Abstract
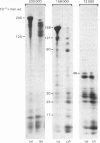
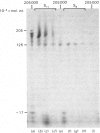
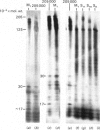
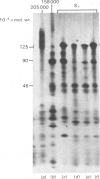
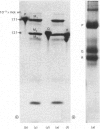
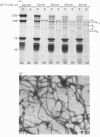
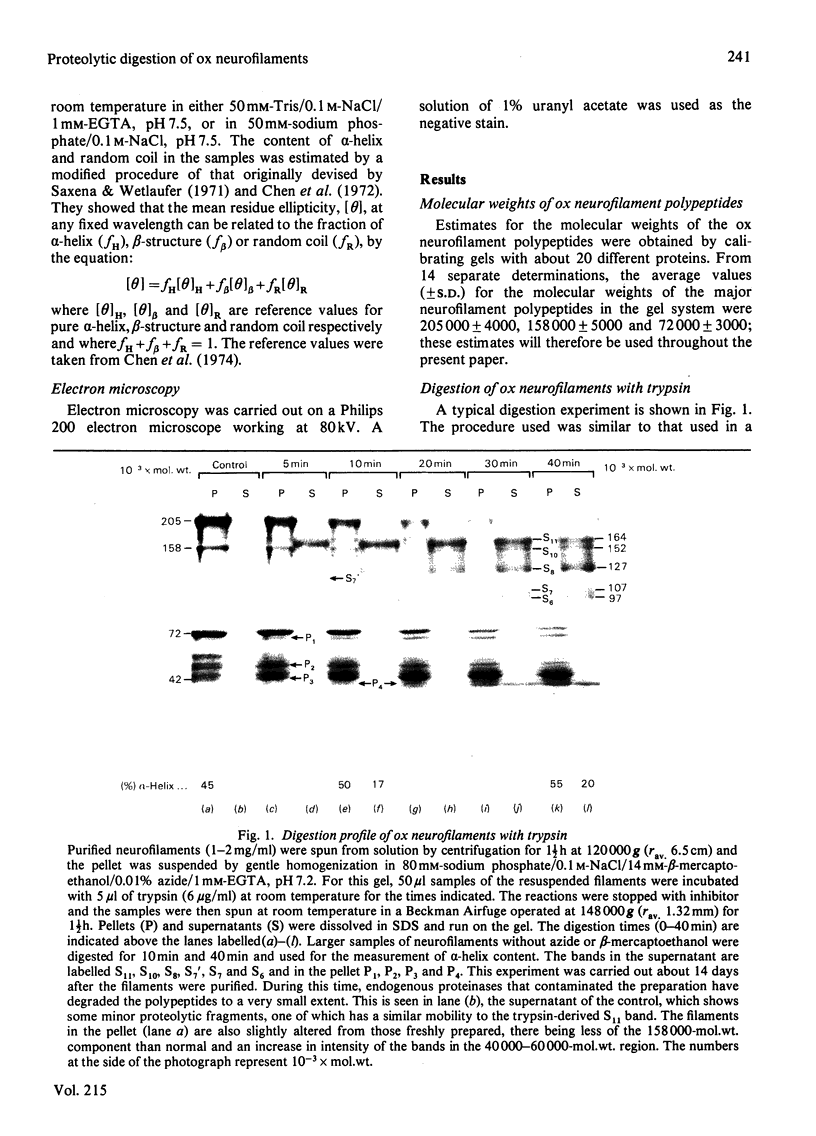
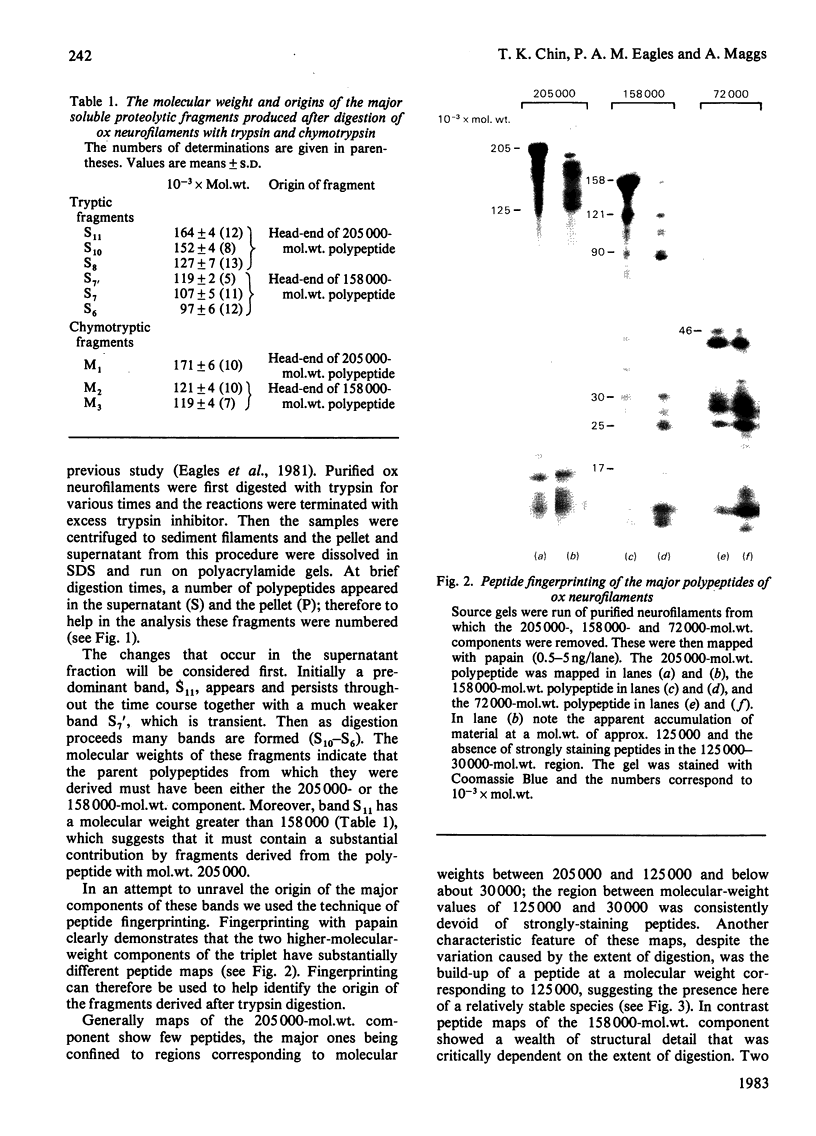
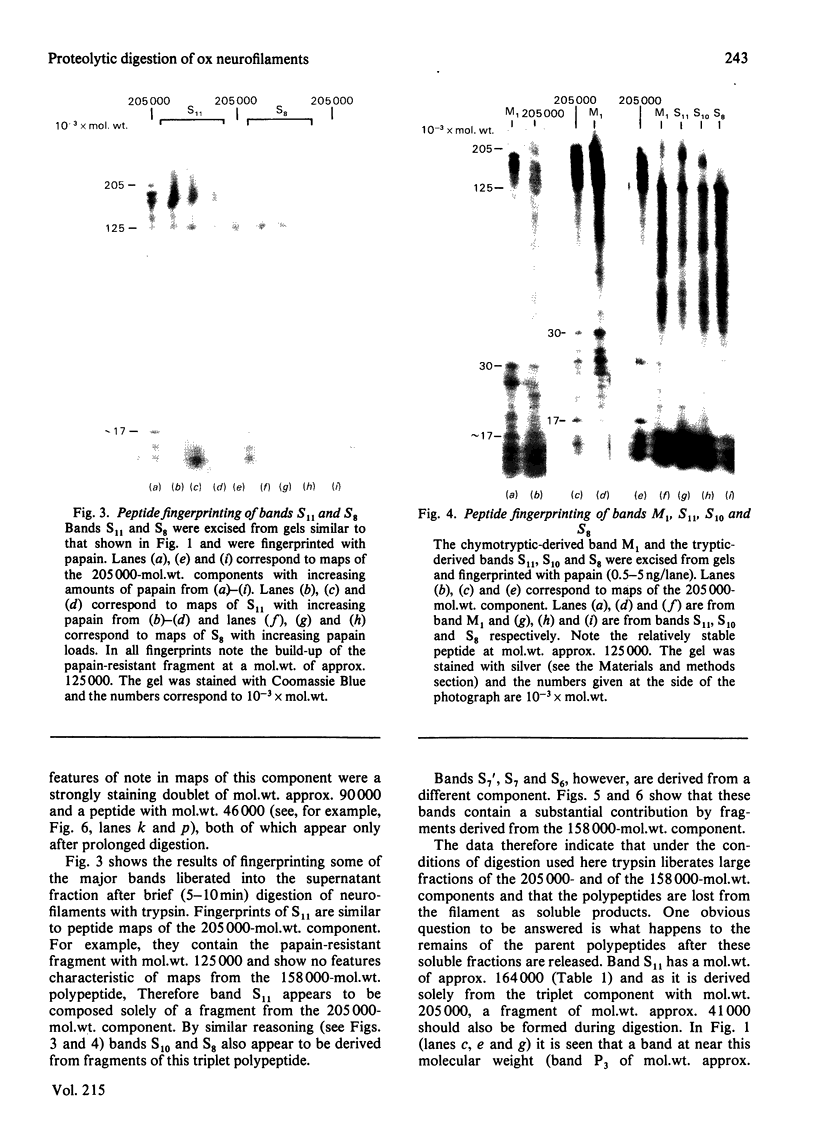
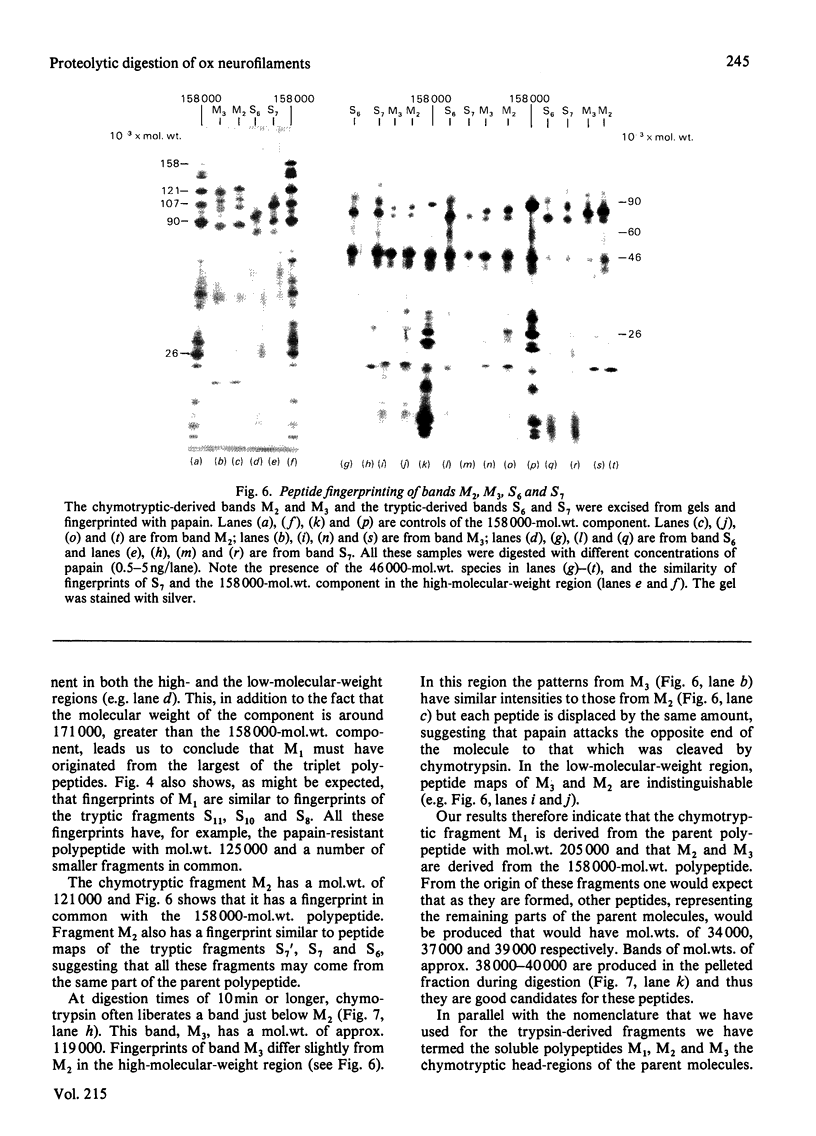
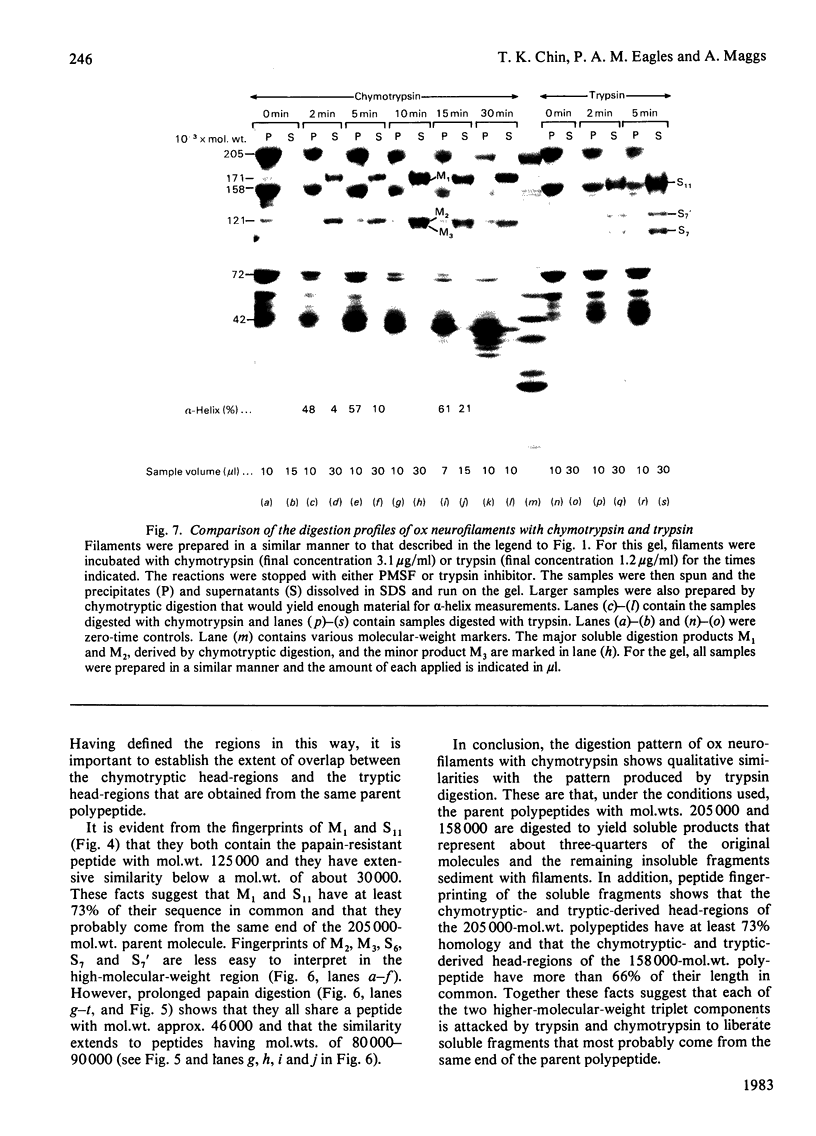
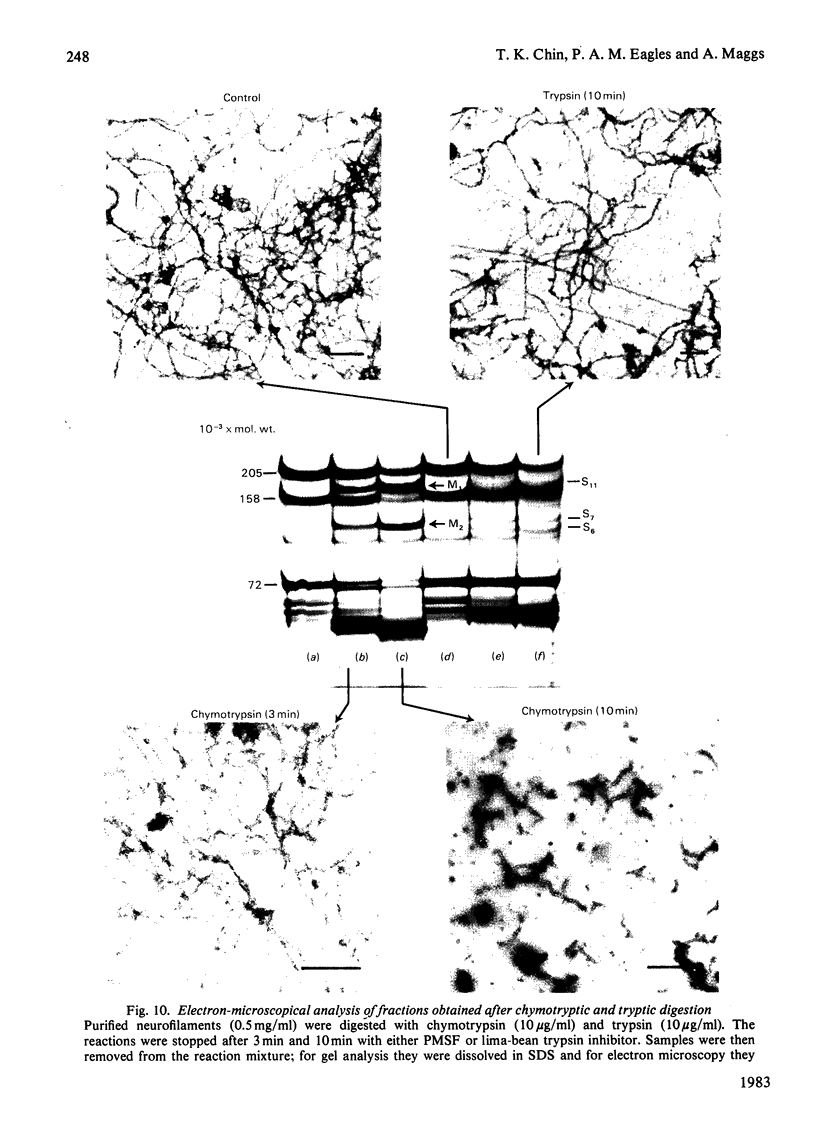
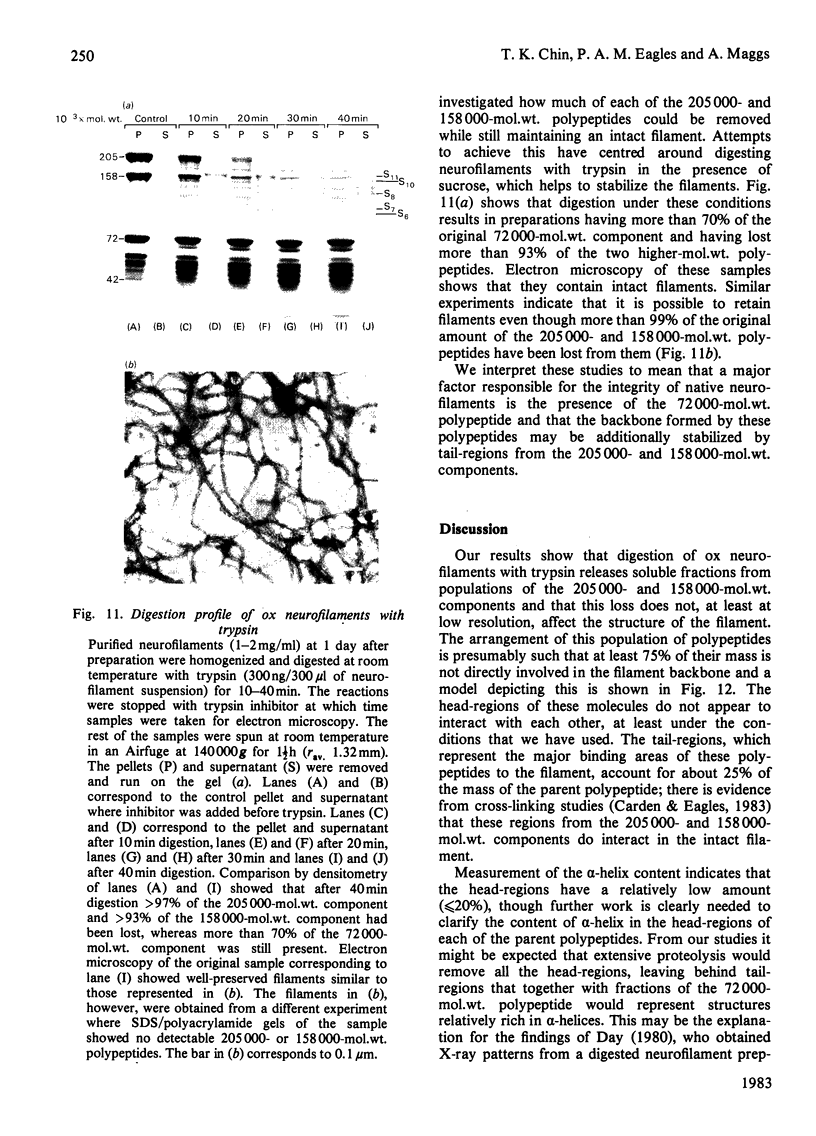
Brief digestion of ox neurofilaments with trypsin liberates fragments that are soluble and have molecular weights ranging from 164 000 to 97 000. Peptide fingerprinting indicates that these regions, termed the tryptic head-regions, arise from the 205 000- and 158 000-mol.wt. components of the triplet. The remains of the parent polypeptides sediment with normal filaments and have been termed tail-regions. Digestion of neurofilaments with chymotrypsin also liberates soluble fragments (chymotryptic head-regions) but these have mol.wts. 171 000 and 119 000, though they too originate from the higher-molecular-weight triplet polypeptides. Tryptic and chymotryptic head-regions have extensive homology, and a low (less than or equal to 20%) helix content. Electron microscopy shows that chymotryptic digestion rapidly reduces the length of filaments, probably because this enzyme preferentially attacks the 72 000-mol.wt. polypeptide. In contrast, brief digestion with trypsin does not reduce filament length even though more than 90% of the two higher-molecular-weight components have been cleaved. These results indicate that the backbone of native filaments is formed from the 72 000-mol.wt. polypeptide together with the tail-regions from the 205 000- and 158 000-mol.wt. polypeptides. The corresponding head-regions of these components, which can represent nearly 75% of each molecule, are not necessary for preserving the backbone of native neurofilaments and are therefore good candidates for being the side arms that connect these filaments in nerve cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Thorpe R., Cohen J., Selvendran S., Woodhams P. Specific neuronal localization by immunofluorescence of 10 nm filament polypeptides. J Neurocytol. 1980 Dec;9(6):835–844. doi: 10.1007/BF01205022. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Velasco M. E., Sipple J., Gambetti P. Immunochemical characterization of antisera to rat neurofilament subunits. J Neurochem. 1981 Nov;37(5):1260–1265. doi: 10.1111/j.1471-4159.1981.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Majocha R. E., Staton D. M., Marotta C. A. Axonal polypeptides cross-reactive with antibodies to neurofilament proteins. J Neurochem. 1983 Feb;40(2):299–308. doi: 10.1111/j.1471-4159.1983.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Eagles P. A. Neurofilaments from ox spinal nerves. Isolation, disassembly, reassembly and cross-linking properties. Biochem J. 1983 Nov 1;215(2):227–237. doi: 10.1042/bj2150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dahl D. Isolation of neurofilament proteins and of immunologically active neurofilament degradation products from extracts of brain, spinal cord and sciatic nerve. Biochim Biophys Acta. 1981 Apr 28;668(2):299–306. doi: 10.1016/0005-2795(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Day W. A. CNS neurofilament proteolysis and the 58 000-dalton fragment. J Ultrastruct Res. 1980 Jan;70(1):1–7. doi: 10.1016/s0022-5320(80)90016-7. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Gilbert D. S., Maggs A. Neurofilament structure and enzymic modification. Biochem Soc Trans. 1980 Oct;8(5):484–487. doi: 10.1042/bst0080484. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Gilbert D. S., Maggs A. The polypeptide composition of axoplasm and of neurofilaments from the marine worm Myxicola infundibulum. Biochem J. 1981 Oct 1;199(1):89–100. doi: 10.1042/bj1990089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Porter K. R. Microtrabecular structure of the axoplasmic matrix: visualization of cross-linking structures and their distribution. J Cell Biol. 1980 Nov;87(2 Pt 1):464–479. doi: 10.1083/jcb.87.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol. 1981 Sep 25;151(3):565–571. doi: 10.1016/0022-2836(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Metuzals J., Montpetit V., Clapin D. F. Organization of the neurofilamentous network. Cell Tissue Res. 1981;214(3):455–482. doi: 10.1007/BF00233488. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. A new basis for interpreting the circular dichroic spectra of proteins. Proc Natl Acad Sci U S A. 1971 May;68(5):969–972. doi: 10.1073/pnas.68.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Cytoplasmic structure in rapid-frozen axons. J Cell Biol. 1982 Sep;94(3):667–669. doi: 10.1083/jcb.94.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. A., Shaw G., Weber K. Immunoelectronmicroscopical localization of the three neurofilament triplet proteins along neurofilaments of cultured dorsal root ganglion neurones. Exp Cell Res. 1982 Feb;137(2):403–413. doi: 10.1016/0014-4827(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Liem R. K. Neurofilaments. J Neurochem. 1979 Jul;33(1):5–13. doi: 10.1111/j.1471-4159.1979.tb11699.x. [DOI] [PubMed] [Google Scholar]
- TOMBS M. P., SOUTER F., MACLAGAN N. F. The spectrophotometric determination of protein at 210 millimicrons. Biochem J. 1959 Sep;73:167–171. doi: 10.1042/bj0730167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. Electron microscopy of MAP 2 (microtubule-associated protein 2). J Ultrastruct Res. 1982 Sep;80(3):374–382. doi: 10.1016/s0022-5320(82)80051-8. [DOI] [PubMed] [Google Scholar]
- Wuerker R. B., Kirkpatrick J. B. Neuronal microtubules, neurofilaments, and microfilaments. Int Rev Cytol. 1972;33:45–75. doi: 10.1016/s0074-7696(08)61448-5. [DOI] [PubMed] [Google Scholar]
- Zackroff R. V., Idler W. W., Steinert P. M., Goldman R. D. In vitro reconstitution of intermediate filaments form mammalian neurofilament triplet polypeptides. Proc Natl Acad Sci U S A. 1982 Feb;79(3):754–757. doi: 10.1073/pnas.79.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]