Abstract
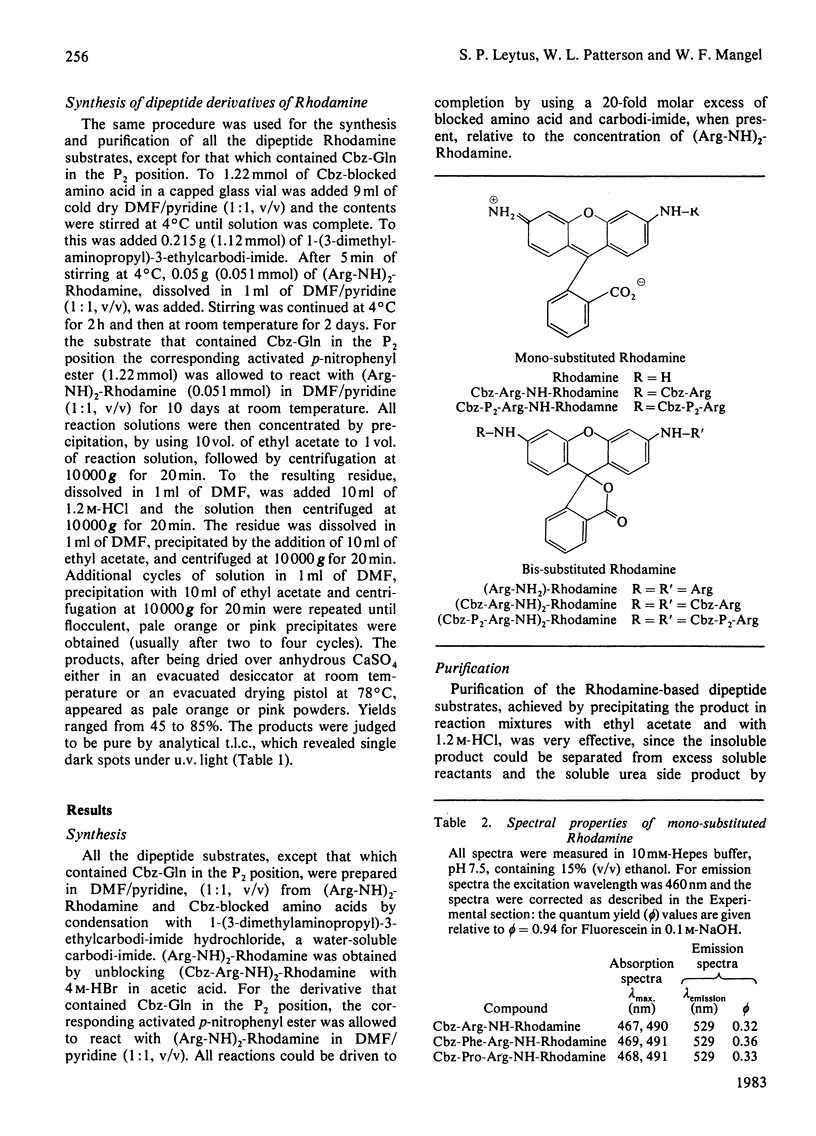
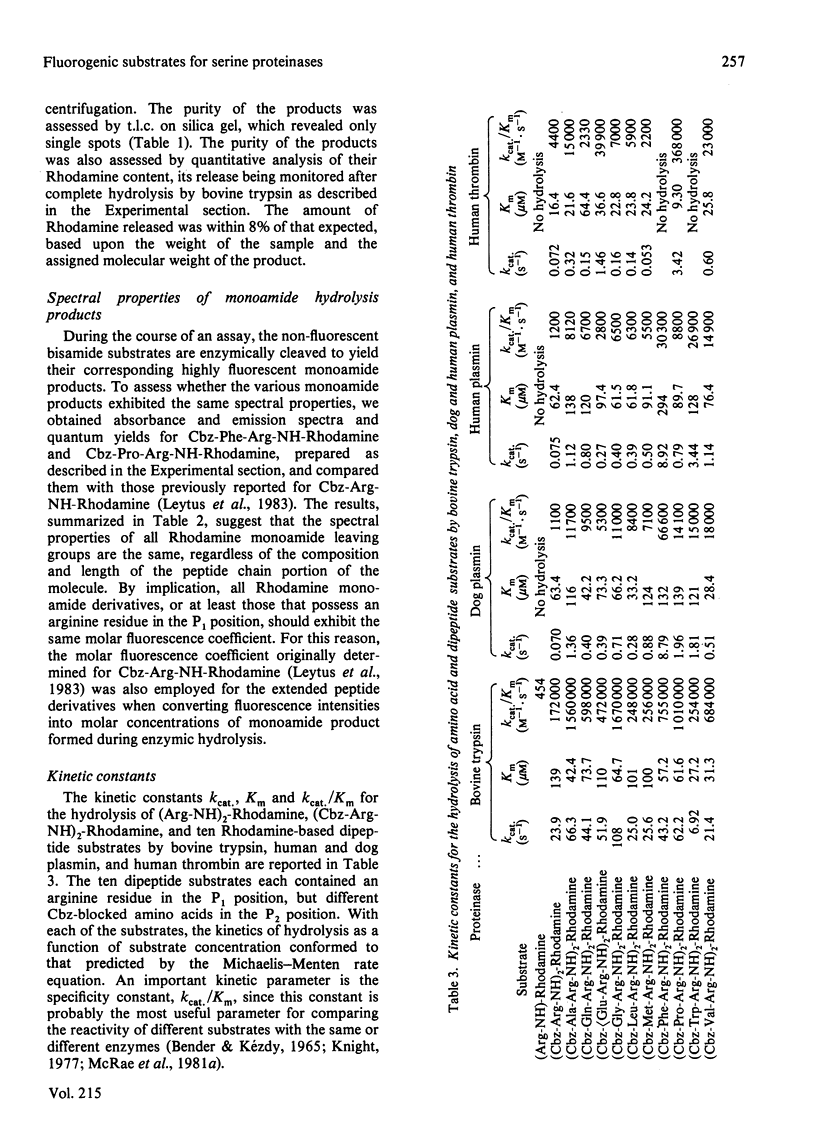
A series of dipeptide derivatives of Rhodamine, each containing an arginine residue in the P1 position and one of ten representative benzyloxycarbonyl (Cbz)-blocked amino acids in the P2 position, has been synthesized, purified and characterized as substrates for serine proteinases. These substrates are easily prepared with high yields. Cleavage of a single amide bond converts the non-fluorescent bisamide substrate into a highly fluorescent monoamide product. Macroscopic kinetic constants for the interaction of these substrates with bovine trypsin, human and dog plasmin, and human thrombin are reported. Certain of these substrates exhibit extremely large specificity constants. For example, the kcat./Km for bovine trypsin with bis-(N-benzyloxycarbonylglycyl-argininamido)-Rhodamine [(Cbz-Gly-Arg-NH)2-Rhodamine] is 1 670 000 M-1 X S-1. Certain of these substrates are also highly selective. For example, the most specific substrate for human plasmin, (Cbz-Phe-Arg-NH2)-Rhodamine, is not hydrolysed by human thrombin, and the most specific substrate for human thrombin, (Cbz-Pro-Arg-NH)2-Rhodamine, is one of the least specific substrates for human plasmin. Comparison of the kinetic constants for hydrolysis of the dipeptide substrates with that of the single amino acid derivative, (Cbz-Arg-NH)2-Rhodamine, indicates that selection of the proper amino acid residue in the P2 position can effect large increases in substrate specificity. This occurs primarily as a result of an increase in kcat. as opposed to a decrease in Km and, in certain cases, is accompanied by a large increase in selectivity. Because of their high degree of sensitivity and selectivity, these Rhodamine-based dipeptide compounds should be extremely useful substrates for studying serine proteinases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Sodetz J. M. Rabbit plasminogen and plasmin isozymes. Methods Enzymol. 1976;45:273–286. doi: 10.1016/s0076-6879(76)45026-7. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Melhado L. L., Mangel W. F. Rhodamine-based compounds as fluorogenic substrates for serine proteinases. Biochem J. 1983 Feb 1;209(2):299–307. doi: 10.1042/bj2090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leytus S. P., Peltz G. A., Liu H. Y., Cannon J. F., Peltz S. W., Livingston D. C., Brocklehurst J. R., Mangel W. F. A quantitative assay for the activation of plasminogen by transformed cells in situ and by urokinase. Biochemistry. 1981 Jul 21;20(15):4307–4314. doi: 10.1021/bi00518a011. [DOI] [PubMed] [Google Scholar]
- Livingston D. C., Brocklehurst J. R., Cannon J. F., Leytus S. P., Wehrly J. A., Peltz S. W., Peltz G. A., Mangel W. F. Synthesis and characterization of a new fluorogenic active-site titrant of serine proteases. Biochemistry. 1981 Jul 21;20(15):4298–4306. doi: 10.1021/bi00518a010. [DOI] [PubMed] [Google Scholar]
- McRae B. J., Lin T. Y., Powers J. C. Mapping the substrate binding site of human C1r and C1s with peptide thioesters. Development of new sensitive substrates. J Biol Chem. 1981 Dec 10;256(23):12362–12366. [PubMed] [Google Scholar]
- Zimmerman M., Ashe B., Yurewicz E. C., Patel G. Sensitive assays for trypsin, elastase, and chymotrypsin using new fluorogenic substrates. Anal Biochem. 1977 Mar;78(1):47–51. doi: 10.1016/0003-2697(77)90006-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Yurewicz E., Patel G. A new fluorogenic substrate for chymotrypsin. Anal Biochem. 1976 Jan;70(1):258–262. doi: 10.1016/s0003-2697(76)80066-8. [DOI] [PubMed] [Google Scholar]


