Abstract
Objective
This study explored the predictors of abemaciclib discontinuation, a cyclin-dependent kinase 4 and 6 inhibitor, in patients with breast cancer.
Material and Methods
Between November 2018 and March 2023, 147 patients with breast cancer treated with abemaciclib at Osaka Medical and Pharmaceutical University Hospital and Kindai University Nara Hospital were included. The exclusion criteria were as follows: lack of blood testing within 2 weeks prior to starting abemaciclib therapy, transfer to another facility after the commencement of abemaciclib therapy, and discontinuation of abemaciclib therapy due to the diagnosis of another cancer. The duration from the initiation of abemaciclib to discontinuation for any reason and to temporary suspension or dose reduction due to adverse events were analyzed as outcome variables using multivariate Cox regression analysis.
Results
Baseline weight < 54 kg, bone metastases, and hemoglobin level ≤ 12.4 g/dL were independent predictors of abemaciclib discontinuation for any reason. The main adverse events leading to abemaciclib discontinuation were liver enzyme elevation and gastrointestinal symptoms. Additionally, focusing on the adverse event of abemaciclib, a baseline weight < 54 kg was an independent predictor of temporary suspension or dose reduction due to adverse events. The most common adverse events leading to temporary suspension or dose reduction were neutropenia and diarrhea.
Conclusion
Patients with lower body weight are more susceptible to the adverse events of abemaciclib, increasing their risk of treatment discontinuation. In such patients, strict monitoring of adverse events and consideration of more frequent medical visits are necessary from the start of abemaciclib therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13091-y.
Keywords: Abemaciclib, Breast cancer, Chemotherapy, Discontinuation, Predictor
Introduction
Breast cancer is diagnosed at an early stage in over 90% of cases, with a mortality rate of approximately 15–20% relative to the incidence, indicating a higher survival rate compared to other cancers [1, 2]. However, cases with breast cancer that recur are predominantly incurable, and patients achieving surgical remission are required to complete postoperative pharmacotherapy to suppress recurrence [3]. Moreover, even in cases of metastasis, pharmacotherapy can yield a five-year survival rate of approximately 25%, significantly affecting overall survival [4].
Cyclin-dependent kinases (CDKs) 4 and 6 form complexes with cyclin D, inactivating the tumor suppressor retinoblastoma protein by phosphorylation, and facilitating cell cycle progression from G1 to S phase [5]. Abemaciclib targets cyclin D, preventing the formation of complexes between CDK4 and 6 and cyclin D, and preventing the phosphorylation of retinoblastoma protein, thereby stopping the progression of the cell cycle from G1 to S phase and suppressing tumor growth [6, 7]. The adverse event profiles of CDK4/6 inhibitors differ depending on their selectivity for these kinases, and because abemaciclib has lower selectivity for CDK6 than other CDK4/6 inhibitors, it is expected to have lower hematological toxicity [8]. Over 50% of patients with breast cancer exhibit overexpression of cyclin D [9], and excessive expression of cyclin D has been reported in estrogen receptor-positive cells [10].
Since 2014, international Phase III trials, specifically the MONARCH2 and MONARCH3 trials, have targeted hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative inoperable or recurrent breast cancer. The MONARCH2 trial demonstrated the efficacy and safety of abemaciclib in combination with fulvestrant, a selective estrogen receptor degrader, whereas the MONARCH3 trial demonstrated its effectiveness when combined with nonsteroidal aromatase inhibitors (NSAI), such as anastrozole or letrozole [11, 12]. Consequently, in September 2018, abemaciclib was approved in Japan for the treatment of hormone receptor-positive and HER2-negative inoperable or recurrent breast cancers.
Additionally, since 2017, an international Phase III monarchE trial has been conducted for adjuvant pharmacotherapy in patients with hormone receptor-positive and HER2-negative breast cancer at high risk of recurrence. The trial demonstrated that the combined administration of abemaciclib and adjuvant endocrine therapy significantly extended invasive disease-free survival (IDFS) compared to endocrine therapy alone in postoperative patients with hormone receptor-positive and HER2-negative breast cancer at high risk of recurrence. Consequently, in December 2021, abemaciclib in combination with endocrine therapy was approved in Japan as an adjuvant pharmacotherapy for high-risk recurrent hormone receptor–positive and HER2-negative breast cancer [13].
Abemaciclib is one of the available CDK4/6 inhibitors used as a first-line therapy for inoperable or recurrent hormone receptor-positive, HER2-negative breast cancer, along with other agents such as palbociclib and ribociclib (not yet approved in Japan). In the MONARCH2 trial, over 60% of patients treated with abemaciclib experienced Grade 3 or higher adverse events. Adverse events led to abemaciclib discontinuation in 15.9% of patients and dose reduction in 42.9% of patients [14]. In the MONARCH3 study, 76.3% of patients experienced diarrhea, with 2.3% abemaciclib discontinuation [12]. Additionally, 41.3% of patients developed neutropenia, which improved with temporary suspension of the therapy. In the monarchE study, 45.5% of patients experienced Grade 3 or higher adverse events, leading to abemaciclib discontinuation in 16.6% of patients, with 4.8% of abemaciclib discontinuation due to diarrhea [13]. These findings indicate a high incidence of Grade 3 or higher adverse events with abemaciclib.
To extend the duration of abemaciclib treatment and significantly contribute to prolonged survival in patients with hormone receptor-positive and HER2-negative breast cancer, it is crucial to detect adverse events early and respond appropriately with timely interventions such as temporary suspension or dose reduction. Information on which patients are prone to temporary suspension or dose reduction, followed by therapy discontinuation, and the adverse events associated with these outcomes, is therefore valuable. However, there is limited research on the predictors of abemaciclib discontinuation from abemaciclib therapy, and it is unclear which patient characteristics are associated with discontinuation. Therefore, this study aimed to investigate the predictors of abemaciclib discontinuation therapy due to any reason.
Materials and methods
Patients
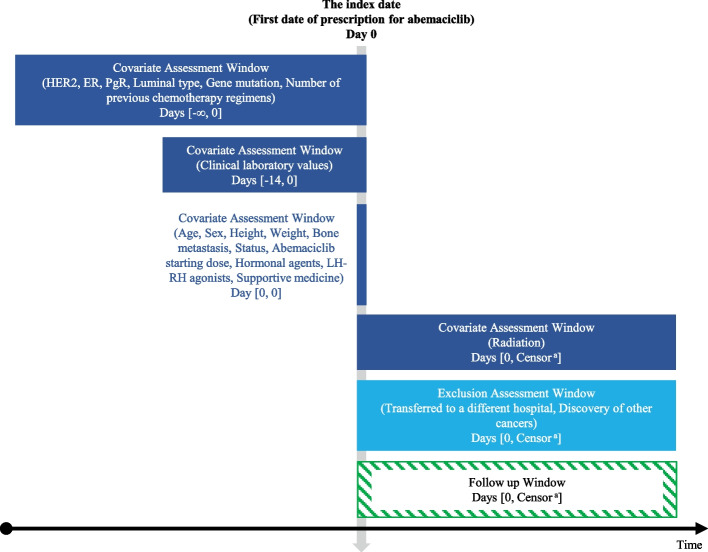
Patients with breast cancer who received abemaciclib therapy at Osaka Medical and Pharmaceutical University Hospital and Kindai University Nara Hospital between November 2018 and March 2023 were included. Figure 1demonstrates the study diagram [15]. The exclusion criteria were lack of blood testing within 2 weeks prior to starting abemaciclib therapy, transfer to another facility after treatment initiation, and discontinuation of abemaciclib therapy due to the diagnosis of another cancer.
Fig.1.
Study diagram. This diagram illustrates the timeline of covariate assessments, exclusion assessments, and follow-up periods for patients treated with abemaciclib. Covariates such as clinical characteristics (HER2, ER, PgR, luminal type, gene mutations, etc.) and laboratory values were assessed within specific windows prior to and at the start of therapy. Patients were followed up from the initiation of abemaciclib therapy until the occurrence of an event, such as therapy discontinuation, or censoring. The exclusion assessment window includes events like transfer to a different hospital or diagnosis of other cancers. BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LH-RH, luteinizing hormone-releasing hormone; PgR, progesterone receptor. aEarliest outcome of interest: discontinuation of abemaciclib therapy, end of the study period
Measures
We retrospectively collected from electronic health records (EHR) the following variables: age, sex, height, weight, body mass index (BMI), HER2, estrogen receptor (ER), progesterone receptor (PgR), bone metastases, status, number of previous chemotherapy regimens, abemaciclib starting dose, hormonal agents used in combination, combination with luteinizing hormone-releasing hormone (LH-RH) agonists, combination with radiation, prescription of supportive medicine, and baseline levels of albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen, serum creatinine, white blood cell count, red blood cell count, hemoglobin, platelet count, and neutrophil count.
Outcome
The outcome variables included the duration from the initiation of abemaciclib therapy to discontinuation due to any reason, and the time to temporary suspension or dose reduction due to adverse events. The distinction between discontinuation due to any reason and temporary suspension or dose reduction due to adverse events was based on physician records and prescription history from the EHR. In cases where it was noted as temporary suspension, the EHR for the next consultation date was repeatedly checked. The follow-up period for abemaciclib discontinuation was defined as the time from the initiation of abemaciclib therapy until permanent discontinuation of the therapy or the last clinical follow-up. Therapy discontinuation was considered a permanent event, after which follow-up ceased. In contrast, for abemaciclib temporary suspension or dose reduction, the follow-up period was defined as the time from the initiation of therapy until the first occurrence of temporary suspension or dose reduction due to adverse events. If therapy was resumed after a temporary suspension, follow-up was not resumed, as the temporary suspension itself was treated as the event of interest. The assessment of HER2 status followed the the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines [16]. The threshold for ER and PgR positivity was set at 1%, in line with the St. Gallen International Consensus Guidelines for the Treatment of Early Breast Cancer 2021 [17] and the ASCO/CAP guidelines [18].
Statistical analysis
Numerical variables were analyzed using the median as the cut-off value. In the time-to-event analysis, we used the Kaplan–Meier method to estimate survival curves. The log-rank test was applied to compare survival curves between groups. Variables for the multivariate Cox regression analysis were selected using the stepwise method based on the Akaike information criterion (AIC), considering explanatory variables with a univariate Cox regression p-value < 0.5. To account for multicollinearity, weight and BMI, as well as red blood cell count and hemoglobin, were not both included, and only the variable with the lower p-value was incorporated into the stepwise method. To verify the proportional hazards assumption of our Cox proportional hazards model, tests were conducted based on the Schoenfeld residuals. To assess multicollinearity among the predictors, the variance inflation factor (VIF) was calculated for each variable, with VIF values above 10 indicating significant multicollinearity.
For sensitivity analysis, 10,000 bootstrap resampling iterations were performed to estimate the hazard ratio (HR) [19]. In addition, the influence of individual observations on the HRs was assessed using the HRs obtained from subsets resampled using the jackknife method [20]. Next, we conduct a Cox regression analysis with elastic net regularization, which addresses variable selection and multicollinearity by combining the characteristics of ridge and lasso regressions [21]. To evaluate the impact of different regularization parameters, we performed a tenfold cross-validation. We varied the mixing parameter α from 0 to 1 to balance regularization strength and variable selection, determining the optimal α value by minimizing the cross-validation error. Ridge regression occurs when α = 0, and lasso regression occurs when α = 1. Furthermore, we chose the regularization parameter λ that minimized the cross-validation error for model selection. The coefficients of the final model were estimated based on the selected α and λ. In addition to the main analysis, we performed a supplementary analysis to explore the predictors of abemaciclib discontinuation due to progressive disease and adverse events as separate outcomes. Through these analyses, we evaluated the robustness of the main results. All reported p-values were two-sided, with a significance level of p< 0.05. Statistical analyses were performed using the R version 4.2.2 (R Development Core Team, Vienna, Austria) [22]. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [23].
Results
Study population
Of the 147 patients who met the inclusion criteria, 137 patients were analyzed after excluding 7 patients who did not undergo blood tests within 2 weeks before starting abemaciclib therapy, 2 patients who were transferred after treatment initiation, and 1 patient in whom abemaciclib therapy was discontinued due to the diagnosis of another cancer (Figure S1).
Background of the patients
Table 1 presents patient characteristics. The median (interquartile range) age of the 137 patients was 61 years (50–72 years). Only patients who are HER2-negative (2 + / fluorescence in situ hybridization (FISH)- or below) and hormone receptor-positive (ER or PgR positive) are eligible for abemaciclib therapy. Among the patients, 23 (17.4%), 65 (49.2%), 44 (33.3%), and five (3.6%) had HER2 2 + /FISH-, HER2 1 + , HER2 0, and an unknown HER2 status, respectively. Additionally, 124 (90.5%) patients were ER-positive, and 109 (79.6%) were PgR positive. There were 46 (33.6%) postoperative patients and 91 (66.4%) patients with recurrent disease progression, of whom 66 (48.2%) showed bone metastases. Hormone therapy is the first-line treatment for patients who are hormone receptor-positive. Of 137 patients, 86 (63.2%) had no history of chemotherapy. Among the 137 patients, 41 (29.9%) started treatment with a reduced initial dose, based on the physician’s judgment. In addition, 28 patients (20.4%) underwent radiotherapy at some point after the initiation of abemaciclib therapy. Abemaciclib often causes a high incidence of diarrhea, and physicians may prescribe probiotics or antidiarrheals at the beginning of the treatment. In this study, probiotics were prescribed to 78 patients (56.9%), and 123 patients (89.8%) received loperamide or other antidiarrheals. All other variables had complete data, except for HER2, ER, and PgR statuses, which are explicitly listed as ‘Unknown’ in Table 1 where applicable.
Table 1.
Clinical and demographic characteristics of patients
| Characteristics | Statistics | Overall |
|---|---|---|
| Patients | n (%) | 137 (100) |
| Age (years) | median (IQR) | 61 (50–72) |
| Sex | ||
| Female | n (%) | 136 (99.3) |
| Male | n (%) | 1 (0.7) |
| Height (m) | median (IQR) | 1.56 (1.50–1.60) |
| Weight (kg) | median (IQR) | 54 (49–60) |
| Body mass index (kg/m2) | median (IQR) | 22.1 (20.0–24.9) |
| HER2a | ||
| 0 | n (%) | 44 (33.3) |
| 1 + | n (%) | 65 (49.2) |
| 2 + FISH- | n (%) | 23 (17.4) |
| Unknown | n (%) | 5 (3.6) |
| ERa | ||
| Positive | n (%) | 124 (90.5) |
| Negative | n (%) | 1 (0.7) |
| Unknown | n (%) | 12 (8.8) |
| PgRa | ||
| Positive | n (%) | 109 (79.6) |
| Negative | n (%) | 16 (11.7) |
| Unknown | n (%) | 12 (8.8) |
| Bone metastasis | n (%) | 66 (48.2) |
| Status | ||
| Postoperative | n (%) | 46 (33.6) |
| Recurrent progression | n (%) | 91 (66.4) |
| Number of previous chemotherapy regimens | ||
| 0 | n (%) | 86 (63.2) |
| 1 | n (%) | 27 (19.1) |
| 2 | n (%) | 19 (14.0) |
| 3 | n (%) | 4 (2.9) |
| 4 | n (%) | 1 (0.7) |
| Abemaciclib starting dose (mg) | ||
| 300 | n (%) | 96 (70.1) |
| 200 | n (%) | 35 (25.5) |
| 100 | n (%) | 6 (4.4) |
| Hormonal agents used in combination | ||
| Antiestrogen (FUL, TAM) | n (%) | 72 (52.6) |
| FUL | n (%) | 65 (47.4) |
| TAM | n (%) | 7 (5.1) |
| Aromatase inhibitor (LET, ANA) | n (%) | 65 (47.4) |
| LET | n (%) | 53 (38.8) |
| ANA | n (%) | 12 (8.7) |
| Combination with LH-RH agonists (LEU, GOS) | n (%) | 16 (11.7) |
| LEU | n (%) | 15 (10.9) |
| GOS | n (%) | 1 (0.8) |
| Combination with radiation | n (%) | 28 (20.4) |
| Prescription of supportive medicine | ||
| Probiotics | n (%) | 78 (56.9) |
| Antidiarrheal | n (%) | 123 (89.8) |
| Clinical laboratory values | ||
| Alanine aminotransferase (U/L) | median (IQR) | 16 (12–23) |
| Albumin (g/dL) | median (IQR) | 4.1 (3.7–4.3) |
| Aspartate aminotransferase (U/L) | median (IQR) | 23 (18–28) |
| Blood urea nitrogen (mg/dL) | median (IQR) | 13 (11–16) |
| Hemoglobin (g/dL) | median (IQR) | 12.4 (11.7–13.3) |
| Neutrophil (/μL) | median (IQR) | 2,735 (1,953–4,432) |
| Platelet (104/μL) | median (IQR) | 220 (168–259) |
| Red blood cell (103/μL) | median (IQR) | 4,000 (3,725–4,340) |
| Serum creatinine (mg/dL) | median (IQR) | 0.65 (0.56–0.76) |
| Total bilirubin (mg/dL) | median (IQR) | 0.5 (0.4–0.6) |
| White blood cell (/μL) | median (IQR) | 4,900 (3,755–6,340) |
ANA anastrozole, ER estrogen receptor, FUL fulvestrant, GOS goserelin, HER2 human epidermal growth factor receptor 2, IQR interquartile range, LET letrozole, LEU leuprorelin, LH-RH luteinizing hormone-releasing hormone, PgR progesteron receptor, TAM tamoxifen
aER/PgR/HER2 evaluation was performed at the time of diagnosis
Predictors of abemaciclib discontinuation
Of the 137 patients, 71 discontinued of abemaciclib during the follow-up period. Reasons for abemaciclib discontinuation were adverse events and progressive disease in 36 (26.3%) and 35 (25.5%) patients, respectively. Adverse events leading to abemaciclib discontinuation were most frequently Grade 2 (21 patients, 58.3%) and Grade 3 (14 patients, 38.9%). The adverse events included increased liver enzymes in 7 patients (19.4%); gastrointestinal symptoms such as nausea, vomiting, and anorexia in 6 patients (16.7%); diarrhea, fatigue, and lung infection in 5 patients (13.9%); and others in 11 patients (30.6%). Some patients experienced multiple adverse events simultaneously, which led to their abemaciclib discontinuation (Table S1). Figure 2a shows the Kaplan–Meier curves for the duration of abemaciclib therapy dropout, with a median survival time (MST) of 386 days. Multivariate Cox regression analysis using explanatory variables obtained through the stepwise method based on the univariate Cox regression results showed that a baseline weight < 54 kg, bone metastases, and hemoglobin levels < 12.4 g/dL were independent predictors of abemaciclib discontinuation (Table 2).
Fig. 2.
Kaplan–Meier curve for time to (a) abemaciclib discontinuation due to any reason and (b) abemaciclib temporary suspension or dose reduction due to adverse events
Table 2.
Cox regression analysis for independent predictors of abemaciclib discontinuation due to any reason
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95%CI | p-value | Hazard ratio | 95%CI | p-value | VIF |
| Age (≥ 61 years) | 1.273 | 0.798–2.031 | 0.311 | ||||
| Height (< 1.56 m) | 1.188 | 0.748–1.886 | 0.466 | ||||
| Weight (< 54 kg) | 2.021 | 1.262–3.238 | 0.003* | 1.962 | 1.216–3.167 | 0.006* | 1.027 |
| Body mass index (< 22.06 kg/m2) | 1.701 | 1.065–2.716 | 0.026* | ||||
| Bone metastasis | 1.986 | 1.226–3.217 | 0.005* | 1.708 | 1.048–2.784 | 0.032* | 1.019 |
| Status (Postoperative) | 0.563 | 0.322–0.986 | 0.044* | ||||
| Number of previous chemotherapy regimens (≥ 1) | 1.010 | 0.617–1.654 | 0.967 | ||||
| Starting dose down (< 300 mg) | 0.996 | 0.603–1.646 | 0.988 | ||||
| Hormonal agents used in combination (antiestrogen) | 1.294 | 0.807–2.073 | 0.285 | ||||
| Combination with LH-RH agonists (LEU, GOS) | 1.012 | 0.503–2.036 | 0.974 | ||||
| Combination with radiation | 0.784 | 0.410–1.502 | 0.464 | ||||
| Prescription of supportive medicine | |||||||
| Probiotics | 1.294 | 0.808–2.072 | 0.284 | ||||
| Antidiarrheal | 1.171 | 0.536–2.559 | 0.692 | ||||
| Clinical laboratory values | |||||||
| Alanine aminotransferase (≥ 16 U/L) | 1.069 | 0.667–1.713 | 0.782 | ||||
| Albumin (< 4.1 g/dL) | 1.198 | 0.750–1.915 | 0.449 | ||||
| Aspartate aminotransferase (≥ 23 U/L) | 0.875 | 0.549–1.395 | 0.574 | ||||
| Blood urea nitrogen (≥ 13 mg/dL) | 1.184 | 0.745–1.882 | 0.474 | ||||
| Hemoglobin (≤ 12.4 g/dL) | 1.704 | 1.056–2.751 | 0.029* | 1.848 | 1.129–3.024 | 0.028* | 1.010 |
| Neutrophil (< 2,735 /μL) | 0.911 | 0.573–1.447 | 0.692 | ||||
| Platelet (< 220 × 104 /μL) | 1.438 | 0.903–2.292 | 0.126 | 1.467 | 0.912–2.358 | 0.114 | 1.030 |
| Red blood cell (< 4,000x103 /μL) | 1.555 | 0.975–2.479 | 0.064 | ||||
| Serum creatinine (≥ 0.65 mg/dL) | 1.163 | 0.727–1.858 | 0.529 | ||||
| Total bilirubin (≥ 0.5 mg/dL) | 0.988 | 0.616–1.587 | 0.962 | ||||
| White blood cell (< 4,900 /μL) | 1.024 | 0.645–1.627 | 0.919 | ||||
CI Confidence interval, GOS Goserelin, LEU Leuprorelin, LH-RH Luteinizing hormone-releasing hormone, VIF Variance inflation factor
*p < 0.05
Predictors of abemaciclib temporary suspension or dose reduction due to adverse events
Among the 137 patients, 86 experienced temporary suspension or dose reduction because of adverse events during the follow-up period. The adverse events leading to temporary suspension or dose reduction of abemaciclib therapy were most frequent in Grades 3 (50 patients, 58.1%) and 2 (42 patients, 48.8%). The adverse events included neutropenia in 41 patients (47.7%), diarrhea in 30 patients (34.9%), increased liver enzymes in 5 patients (5.8%), fatigue in 4 patients (4.7%), and others in 18 patients (20.9%). Some patients experienced multiple adverse events simultaneously, which led to a temporary suspension or dose reduction (Table S2). Figure 2b shows the Kaplan–Meier curve for the duration of temporary suspension or dose reduction owing to adverse events, with an MST of 45 days. Multivariate Cox regression analysis, using explanatory variables obtained through the stepwise method based on the univariate Cox regression results, showed that a baseline weight of < 54 kg was an independent predictor of temporary suspension or dose reduction due to adverse events in abemaciclib therapy (Table 3).
Table 3.
Cox regression analysis for independent predictors of abemaciclib temporary suspension or dose reduction due to adverse events
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95%CI | p-value | Hazard ratio | 95%CI | p-value | VIF |
| Age (≥ 61 years) | 0.929 | 0.612–1.410 | 0.730 | ||||
| Height (< 1.56 m) | 1.057 | 0.697–1.602 | 0.795 | ||||
| Weight (< 54 kg) | 1.941 | 1.271–2.963 | 0.002* | 2.022 | 1.323–3.091 | 0.010* | 1.022 |
| Body mass index (< 22.06 kg/m2) | 1.064 | 0.702–1.614 | 0.769 | ||||
| Bone metastasis | 1.050 | 0.692–1.592 | 0.820 | ||||
| Status (Postoperative) | 0.980 | 0.626–1.533 | 0.929 | ||||
| Number of previous chemotherapy regimens (≥ 1) | 0.897 | 0.579–1.391 | 0.628 | ||||
| Starting dose down (< 300 mg) | 0.784 | 0.490–1.254 | 0.310 | ||||
| Hormonal agents used in combination (antiestrogen) | 1.285 | 0.844–1.958 | 0.242 | ||||
| Combination with LH-RH agonists (LEU, GOS) | 0.884 | 0.443–1.764 | 0.726 | ||||
| Combination with radiation | 0.868 | 0.516–1.459 | 0.593 | ||||
| Prescription of supportive medicine | |||||||
| Probiotics | 1.154 | 0.755–1.762 | 0.508 | ||||
| Antidiarrheal | 1.736 | 0.802–3.760 | 0.162 | 1.954 | 0.900–4.242 | 0.091 | 1.022 |
| Clinical laboratory values | |||||||
| Alanine aminotransferase (≥ 16 U/L) | 1.151 | 0.758–1.750 | 0.509 | ||||
| Albumin (< 4.1 g/dL) | 1.213 | 0.799–1.842 | 0.364 | ||||
| Aspartate aminotransferase (≥ 23 U/L) | 1.154 | 0.759–1.754 | 0.504 | ||||
| Blood urea nitrogen (> 13 mg/dL) | 0.875 | 0.577–1.327 | 0.530 | ||||
| Hemoglobin (< 12.4 g/dL) | 1.055 | 0.662–1.528 | 0.980 | ||||
| Neutrophil (< 2,735 /μL) | 0.996 | 0.657–1.510 | 0.985 | ||||
| Platelet (< 220 × 104 /μL) | 1.337 | 0.880–2.032 | 0.174 | ||||
| Red blood cell (< 4,000 × 103 /μL) | 1.410 | 0.929–2.140 | 0.107 | ||||
| Serum creatinine (≥ 0.65 mg/dL) | 1.130 | 0.741–1.722 | 0.570 | ||||
| Total bilirubin (≥ 0.5 mg/dL) | 1.356 | 0.878–2.092 | 0.169 | ||||
| White blood cell (< 4,900 /μL) | 0.977 | 0.644–1.481 | 0.911 | ||||
*p<0.05
Sensitivity analysis
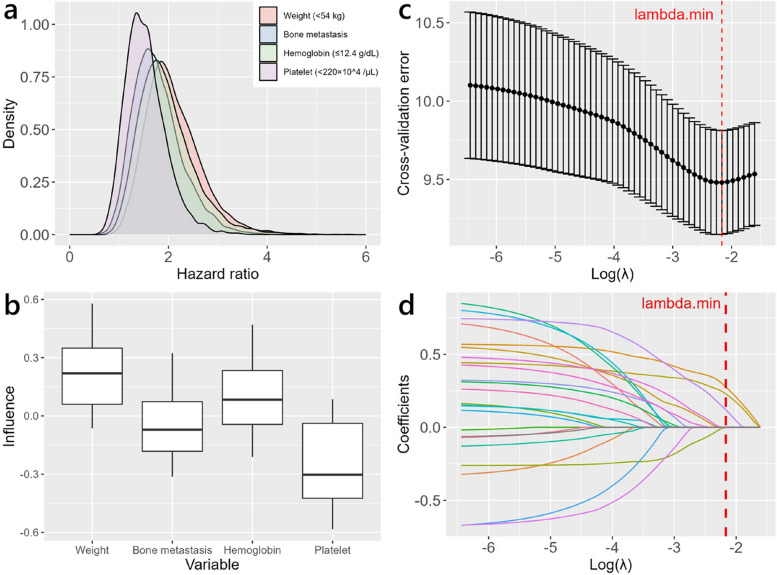
In the multivariate Cox regression analysis shown in Table 2, which considered abemaciclib discontinuation as an outcome, the distribution of 10,000 HRs for each explanatory variable was estimated using the bootstrap method. The median HR and 95% confidence interval (CI) were 2.015 (1.226–3.433) for weight (< 54 kg), 1.719 (1.018–2.953) for bone metastases, and 1.873 (1.119–3.352) for hemoglobin (≤ 12.4 g/dL) (Table S3 and Fig. 3a). Next, using the jackknife method, we estimated the distribution of HRs for each explanatory variable and investigated the influence of each observation on the individual HRs. The influence of individual observations on the HRs for weight was greater than that of the other independent predictors (Table S4 and Fig. 3b). Furthermore, we built a Cox regression model using elastic net regularization and through the optimized model, identified weight, bone metastasis, and hemoglobin level as significant predictors (Figs. 3c and d, Tables S5 and S6).
Fig. 3.
Sensitivity analysis for predictors of abemaciclib discontinuationdue to any reason. a Density plot of hazard ratios from 10,000 bootstrap samples. b Influence plot for hazard ratios from subsets generated by jackknife resampling. Box plots show the median, third quartile, first quartile, and range of data points outside the quartile range, but excluding outliers. c Cross-validation error as a function of λ. Error bars indicate the standard error of the cross-validation error at each λ value. The hyperparameter α, a measure of the mixture between ridge regression and lasso regression, was set to 0.9 based on the tenfold cross-validation error. The vertical dashed red line corresponds to the value of regularization parameter λ that minimizes cross-validation error. d Regularization path for elastic net-regularized Cox regression
Similarly, for the multivariate Cox regression analysis shown in Table 3, with the outcome being temporary suspension or dose reduction of abemaciclib, the median HR and 95% CI obtained using the bootstrap method for weights (< 54 kg) were 2.035 (1.332–3.165) (Table S7 and Fig. 4a). Using the jackknife method, we estimated the distribution of the HRs for each explanatory variable and investigated the influence of individual observations on the HRs for weight (< 54 kg). The influence of individual observations on the HRs for weight was smaller than that when abemaciclib discontinuation was considered the outcome (Table S8 and Fig. 4b). Furthermore, the results from the Cox regression model using elastic net regularization identified weight as an important predictor. The sensitivity analyses were consistent with the main analysis and further supported the reliability of our conclusions (Figs. 4c and d, Tables S9 and S10).
Fig. 4.
Sensitivity analysis for predictors of abemaciclib temporary suspension or dose reduction due to adverse events. a Density plot of hazard ratios from 10,000 bootstrap samples. b Influence plot for hazard ratios from subsets generated by jackknife resampling. Box plots show the median, third quartile, first quartile, and range of data points outside the quartile range, but excluding outliers. c Cross-validation error as a function of λ. Error bars indicate the standard error of the cross-validation error at each λ value. The hyperparameter α, a measure of the mixture between ridge regression and lasso regression, was set to 0.7 based on the tenfold cross-validation error. The vertical dashed red line corresponds to the value of regularization parameter λ that minimizes cross-validation error. d Regularization path for elastic net-regularized Cox regression
Supplementary analysis
The Cox regression analysis shown in Table 2 presents the predictors of abemaciclib discontinuation for any reason. Of the 137 patients, 35 (25.5%) discontinued treatment due to progressive disease, and 36 (26.3%) discontinued treatment due to adverse events. We therefore performed a separate analysis for abemaciclib discontinuation based on these two reasons. Multivariate Cox regression analysis, using explanatory variables obtained through stepwise selection from the results of univariate Cox regression analysis, showed that baseline body weight less than 54 kg, bone metastasis, ALT levels ≥ 16 U/L, and hemoglobin levels < 12.4 g/dL were independent predictors of abemaciclib discontinuation due to progressive disease (Table S11).
In addition, multivariate Cox regression analysis for discontinuation due to adverse events identified baseline age ≥ 61 years, body weight < 54 kg, hemoglobin levels < 12.4 g/dL, neutrophil count ≥ 2,735 /μL, and platelet count ≤ 220×104 /μL as independent predictors of discontinuation due to adverse events during abemaciclib therapy (Table S12).
The results of the Kaplan–Meier analysis are presented in Figure S2. The MST for abemaciclib discontinuation due to any reason was longer in the postoperative group (537 days) compared to the recurrent progression group (274 days) (log-rank test, p = 0.041). The primary reasons for abemaciclib discontinuation in the postoperative group were progressive disease in 10.9% and adverse events in 23.9% of patients, while in the recurrent progression group, progressive disease accounted for 33.0% and adverse events for 27.5% of patients (Table S13). No significant difference in MST for temporary suspension or dose reduction was observed between the postoperative and recurrent progression groups (log-rank test, p = 0.903), with MST values of 43.5 days and 64 days, respectively (Table S13).
Of the 137 patients, nearly 30% (29.9%) started abemaciclib treatment with a reduced initial dose based on physician judgment. To explore the reasons behind this dose reduction, we analyzed the baseline clinical and demographic characteristics of patients in both the standard dose and reduced dose groups. Statistically significant differences were observed between the groups in several variables, including age, height, probiotics, antidiarrheal, and clinical laboratory values such as AST, blood urea nitrogen, neutrophil, platelet, and white blood cell counts (Table S14). These characteristics may have influenced the decision to reduce the starting dose and could potentially impact the treatment outcomes, including adherence and completion.
Discussion
This study, which focused on patients with breast cancer undergoing abemaciclib therapy, identified baseline weight below 54 kg, hemoglobin levels of 12.4 g/dL or less, and bone metastasis as predictors of abemaciclib discontinuation. Additionally, a baseline weight < 54 kg was a predictor of temporary suspension or dose reduction due to adverse events. These findings were corroborated by multiple sensitivity analyses.
According to a previous study in patients with hormone receptor-positive and HER2-negative inoperable or recurrent breast cancer treated with abemaciclib, the risk of Grade 3 or higher neutropenia is significantly lower in overweight or obese patients compared to underweight or normal-weight patients [24]. As the package insert recommends temporary suspension of abemaciclib in case of Grade 3 or higher neutropenia, this findings align with our observation that low body weight is a predictor of abemaciclib temporary suspension or dose reduction due to adverse events. While this may suggest a protective effect in terms of neutropenia, it does not necessarily imply a lower overall risk of abemaciclib discontinuation. In fact, contradictory results have been reported for other CDK4/6 inhibitors like palbociclib, where some studies show a higher incidence of hematologic toxicities in patients with lower BMI [25], while others suggest a higher BMI is associated with increased neutropenia [26]. On the other hand, it is important to note that adverse events other than neutropenia, such as gastrointestinal issues or liver enzyme elevation, could also lead to temporary suspension. In our study, we found that the impact of individual weight observations on HR was smaller when temporary suspension or dose reduction was the outcome compared to discontinuation. This suggests that while low body weight is a predictor of adverse events, the correlation between weight and abemaciclib discontinuation might be influenced by a broader range of factors. Furthermore, although abemaciclib dosing is standardized regardless of body weight or height, it is possible that differences in blood concentration may exist between smaller patients and those with standard body sizes, potentially contributing to adverse event occurrence. However, there is no pharmacokinetic or pharmacodynamic data to confirm this, and further research is required to clarify these relationships.
Reports suggest that lower baseline hemoglobin levels may reduce the efficacy of chemotherapy in patients with breast cancer by limiting tumor oxygenation and thus impairing antiproliferative activity [27]. While this effect has been demonstrated in the context of chemotherapy, it is unclear whether the same mechanism applies to targeted therapies like abemaciclib. In our study,, low baseline hemoglobin levels were identified as a predictor of abemaciclib discontinuation. This suggests that patients with lower hemoglobin levels may be more vulnerable to disease progression or adverse events, potentially leading to abemaciclib discontinuation. Furthermore, baseline hemoglobin levels have been reported as prognostic biomarkers for clinical outcomes across various cancers [28]. For instance, a low hemoglobin, albumin, lymphocyte, and platelet (HALP) score, calculated as [hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)]/platelets (/L), has been associated with shorter overall survival in multiple cancer types. However, while these associations are based on clinical data, no direct pharmacokinetic or pharmacodynamic evidence linking hemoglobin levels to abemaciclib treatment outcomes is currently available. Therefore, further research is needed to explore the underlying mechanisms and to establish whether hemoglobin levels directly influence the efficacy or tolerability of abemaciclib therapy.
In our study, 36 of 137 patients with breast cancer who underwent abemaciclib therapy discontinued treatment due to adverse events. The most common adverse events leading to abemaciclib discontinuation were increased liver enzymes in 7 patients (19.4%), gastrointestinal symptoms such as nausea, vomiting, and anorexia in 6 patients (16.7%), and diarrhea in 5 patients (13.9%). In the MONARCH2 trial, 70 of 441 patients (15.9%) discontinued treatment due to adverse events [14]. Among these, drug-induced liver injury was observed in 2 patients (2.9%), and ALT and AST elevations were observed in 1 patient each (1.4%), which was less frequent than in our study [29]. This could partially explain the higher rate of increased liver enzyme observed in our study, as approximately 30% of patients in the MONARCH2 trial were Asian [14]. However, it is important to note that the MONARCH 2 trial did not specifically investigate ethnic differences in the risk of liver toxicity. Further research is required to explore this potential relationship.
Our analysis reveals two distinct sets of predictors for abemaciclib discontinuation, depending on the event type. Patients who discontinued due to progressive disease were generally characterized by more advanced cancer or compromised baseline health, including factors like bone metastasis and lower hemoglobin levels, which are indicative of more aggressive disease states. In contrast, discontinuation due to adverse events was predominantly associated with older age and abnormalities in blood cell lineages: low hemoglobin and low platelet count. These hematologic vulnerabilities suggest an increased susceptibility to treatment-related toxicity in these patients. The distinct predictors for progressive disease and adverse events indicate that personalized management strategies may be necessary, with enhanced monitoring for both disease progression and treatment-related side effects in vulnerable patient subgroups.
Among the 137 patients who received abemaciclib therapy, 86 experienced temporary suspension or dose reduction due to adverse events during the follow-up period. Adverse events play a significant role in abemaciclib discontinuation. Therefore, the present study investigated the predictors of adverse events leading to temporary suspension or dose reduction of abemaciclib therapy. These findings indicate that a low baseline body weight is a predictor. The most common adverse events that resulted in temporary suspension or dose reduction were neutropenia (41 patients, 47.7%), diarrhea (30 patients, 34.9%), and increased liver enzyme levels (5 patients, 5.8%). The MST for temporary suspension or dose reduction owing to adverse events was 45 days. Therefore, patients weighing less than 54 kg have a higher risk of adverse events, leading to temporary suspension or dose reduction. More stringent monitoring of adverse events and more frequent visits should be considered for at least the first 45 days of treatment.
This study had several limitations. First, there are unmeasured confounding factors. Since this was a retrospective observational study, we could not adjust for confounding factors, such as smoking and drinking habits or medication adherence. Second, although this was a multicenter collaborative study, the sample size was relatively small. The eligible population for abemaciclib therapy was limited to patients with hormone receptor-positive and HER2-negative breast cancer; therefore, the number of users was low. As a result, the number of variables that could be analyzed in the multivariate analysis was limited. Third, this study only included Japanese patients. As mentioned previously, racial differences may influence the risk of adverse events [29]. As explored using the jackknife method, the influence of individual observations on the HR for weight when abemaciclib discontinuation was relatively large. Analyzing populations with different demographic characteristics could result in different HRs. In the future, we believe that it will be necessary to conduct more detailed investigations considering the medical history, genetic mutations, and racial differences by accumulating more cases.
In conclusion, low baseline body weight, bone metastasis, and low hemoglobin levels may serve as markers for abemaciclib discontinuation. Additionally, a low baseline body weight may be a marker of adverse events that lead to temporary suspension or dose reduction of abemaciclib therapy. Therefore, patients with low body weight are more susceptible to adverse events during abemaciclib treatment, increasing their risk of discontination and potentially limiting their ability to fully benefit from the therapy. Since more than half of these adverse events occur within the first 45 days of treatment, patients with low body weight should be closely monitored for adverse event for at least 45 days after starting abemaciclib. Previous studies have reported that the MONARCH2 and 3 trials demonstrated progression free survival with abemaciclib, regardless of dose reduction or the early onset of diarrhea and neutropenia [30]. Regular symptom assessments for patients receiving the CDK4/6 inhibitor palbociclib in combination with endocrine therapy have also been shown to improve quality of life [31]. Based on these findings, it is considered preferable to continue abemaciclib therapy, even with early dose reduction if adverse events are suspected, rather than risking abemaciclib discontinuation or prolonged treatment suspension due to worsening adverse events. Therefore, identifying patients at high risk for adverse events and increasing the frequency of adverse event monitoring and medical visits for these patients is crucial for the early detection and appropriate management of adverse events. However, these results alone do not recommend a temporary suspension or dose reduction to continue abemaciclib therapy. Further research is required to understand the effect of dose reduction on the efficacy of abemaciclib therapy in low-weight patients.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AIC
Akaike Information Criterion
- ALT
Alanine aminotransferase
- ASCO
American Society of Clinical Oncology
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CAP
College of American Pathologists
- CDKs
Cyclin-dependent kinases
- CI
Confidence interval
- EHR
Electronic health records
- ER
Estrogen receptor
- FISH
Fluorescence in situ hybridization
- HALP
Hemoglobin albumin lymphocyte platelet
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- IDFS
Invasive disease-free survival
- LH-RH
Luteinizing hormone-releasing hormone
- MST
Median survival time
- NSAI
Nonsteroidal aromatase inhibitors
- PgR
Progesterone receptor
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- VIF
Variance inflation factor
Authors’ contributions
Noriaki Kataoka: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing—original draft. Takeo Hata: Methodology, Software. Validation, Formal analysis, Investigation, Writing—original draft, Writing—review and editing, and Supervision. Kouichi Hosomi: Methodology, Validation, Investigation, Writing—review and editing. Atsushi Hirata: Methodology, Validation, Investigation, Data curation, Writing—reviewing and editing. Ryosuke Ota: Methodology, Validation, Investigation, Data curation, Writing—review and editing. Masami Nishihara: Writing—review and editing. Kosei Kimura: Resources, Writing—review and editing. Mitsuhiko Iwamoto: Resources, Writing—review and editing. Akira Ashida: Writing—review and editing. Masashi Neo: Writing—review and editing, Project administration.
Funding
No source of funding was used to conduct this study.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Osaka Medical and Pharmaceutical University (Approval ID:2023–127). As this was a retrospective observational study without intervention or invasion, using only existing electronic health record information, the requirement for patient consent was waived.
Consent of publication
Not applicable.
Competing of interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Chan PS, Lok V, et al. Global incidence and mortality of breast cancer: a trend analysis. Aging. 2021;13(4):5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen RN, Mellemkjær L, Ejlertsen B, et al. Mortality after late breast cancer recurrence in Denmark. J Clin Oncol. 2022;40(13):1450. [DOI] [PubMed] [Google Scholar]
- 4.Sambi M, Qorri B, Harless W, Szewczuk MR. Therapeutic options for metastatic breast cancer. Adv Exp Med Biol. 2019;1152:131. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417. [DOI] [PubMed] [Google Scholar]
- 6.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig New Drugs. 2014;32(5):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Guzmán R, Calsina B, Hermoso A, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017;8(41):69493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braal CL, Jongbloed EM, Wilting SM, et al. Inhibiting CDK4 / 6 in breast cancer with Palbociclib, Ribociclib, and Abemaciclib: similarities and differences. Drugs. 2021;81(3):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold A, Papanikolaou A. Cyclin D1 in Breast Cancer Pathogenesis. J Clin Oncol. 2005;23(18):4215–24. 10.1200/JCO.2005.05.064. [DOI] [PubMed]
- 10.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol. 2020;6(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sledge GW Jr, Toi M, Neven P, et al. MONARCH2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875. [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss S, Rassen JA, Brown JS, et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med. 2019;170(6):398. [DOI] [PubMed] [Google Scholar]
- 16.Wolff AC, Somer MR, Dowsett M, et al. Human epidermal growth factor receptor 2 testing in breast cancer : ASCO – College of American Pathologists Guideline Update. J Clin Oncol. 2023;41(22):3867. [DOI] [PubMed] [Google Scholar]
- 17.Burstein HJ, Curigliano G, Thurlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer. Ann Oncol. 2021;32(10):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer : ASCO / CAP Guideline Update. J Clin Oncol. 2020;38(12):1346. [DOI] [PubMed] [Google Scholar]
- 19.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1976;7(1):1. [Google Scholar]
- 20.Miller RG. The JackKnife a review. Biometrika. 1974;61(1):1. [Google Scholar]
- 21.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Statist Soc B. 2005;67:301–20. [Google Scholar]
- 22.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2024. [Google Scholar]
- 23.von Elm EV, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzoi MA, Eiger D, Ameye L, et al. Clinical implications of body mass index in metastatic breast cancer patients treated with abemaciclib and endocrine therapy. J Natl Cancer Inst. 2021;113(4):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncato R, Peruzzi E, Gerratana L, et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed Pharmacother. 2023;164: 114906. [DOI] [PubMed] [Google Scholar]
- 26.Kanbayashi Y, Sakaguchi K, Ishikawa T, et al. Predictors for development of palbociclib - induced neutropenia in breast cancer patients as determined by ordered logistic regression analysis. Sci Rep. 2021;11(1):20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottini A, Berruti A, Brizzi MP, et al. Pretreatment haemoglobin levels significantly predict the tumour response to primary chemotherapy in human breast cancer. Br J Cancer. 2003;89(6):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farag CM, Antar R, Akosman S, et al. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget. 2023;14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue K, Masuda N, Iwata H, et al. Japanese subpopulation analysis of MONARCH 2: phase 3 study of abemaciclib plus fulvestrant for treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer that progressed on endocrine therapy. Breast Cancer. 2021;28(5):1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rugo HS, Huober J, Garcia-Saenz JA, et al. Management of abmaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26:e53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harbeck N, Fasching PA, Wuerstlein R, et al. Significantly longer time to deterioration of quality of life due to CANKADO PRO-React eHealth support in HRD HER2L metastatic breast cancer patients receiving palbociclib and endocrine therapy: primary outcome analysis of the multicenter randomized. Ann Oncol. 2023;34(8):660–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.