Abstract
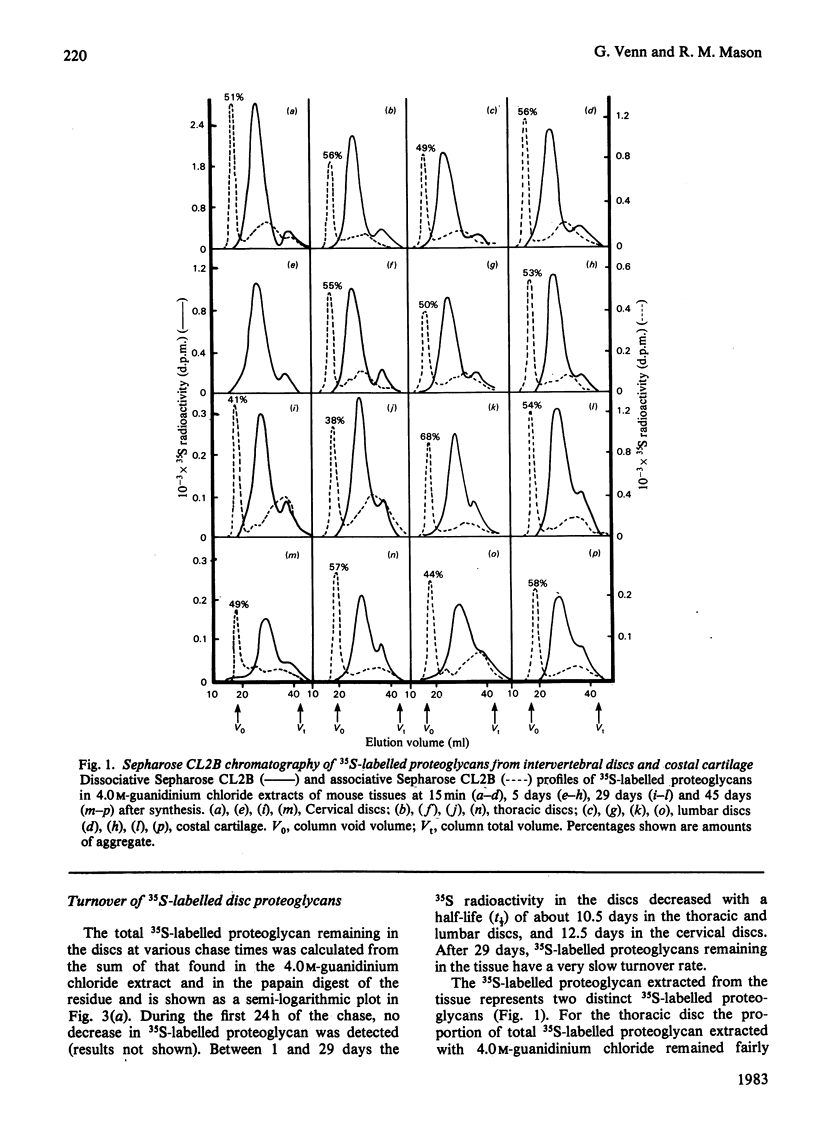
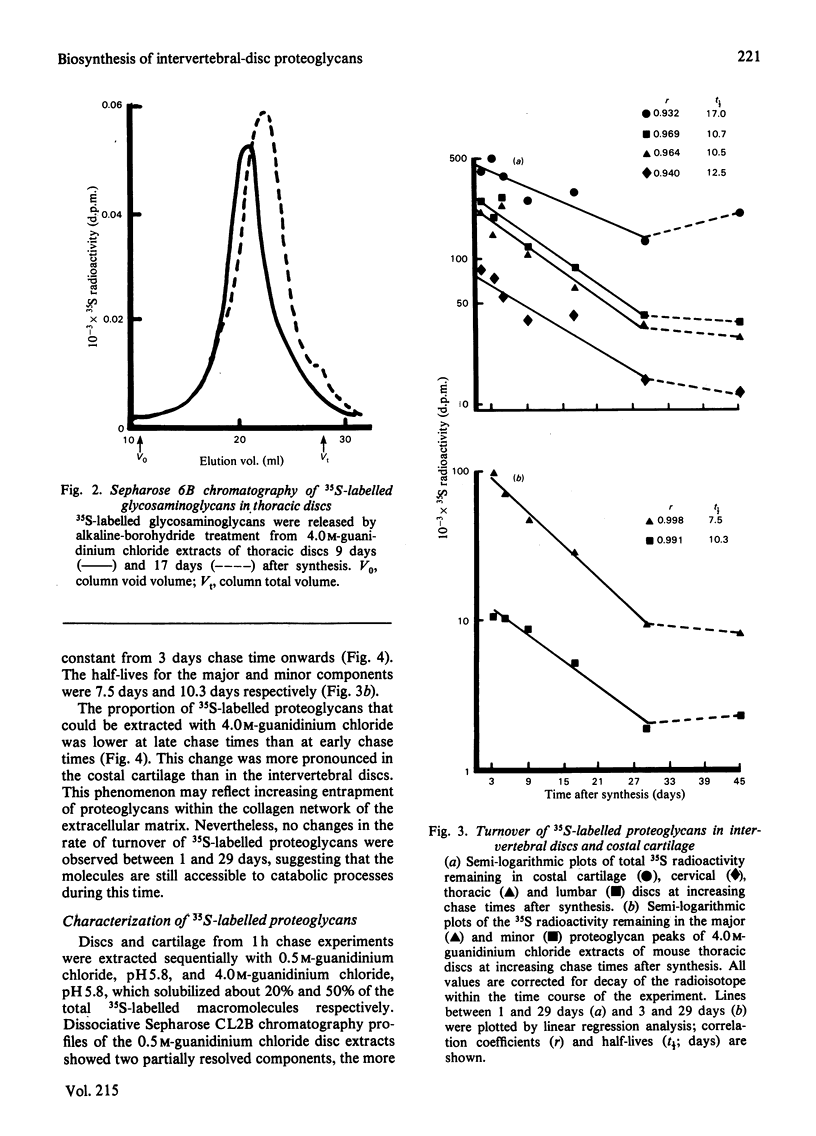
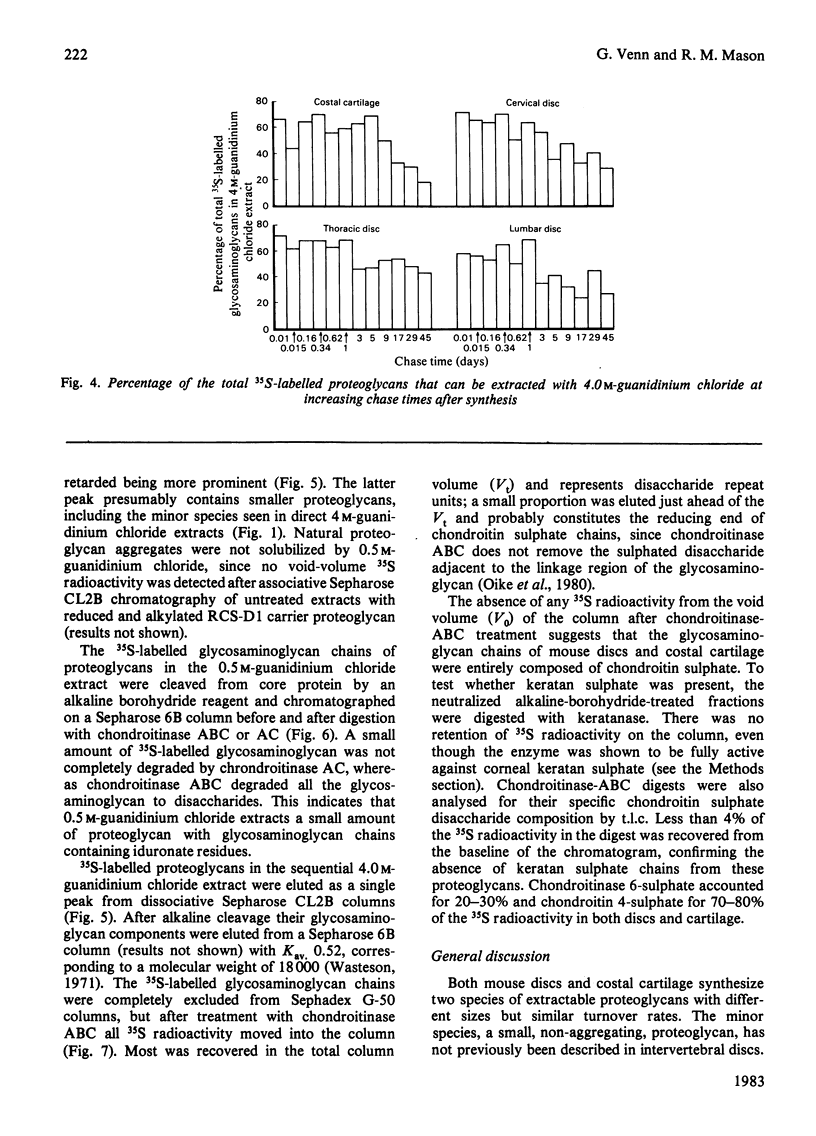
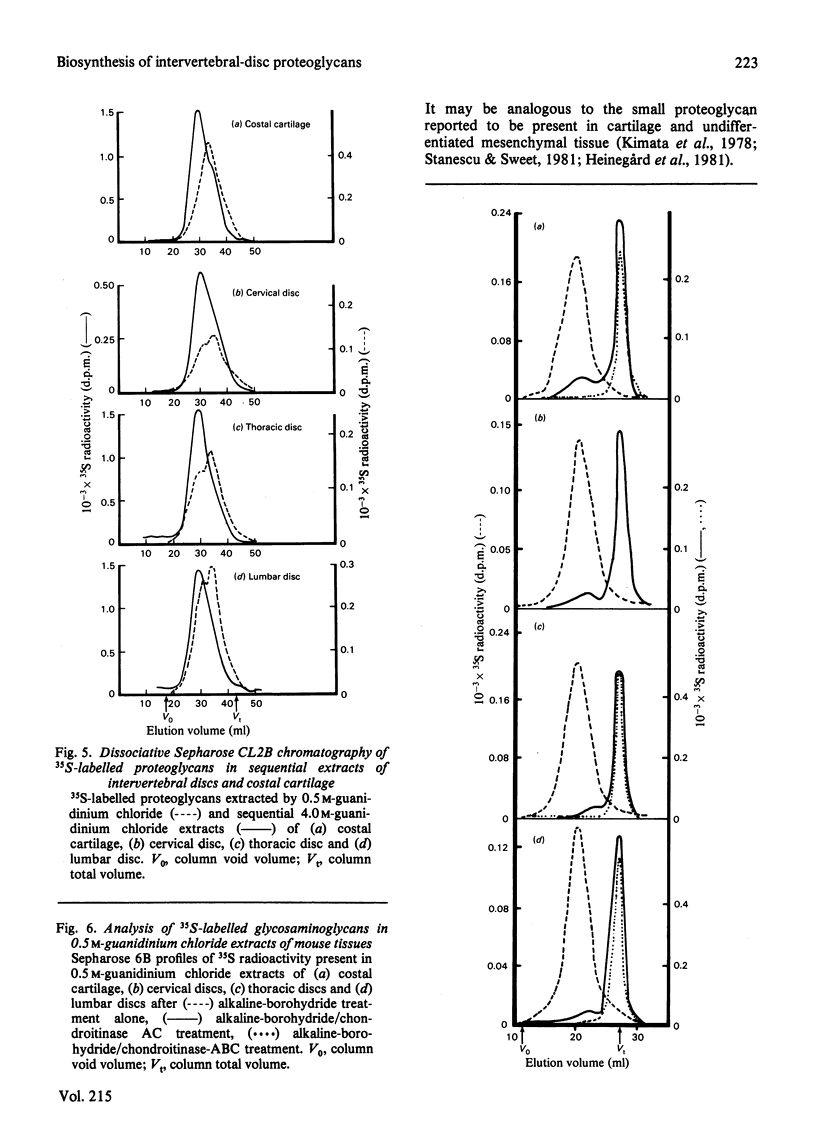
The synthesis and turnover in vivo of 35S-labelled proteoglycans in mouse cervical, thoracic and lumbar intervertebral discs, and in costal cartilage, was investigated after intraperitoneal injection of [35S]sulphate. Intervertebral discs and costal cartilage synthesize similar amounts of 35S-labelled proteoglycans per microgram of DNA. Discs and cartilage all synthesize a major proteoglycan species (approx. 85%) of large hydrodynamic size and a minor species (approx. 15%) of small size. Both proteoglycans carry chondroitin sulphate chains. Keratan sulphate was not found associated with either species. The total 35S-labelled proteoglycan pool had a metabolic half-life (t1/2) of 10-12 days in discs, and 17 days in cartilage. The extractable major and minor species turned over at similar rates. Those proteoglycans left in the tissue after 29 days turn over very slowly. Approx. 50% of the major 35S-labelled proteoglycan species formed mixed aggregates with hyaluronic acid and rat chondrosarcoma proteoglycan. The ability to form aggregates did not decrease up to 45 days after synthesis. Of the heterogeneous population of proteoglycans comprising the major species, those remaining in the tissue 9 days after synthesis were of smaller average hydrodynamic size and had shorter chondroitin sulphate side chains than the average size at the time of synthesis. With increasing time after synthesis, proteoglycans were less readily extracted from the tissue by 4.0 M-guanidinium chloride than at the time of synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOSTROM H. On the metabolism of the sulfate group of chondroitinsulfuric acid. J Biol Chem. 1952 May;196(2):477–481. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- DAVIDSON E. A., SMALL W. Metabolism in vivo of connective-tissue mucopolysaccharides. I. Chondroitin sulfate C and keratosulfate of nucleus pulposus. Biochim Biophys Acta. 1963 Mar 5;69:445–452. doi: 10.1016/0006-3002(63)91292-7. [DOI] [PubMed] [Google Scholar]
- Emes J. H., Pearce R. H. The proteoglycans of the human intervertebral disc. Biochem J. 1975 Mar;145(3):549–556. doi: 10.1042/bj1450549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977 May 27;492(1):29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Faltz L. L., Reddi A. H., Hascall G. K., Martin D., Pita J. C., Hascall V. C. Characteristics of proteoglycans extracted from the Swarm rat chondrosarcoma with associative solvents. J Biol Chem. 1979 Feb 25;254(4):1375–1380. [PubMed] [Google Scholar]
- Ghosh P., Taylor T. K., Braund K. G. Variation of the glycosaminoglycans of the intervertebral disc with ageing. II. Non-chondrodystrophoid breed. Gerontology. 1977;23(2):99–109. doi: 10.1159/000212178. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Gardell S. Studies on protein-polysaccharide complex (proteoglycan) from human nucleus pulposus. I. Isolation and preliminary characterisation. Biochim Biophys Acta. 1967 Oct 9;148(1):164–171. doi: 10.1016/0304-4165(67)90292-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Kimata K., Oike Y., Ito K., Karasawa K., Suzuki S. The occurrence of low buoyant density proteoglycans in embryonic chick cartilage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1431–1439. doi: 10.1016/0006-291x(78)91163-4. [DOI] [PubMed] [Google Scholar]
- Lohmander S., Antonopoulos C. A., Friberg U. Chemical and metabolic heterogeneity of chondroitin sulfate and keratin sulfate in guinea pig cartilage and nucleus pulposus. Biochim Biophys Acta. 1973 Apr 28;304(2):430–448. doi: 10.1016/0304-4165(73)90263-8. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Kimura J. H., Hascall V. C. Biosynthesis of hyaluronic acid in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Mar 10;257(5):2236–2245. [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Nieduszynski I. A., Sheehan J. K., Phelps C. F., Hardingham T. E., Muir H. Equilibrium-binding studies of pig laryngeal cartilage proteoglycans with hyaluronate oligosaccharide fractions. Biochem J. 1980 Jan 1;185(1):107–114. doi: 10.1042/bj1850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Bradford D. S., Cooper K. M. Aggregated proteoglycan synthesis in organ cultures of human nucleus pulposus. J Biol Chem. 1979 Nov 10;254(21):10579–10581. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osebold W. R., Pedrini V. Pepsin-solubilized collagen of human nucleus pulposus and annulus fibrosus. Biochim Biophys Acta. 1976 Jun 15;434(2):390–405. doi: 10.1016/0005-2795(76)90230-0. [DOI] [PubMed] [Google Scholar]
- Rostand K. S., Baker J. R., Caterson B., Christner J. E. Isolation and characterization of mouse articular cartilage proteoglycans using preformed CsCl density gradients in the Beckman Airfuge. A rapid semi-micro procedure for proteoglycan isolation. J Biol Chem. 1982 Jan 25;257(2):703–707. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Sweet M. B. Characterization of a proteoglycan of high electrophoretic mobility. Biochim Biophys Acta. 1981 Feb 18;673(1):101–113. [PubMed] [Google Scholar]
- Stevens R. L., Dondi P. G., Muir H. Proteoglycans of the intervertebral disc. Absence of degradation during the isolation of proteoglycans from the intervertebral disc. Biochem J. 1979 Jun 1;179(3):573–578. doi: 10.1042/bj1790573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. L., Ewins R. J., Revell P. A., Muir H. Proteoglycans of the intervertebral disc. Homology of structure with laryngeal proteoglycans. Biochem J. 1979 Jun 1;179(3):561–572. doi: 10.1042/bj1790561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]



