take advantage of compromised immunity, facilitating infection and progression to active disease. Furthermore, the immune response to TB is often diminished, resulting in atypical presentations and more severe illness. The tuberculosis treatment program, known as DOTS (Directly Observed Treatment, Short-course), employs a comprehensive set of therapeutic regimens specifically designed to combat TB. It is vital to adopt a coordinated approach that manages tuberculosis effectively and addresses any underlying immunocompromising conditions in affected individuals [4–6].
Hydatidosis (parasitic disease) (HC) caused by echinococcus larvae, primarily from the tapeworm Echinococcus granulosus. It mainly affects the liver and lungs, forming hydatid cysts that can lead to symptoms such as pain and nausea or complications if they rupture. Diagnosis of hydatidosis involves imaging tests, cyst fluid analysis, or serological tests. The disease can often be asymptomatic, complicating diagnosis and increasing transmission risk. Pulmonary hydatid cyst treatment may involve pharmacotherapy or surgery, with surgery preferred. The co-infection of HC with TB is commonly observed and prevalent in certain regions, especially where healthcare resources may be limited, whereas instances of co-infection with Lophomonas are considerably rare [7–9].
Comorbid conditions are more prevalent among individuals with autism spectrum disorders (ASD) compared to the general population, negatively impacting their core symptoms, daily activities, and health-related quality of life. These conditions often include mental health disorders as well as physical issues such as infections. Their presence complicates clinical management, increases caregiver stress, and necessitates integrated treatment approaches. There is currently no scientific evidence linking autism to increased risk of infections such as TB, HC, and lophomonas or suggesting that co-infection affects the symptoms or treatment of either condition. However, individuals with autism may encounter unique healthcare challenges, such as barriers to accessing medical services, which can impact their overall health, including infection detection and treatment. Communication and social interaction difficulties often associated with autism may hinder individuals from expressing health concerns or understanding medical advice, leading to delays in diagnosis and treatment for infection and other health issues. Additionally, sensory sensitivities common in autism can complicate medical procedures, like taking chest X-rays or medication administration, resulting in increased anxiety and reluctance to seek necessary healthcare, further worsening their health challenges [10, 11].
Recent developments in medical science have significantly enhanced both treatment options and the overall quality of life for individuals suffering from immunodeficiency disorders. Nonetheless, the heightened susceptibility of these patients to opportunistic infections underscores the necessity for prompt diagnosis and intervention, which demands a high level of clinical awareness and comprehensive expertise in the relevant medical domain. Instances of co-infection, where individuals are simultaneously infected with lophomonas and other infectious diseases, have been documented. However, evidence of co-infection specifically involving lophomonas, HC, and TB is extremely rare globally. This suggests that while co-infections are recognized, the combination of lophomonas, HC and TB is not widely reported. In this case study, we describe the diagnosis and treatment of a 14-year-old boy with ASD co-infected with lophomonas, HC, and TB.
Case presentation
A 14-year-old male patient was admitted to the Department of Respiratory Medicine at Talghani Pediatric Hospital in Gorgan, Iran. His parents reported two instances of hemoptysis prior to admission, during which he expectorated a twelve cubic centimeter (cc) clot on each occasion, accompanied by intermittent fever and productive cough that began approximately ten days earlier. No gastrointestinal or urinary symptoms were reported.
There were no complications noted during the patient’s prenatal or perinatal periods. He has a medical history of ASD and has been under psychiatric care. Due to the ASD, the patient has experienced difficulties in communicating with those around him, which has hindered his ability to articulate his symptoms to both medical professionals and family members.
The patient had stable vital signs, with 96% oxygen saturation without supplemental oxygen, a respiratory rate of 18 per minute, blood pressure of 115/60, and a pulse rate of 78 per minute. Auscultation revealed crackles in the right lung, and heart sounds (S1 and S2) were regular. The abdominopelvic examination was unremarkable, with no signs of splenomegaly or hepatomegaly, and no skin lesions or lymphadenopathy were noted.
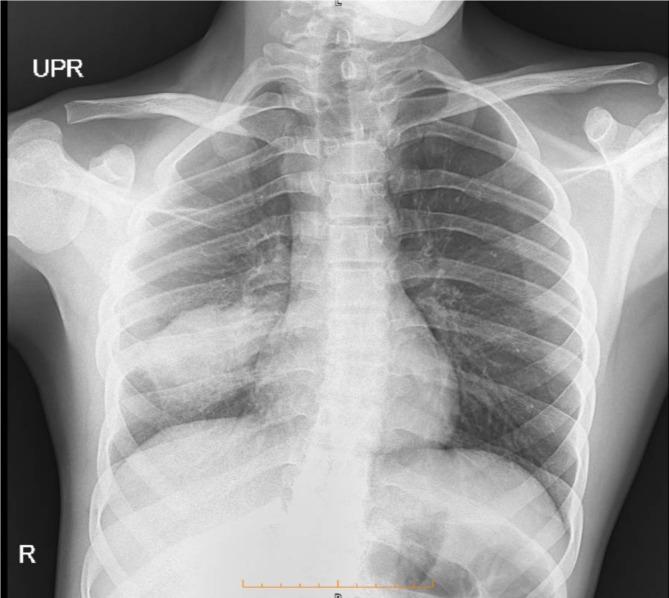
The patient was admitted to the hospital due to persistent symptoms and abnormal findings during the physical examination. Initial assessments included laboratory analyses and chest X-rays, which indicated the presence of a mass-like consolidation in the right middle lobe measuring 46 × 26 millimeters (Fig. 1). The complete blood count revealed anemia and leukocytosis, with an erythrocyte sedimentation rate (ESR) of 36 mm/s and a C-reactive protein (CRP) concentration of + 2. Given the elevated inflammatory markers and the abnormal X-ray findings, further laboratory investigations were performed. Additionally, a spiral CT scan was carried out to obtain more detailed diagnostic insights. Results for serum levels of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA), cytoplasmic anti-neutrophil cytoplasmic antibodies (C-ANCA), and viral markers (HIV, hepatitis B, and hepatitis C) were within normal ranges. Furthermore, the SARS-CoV-2 PCR test was negative in the context of the COVID-19 pandemic.
Fig. 1.
The observation of a mass-like consolidation in the right middle lobe, measuring 46 × 26 millimeters in initial Chest X-ray
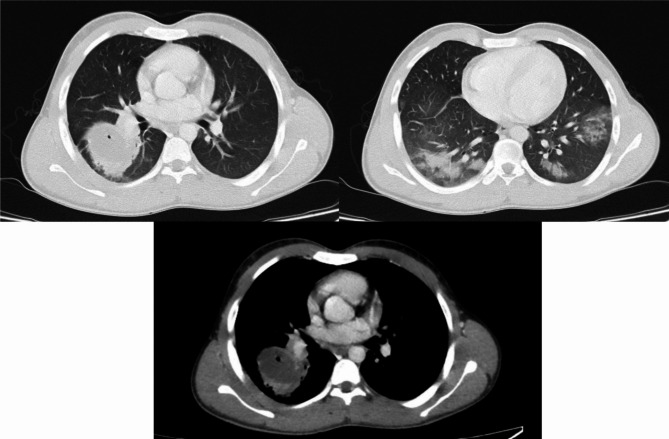
The contrast-enhanced lung CT scan revealed a thick-walled cystic shadow measuring 50 × 34 mm in the right lower lobe, situated adjacent to the hilium, suggestive of a hydatid cyst. An air bubble within the cyst suggests a potential connection to the bronchus. Additionally, ground-glass opacities were noted in the lower lobes of both lungs (Fig. 2).
Fig. 2.
The contrast-enhanced lung CT scan identified a thick-walled cystic lesion, measuring 50 × 34 millimeters, in the right lower lobe along with ground-glass opacities in the lower lobes of both lungs
In light of the abnormalities observed on the lung CT scan, a fiber optic bronchoscopy was performed, during which a bronchoalveolar lavage (BAL) specimen was collected for the assessment of BK and other infections. Microscopic examination of the BAL sample identified live oval flagellated Lophomonas protozoa, Mycobacterium tuberculosis, and hydatid cyst protoscoleces (the larval forms of the parasites). Testing revealed the presence of anti-Echinococcus antibodies (IgG and IgM), confirming a co-infection with tuberculosis, hydatid cysts, and Lophomonas (Fig. 3).
Fig. 3.
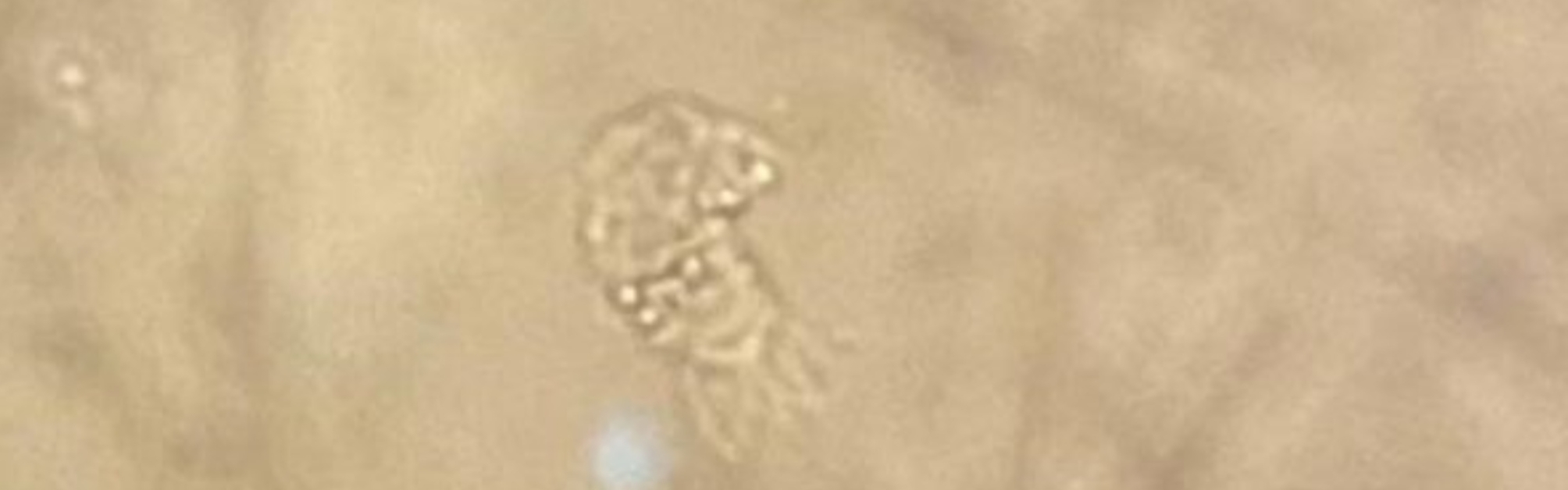
Microscopic analysis of the bronchoalveolar lavage (BAL) sample revealed the presence of live oval flagellated protozoa of the genus Lophomonas
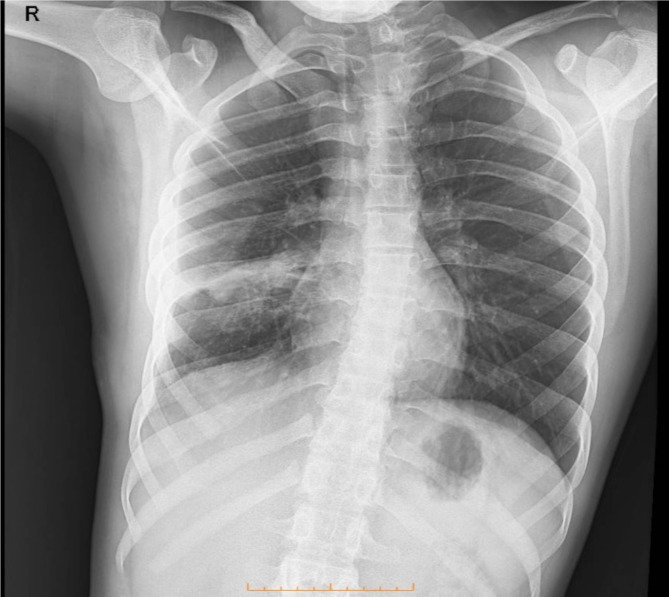
The patient was initiated on a treatment regimen that included metronidazole (500 mg three times daily for two weeks) and oral albendazole (400 mg twice daily), along with the standard tuberculosis therapy. Surgical intervention was performed to reduce the hydatid cyst, involving a comprehensive evaluation and careful planning to minimize complications. Advanced surgical techniques were utilized to ensure both precision and effectiveness. The initial recovery phase was promising, with the patient responding positively to rehabilitation efforts. Upon discharge, the patient was in stable condition, exhibiting improved symptoms and prescribed oral medications (Fig. 4).
Fig. 4.
The radiological follow-up conducted two weeks post-treatment indicates a notable enhancement in the lesion located in the right lung
Following a thorough screening of the contacts, we identified his grandfather as the index case for tuberculosis. Consequently, we administered TB tests to the other family members, and all results were negative.
Discussion
Infections caused by L. blattarum, TB, and HC present with comparable clinical manifestations, which include cough, sputum production, night sweats, weight loss, general weakness, malaise, hemoptysis, and respiratory distress. The symptom overlap and similar imaging findings with other diseases complicate the processes of accurate diagnosis and treatment. Researchers advocate for the consideration of these infections in patients exhibiting elevated eosinophil counts, significant respiratory distress, compromised immune systems, or those who do not respond to antibiotic therapy [4, 5, 7]. Clinicians should maintain a high index of suspicion regarding exposure histories, particularly in immunocompromised patients or individuals who have traveled to endemic areas. Imaging modalities such as chest X-rays or computed tomography (CT) scans may reveal characteristic patterns that assist in distinguishing these infections from other pulmonary disorders; however, the resemblance in radiographic appearances can hinder definitive diagnosis. Confirmatory diagnosis may necessitate serological assays, biopsies, or cultures. The diagnosis of these co-infections presents significant challenges that demand meticulous observation and specialized staining techniques [6–8].
In a review by Mewara A. et al. in 2024 focusing on lophomoniasis infection, the most commonly reported symptoms included cough (70%), fever (60%), expectoration (46%), and hemoptysis (12%). Additionally, a considerable proportion of the patients (89%) presented with comorbidities or associated conditions. BAL samples were predominantly employed for diagnosis (82%), and microscopic examination revealed a 100% detection rate for lophomoniasis, whereas PCR testing identified the infection in approximately 35% of cases [12]. In addition, in a study conducted by Nakhaei M et al. in 2020, the researchers investigated cases of lophomoniasis spanning a period of 27 years, from 1993 to March 2020. The patient demographic ranged in age from 1 month to 84 years, with a mean age of 23.7 years. Among the 307 cases analyzed, 171 patients (55.7%) were classified as juveniles, aged 18 years or younger. Additionally, 111 patients (36.1%) had a documented history of pre-existing medical conditions. BAL samples were the predominant specimen type utilized for the detection of Lophomonas infection, accounting for 87.6% of cases. Moreover, microscopic examination was employed for the diagnosis of the infection in all studies, with the exception of one [13]. This aligns with our patient, who initially demonstrated symptoms of cough, fever, and hemoptysis. Abnormal radiological findings, which include a 50 × 34 mm thick-walled cystic shadow in the right lower lobe and ground glass opacities in the lower lobes of both lungs. His infection was confirmed through BAL sampling and microscopic analysis.
Distinguishing L. blattarum from ciliated epithelial cells under a light microscope can be hard due to the size and morphology. L. blattarum measures approximately 0.5-1.0 micrometers in diameter and has a rod shape, while epithelial cells are much larger, measuring tens of micrometers, and have polygonal or columnar shapes with visible cilia on their surfaces. As a result, medical procedures such as a bronchoscopy biopsy smear, a sputum smear analysis, or bronchoalveolar lavage (BAL) can be conducted to effectively identify the presence of L. blattarum, tuberculosis, and hydatid in patients being evaluated for these conditions. These diagnostic tests play an important role in confirming infections that may affect the respiratory system [1, 2, 14]. In our case, the coinfection of L. blattarum, TB and CE was confirmed by a comprehensive examination that included microscopic examinations and serological tests.
According to a 2015 WHO report in Iran, the tuberculosis incidence rate was 16, resulting in approximately 2,000 deaths annually and the annual incidence rate of hydatidosis is estimated at 0.61 cases per 100,000 people. The geographic distribution of TB and HC underscores the need for epidemiological surveillance in co-endemic regions. Understanding the local dynamics of TB and HC with parasitic infections enables public health initiatives to more effectively target interventions for reducing co-infections. Educational programs that emphasize early recognition and treatment options are essential for healthcare providers in these areas. Although clinically significant pulmonary protozoan infections are rare, they have become more recognized in recent decades due to rising immune suppression rates. Studies show a high prevalence of co-infection between TB and parasitic diseases like CE and Lophomonas, where each infection increases the risk for the other. The patient may have initially had CE or tuberculosis, followed by lophomonas as a superinfection. Since lophomonas commonly affects immunocompromised individuals, TB or HC can weaken the immune system, increasing susceptibility to additional infections and reactivating latent ones. In addition, a recent case report study illustrated the simultaneous occurrence of tuberculosis, pulmonary hydatid cyst, and L. blattarum in a patient with a background of brain tumor and diabetes mellitus [1, 6, 15].
Lophomonas is often overlooked due to its rarity, yet its impact on immunocompromised patients can be significant. In such cases, the body’s diminished defenses create an environment conducive to opportunistic infections. This scenario raises critical questions about the interactions between various pathogens and the host’s immune response [16, 17]. Research by Tajik Jalayeri et al. (2024) identifies factors affecting co-infection rates, including sociodemographic variables (gender, age), underlying health conditions, and living in regions with high parasitic infection rates. This research highlighted that the prevalence of lophomonas infection was found to be notably high among patients who were suspected of having TB and CE. The relationship between these infections calls for a comprehensive patient management approach, especially for at-risk populations. The immunosuppressive effects of corticosteroids facilitate opportunistic infections like L. blattarum and worsen the clinical consequences of coexisting conditions such as cystic echinococcosis and tuberculosis [1]. Similar to our case, the prevalence of lophomonas infection was found to be notably high among a patient who was suspected of having TB and CE. As a result, this overlap in clinical presentations complicates the diagnostic process, drawing attention to the importance of recognizing lophomonas infection in such cases to ensure that patients take the proper treatment.
About 25% of children with ASD exhibit immune deficiency and dysfunction, while most show no signs of immune dysregulation. Therefore, laboratory testing is essential to eliminate this possibility. Additionally, behaviors often associated with autism may indicate other underlying organic disorders. To effectively manage comorbid conditions in individuals with ASD and address infections like L. blattarum, TB, and CE, a series of comprehensive strategies are essential. Regular health screenings should be implemented to identify health issues early. Enhanced hygiene practices are necessary to reduce infection risk, and promoting symptom awareness helps individuals and caregivers recognize underlying health concerns. Finally, providing psychosocial support is vital for offering emotional and psychological resources to navigate these challenges [9, 10, 17].
Our study had strengths, including the reporting of an extremely rare case of TB, CE, and lophomonas co-infection in teen patient with ASD. We also alerted all close contacts and screened them for potential infections. However, we faced limitations, such as the need to send the patient’s bal sample to another center for a diagnosis, which was time-consuming. Because of the patient’s diagnosis of ASD, we found it necessary to seek a psychiatric consultation. This led to various challenges and difficulties during the patient’s doctor visits as well as during evaluations that involved laboratory tests and imaging procedures. The complexities of managing care for a patient with ASD added layers of difficulty during these assessments.
Conclusion
Individuals who are diagnosed with ASD exhibit an increased vulnerability to various opportunistic infections due to their unique health needs and immune system responses. Co-infection involving TB alongside parasitic diseases, such as HC and lophomonas, is an uncommon occurrence in human populations and is generally seen in regions where both diseases are prevalent. Consequently, physicians need to remain vigilant and consider the potential for these co-infections when assessing their patients. This includes thoroughly investigating individuals with ASD who present symptoms that are resistant to standard treatment approaches, as these may indicate the presence of complications from co-infections that require specific attention and intervention.
Acknowledgements
Not applicable.
Author contributions
L.S advised the case report study. M.M. and N.L. gathered the patient’s medical and health records. N.B. did the surgical treatment. M.M. and N.L. wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient’s legal guardian to publish this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The purpose of this case report was completely explained to the patient’s legal guardian, and they were assured that the researchers would keep her information confidential. This case report was performed in line with the Declaration of Helsinki principles. Ethical approval code: IR.GOUMS.REC.1403.210.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jalayeri MH, Sharifi far RA, Lashkarbolouk N, Mazandarani M. The co-infection of pulmonary hydatid cyst, lophomoniasis and tuberculosis in a patient with resistant respiratory symptoms; a case report study. BMC Infect Dis. 2024;24(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Gao ZC. Lophomonas blattarum infection or just the movement of ciliated epithelial cells? Chin Med J. 2016;129(06):739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng SS, Dai ZF, Wang HC, Li YX, Wei DD, Yang RL, Lin XH. Authenticity of pulmonary Lophomonas blattarum infection: a case report. World J Clin cases. 2019;7(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caulfield AJ, Wengenack NL. Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J Clin Tuberculosis Other Mycobact Dis. 2016;4:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean E, Broger T, Yerlikaya S, Fernandez-Carballo BL, Pai M, Denkinger CM. A systematic review of biomarkers to detect active tuberculosis. Nat Microbiol. 2019;4(5):748–58. [DOI] [PubMed] [Google Scholar]
- 6.Jalayeri MH, Zakariaei Z, Fakhar M, Sharifpour A, Banimostafavi ES, Soleymani M. Ruptured pulmonary hydatid cyst and lophomoniasis comorbidity in a young man: a rare case. Oxf Med Case Rep. 2023;2023(3):omad023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruah A, Sarma K, Barman B, Phukan P, Nath C, Boruah P, Rajkhowa P, Baruah M, Dutta A, Naku N. Clinical and laboratory presentation of hydatid disease: a study from Northeast India. Cureus. 2020;12(9). [DOI] [PMC free article] [PubMed]
- 8.Gessese AT. Review on epidemiology and public health significance of hydatidosis. Veterinary Med Int. 2020;2020(1):8859116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydin Y, Ulas AB, Ahmed AG, Eroglu A. Pulmonary hydatid cyst in children and adults: diagnosis and management. Eurasian J Med. 2022;54(1):S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Beltagi M. Autism medical comorbidities. World J Clin Pediatr. 2021;10(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson H, Sjöqvist H, Brynge M, Gardner R, Dalman C. Childhood infections and autism spectrum disorders and/or intellectual disability: a register-based cohort study. J Neurodevelopmental Disorders. 2022;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mewara A, Gile GH, Mathison B, Zhao H, Pritt B, Bradbury RS. Lophomonas as a respiratory pathogen—jumping the gun. J Clin Microbiol. 2024;62(1):e00845–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakhaei M, Fakhar M, Sharifpour A, Ziaei Hezarjaribi H, Banimostafavi ES, Nazar E. Global status of emerging Lophomonas infection: a systematic review of reported cases (1993–2020). Interdisciplinary Perspectives on Infectious Diseases. 2022;2022(1):3155845. [DOI] [PMC free article] [PubMed]
- 14.Jalayeri MH, Aghaei M, Mazandarani M, Lashkarbolouk N, Sharifpour A. Diagnosis of pulmonary lophomoniasis infection in patient with systemic lupus erythematosus; A case report and literature review. Respirol Case Rep. 2024;12(10):e70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golsha R, Mazandarani M, Sohrabi A, Shirzad-Aski H, Kamalinia H, Rezaeifar A, Fattahi M. Risk factors and treatment outcome of smear positive pulmonary tuberculosis patients: A five-year study in the North of Iran. Caspian J Intern Med. 2024;15(2):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajik Jalayeri MH, Lashkarbolouk N, Mazandarani M. Diagnosis of pulmonary lophomoniasis in an elderly anthracosis patient with resistant respiratory symptoms: A literature review and a case report study. Clin Case Rep. 2024;12(6):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao X, Liao Q, Pan T, Li S, Zhang X, Zhu S, Lin Z, Qiu Y, Liu J. Retrospect and prospect of Lophomonas blattarum infections and lophomoniasis reported in China. Open Access Libr J. 2014;1(9):1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.