Abstract
Purpose
Anti-hyperlipidemic drug treatments are effective in reducing the risk of cardiovascular disease. In a long-term retrospective inception cohort study, we aimed to assess the real-world comparative effectiveness of anti-hyperlipidemic monotherapies for primary prevention of cardiovascular events.
Patients and Methods
Patients aged 18 years and older, who initiated primary prevention with anti-hyperlipidemic monotherapy, were selected from the University of Groningen IADB.nl dispensing database. In intention-to-treat (ITT) analysis we included all patients, whereas in per-protocol (PP) analysis we included both all patients independent of adherence (PPIA) and adherent patients (PPA). Study outcome was the time to first prescription of acute cardiac drug therapy measured by valid drug proxies to identify a first major cardiovascular event. We applied inverse probability of treatment-weighted (IPTW) analysis using Cox regression and time-varying Cox regression with simvastatin as the reference category to estimate the average treatment effect hazard ratios (HR) and their corresponding 95% confidence intervals (CI).
Results
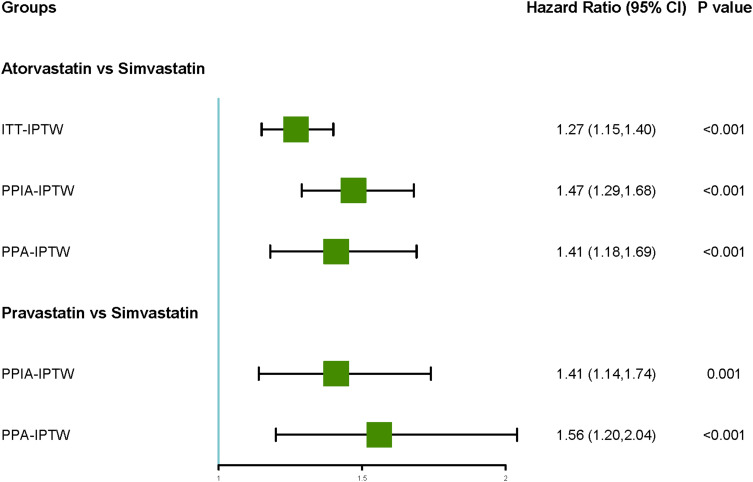
Atorvastatin users had significantly higher hazards compared to simvastatin users (HR range: 1.27 to 1.47, 95% CI: 1.15 to 1.69). Similarly, Pravastatin users also exhibited increased hazards compared to simvastatin users (HR range: 1.41 to 1.56, 95% CI: 1.14 to 2.04). Similar patterns were observed in patients with diabetes, rheumatoid arthritis, and asthma/COPD. No differences were found in the hazards of rosuvastatin, fluvastatin, fibrates, and simvastatin.
Conclusion
Atorvastatin and pravastatin users had higher long-term rates of cardiovascular events compared to simvastatin monotherapy in primary prevention, the difference may be attributed to the confounding by severity, but also possibly due to differences in drug mechanisms or patient response. These findings could influence current guideline recommendations, suggesting a potential preference for simvastatin in primary prevention, underscoring the need for further research to explore long-term impacts and underlying mechanisms, especially in diverse populations.
Keywords: acute cardiac drug therapy, time-varying confounding, inverse probability treatment weighting, Cox regression
Graphical Abstract
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality worldwide.1 According to Statistics Netherlands data,2 from 1950 to 2021, the mortality caused by CVD among all causes showed a trend of initially rising and then slowly decreasing from 2000. However, CVD still account for a substantial proportion of all causes of death in the Netherlands.
Hyperlipidemia is a prevalent risk factor for cardiovascular disease.3 Anti-hyperlipidemic drug treatments such as statins are effective in regulating plasma Low-Density Lipoprotein (LDL) concentrations, thus reducing the risk of morbidity and mortality associated with cardiovascular disease.3,4 It is also important to consider the issue of persistence with statin therapy, as studies have indicated poor adherence to this treatment.5
In clinical practice, statins and fibrates are frequently prescribed as anti-hyperlipidemic drugs, no evidence of a difference between statins and fibrates in reducing cardiovascular events, though statins with fewer adverse effects compared to fibrates.6 Based on cardiovascular risk management guidelines in the Netherlands,7 atorvastatin, rosuvastatin, or simvastatin are recommended for patients with a desired LDL cholesterol (LDL-C) reduction of less than 40%, while only atorvastatin or rosuvastatin are advised to achieve reductions greater than 40%. In comparative effectiveness cohort studies, atorvastatin demonstrated superior performance compared to simvastatin in preventing cardiovascular events.8,9 However, it is important to acknowledge that the observed differences in outcomes between the two statins may not solely be due to the inherent characteristics of the medications, but may also be influenced by the underlying patient profiles and indications for their prescription.
One study,10 based on PHARMO data in the Netherlands, compared baseline conditions among new users of rosuvastatin, atorvastatin, simvastatin, and pravastatin. Pravastatin users had the highest CVD rates (40.7%) at baseline, while users of the other three drugs only had average rates of 5% to 8%. The mean LDL-C level in all three drugs users were similar and slightly higher than pravastatin users; however, the mean LDL-C level of all four drug users in lower risk group was similar (from 4.48 to 4.66), same trend in higher risk group (from 3.84 to 3.95). This may indicate that pravastatin was predominantly utilized for secondary prevention of CVD. In this way, this differential selection of statins based on patient characteristics can introduce bias in research studies comparing their effects, due to pravastatin and atorvastatin, more frequent prescription for secondary prevention and simvastatin for primary prevention.
Furthermore, the real-world effectiveness between two drugs needs to be examined in comparison to other medications in the same therapeutic class. Simvastatin, for instance, has demonstrated superior population-level effectiveness in comparison to gemfibrozil (a type of fibrate) for primary prevention of major adverse cardiovascular events.11 Additionally, a study conducted in France found no difference in the risk of cardiovascular disease events between 5mg of rosuvastatin and 20mg of simvastatin.12 Moreover, a systematic review of several randomized controlled trials concluded that the three main statins (pravastatin, simvastatin, and atorvastatin) at their standard dosages showed no statistically significant difference in their long-term prevention of CVD.13
Confounding by indication is also one problem we should take into consideration. For instance, statins could potentially benefit patients with conditions/diseases like rheumatoid arthritis (RA) and asthma or chronic obstructive pulmonary disease (COPD).14 On the other hand, statins may have a negative impact on glycemic control15 for example increased HbA1c. Furthermore, methotrexate (MTX) is linked to a decreased risk of CVD events in patients with RA,16 while the interplay between asthma/COPD and cardiovascular risk is complicated.17 However, limited evidence exists regarding the comparative influence of drugs used for diabetes, asthma or COPD on anti-hyperlipidemic drug monotherapy.
We therefore set out to investigate the real-world effectiveness of anti-hyperlipidemic monotherapies in primary prevention of CVD taking adherence into account. We further explored any effect modification by frequent comorbidities as asthma/COPD, RA or diabetes.
Materials and Methods
Study Design and Setting
Our study is a retrospective inception cohort study in which we applied both an intention to treat (ITT) and per protocol (PP) analysis approach. We used data from the IADB.nl database of the University of Groningen, which can reflect the real-world information. This pharmacy dispensing database collected information of a population in the Netherlands from 1994 till now. Registration in the database is irrespective of health care insurance and age, sex and prescription rates among the database population have been found to be representative of the Netherlands as a whole, and the database has been widely used for research. Due to the high patient-pharmacy commitment in the Netherlands, the medication records for each patient are virtually complete, except for over the counter drugs and medication dispensed during hospitalization.18,19
The study period was from January 1, 1996 to December 31, 2020. We set index date as the date of first prescription of exposure (start of drug therapy with any anti-hyperlipidemic monotherapy, see exposure definition).
Study Population
Inclusion and Exclusion Criteria
The criteria were similar to a previous IADB database study.20 Patients were eligible for inclusion if they were aged 18 years or older at index date. Furthermore, each patient had at least two years of prescription records before the index date and at least one year of records after it to be included. In order to satisfy the condition that the exposure is monotherapy, every included patient had at least three prescriptions of the same class of anti-hyperlipidemic monotherapy in the first year from the index date.
Patients who were prescribed anti-hypertensive monotherapies within one year after the index date were excluded from the study. Additionally, patients who received at least two prescriptions of either antihypertensive or anti-hyperlipidemic drug fixed-dose combinations in the year following the index date were also excluded. Moreover, patients who received any acute cardiac drug therapy (CDT) within two years prior to or within 90 days after the index date were excluded. Patients who had been on chronic drug therapy for stable heart failure, migraine, adrenal disease, hyperparathyroidism, and thyroid problems (with at least two prescriptions) in the two years prior to or within 90 days after the index date were also excluded from the analysis.
Unlike the ITT analysis, which included all patients, we selected two subsets for PP analysis. One cohort consists of all patients-independent of adherence (PPIA), the other cohort consists of adherent patients with at least one-year follow-up time (PPA).
Exposure
Exposures were defined as using the following anti-hyperlipidemic single-drug compounds with their ATC codes in brackets, simvastatin (C10AA01), pravastatin (C10AA03), fluvastatin (C10AA04), atorvastatin (C10AA05), rosuvastatin (C10AA07) or fibrates (C10AB). Participants within a designated group receiving anti-hyperlipidemic monotherapy were permitted to utilize various chemical compounds, provided they belonged to the same class as per the ATC code (at the level of ATC code level 3/4).
Adherence
Adherence to treatment was defined as the number of days covered by anti- hyperlipidemic drug monotherapy divided by the number of follow-up days.21,22 We distinguish between total adherence, which is adherence in the total follow-up time, and interval adherence, which is the adherence in each 180 days interval. Both adherence measures were categorized as “high adherence” (adherence ≥0.8), “intermediate adherence” (0.5 ≤ adherence < 0.8), and “low adherence” (adherence < 0.5).23
Primary Outcome
The primary outcome is defined as the time to the first prescription of CDT. Acute CDT is a proxy for an incident major cardiovascular event according to Pouwels et al.24 Based on their conclusion, the most accurate combination of acute CDT drugs to identify a CVD is at least two drug prescriptions of either a platelet aggregation inhibitor (B01AC), organic nitrate (C01DA), and/or a vitamin K antagonist (B01AA) or other vasodilators used in acute cardiac disease therapies (C01DX), in a time window of 180 days whichever comes first, after the index date.
Follow-Up Time – ITT versus PP Approach
Since PP analysis has stricter criteria for its participants, the study period is often shorter compared to ITT analysis. Compared with a 25-year study period in ITT analysis, PP analysis used a study period up to 10 years.
In ITT analysis, the end of follow-up time was defined as (1) outcome event (the date of first proxy prescription of CDT), (2) censored event: December 31, 2020 (the end date of our study), or the date of last prescription in the database (before December 31, 2020).
In PP analysis, the end of the follow-up period was determined by the occurrence of one of the following events: (1) outcome event (the date when patients received their first prescription for a drug indicating an CDT), (2) censored event (the date when patients had no outcome but discontinued their treatment, or received an additional anti-hyperlipidemic drug), the conclusion of a 10-year follow-up period, or reaching 31 December 2020, whichever event occurred first.
Discontinuation, Switch, Add-on
Discontinuation was defined as a period of more than 180 days without receiving a prescription from same class for initial monotherapy of anti-hyperlipidemic drugs.21,23 A drug switch occurred when patients discontinued a specific drug therapy for a duration exceeding 180 days and subsequently received a prescription for a new class of anti-hyperlipidemic drug monotherapy or anti-hyperlipidemic drug fixed-dose combinations within 180 days.25 Drug add-on was defined as the addition of a new drug class or anti-hyperlipidemic drug fixed-dose combinations to an existing drug class before drug discontinuation.26 If patients experienced discontinuation or add-on events prior to the occurrence of the outcome, they were considered to be censored in the analysis (drug switch was not considered as censored because it happened only after drug discontinuation).
Potential Confounders
Besides demographic characteristics including age, sex, and calendar year periods, we also considered other potential confounders such as comorbidities drug history and drug used for common comorbidities. Calendar year periods were categorized into three periods from 1996 to 2020 which reflected the time of the first prescription of an anti-hyperlipidemic drug monotherapy. Drugs used for common comorbidities including diabetes, RA, asthma, or COPD were assessed in both the ITT and PP analysis. In the ITT analysis, the time-constant variable “comorbidities drug history” represents at least two prescriptions for any of these three diseases within two years before the index date. In the PP analysis, the time-constant variable “initial drugs for common comorbidities” indicated whether patients had prescriptions for any of these three diseases within the first 180 days after the index date. Additionally, the time-varying variable “drugs for common comorbidities” represents whether patients had prescriptions for these diseases within each subsequent 180-day period.
Statistical Analysis
R statistical software was used for both data preprocessing and analysis purposes. Baseline characteristics were succinctly summarized as mean ± standard deviations (sd) for continuous variables and frequencies presented as proportions and percentages for categorical variables. To test for statistically significant differences in baseline characteristics between comparison groups, we applied the Pearson’s χ2 test, ANOVA test, and Welch’s ANOVA test. A significance level of α = 0.05 was set for the two-sided tests to indicate statistical significance.
We used survfit function in “survival package” to plot Kaplan–Meier survival curves of time to acute CDT for the five different classes of drugs compared with simvastatin, both with and without Inverse Probability Treatment Weighting (IPTW) for patients in three cohorts. We used ipwpoint and ipwtm function from the “ipw” R-package27 to calculate ATE estimand propensity scores as well as to fit marginal structural models, one with time-constant confounders and another with time-varying confounders, in ITT and PP analysis, respectively.
We used the coxph function in the “survival” R-package to construct the Cox regression with time constant variables and the time-varying Cox regression model28, with and without IPTW, to estimate the total relative effectiveness and relative effectiveness in subgroups of six anti- hyperlipidemic drugs monotherapy. We performed subgroup analysis, using sex, age, drugs used for diabetes, drugs used for RA, drugs used for asthma or COPD, calendar year, and total adherence as variables, we conducted the same analysis with IPTW between five other classes of anti-hyperlipidemic drugs and simvastatin. All R code is available from the authors upon request.
Sensitivity Analysis
In both ITT and PP analysis, a sensitivity analysis was done for comparison among equalized anti-hyperlipidemic drugs monotherapy doses. The equivalent doses (EQD) scheme was used to ensure that the percentage reduction in LDL-C was approximately the same across different anti-hyperlipidemic drugs.29,30 Based on three categories of EQD (less than 30%, 30–45%, and more than 45%), we divided our patients into three levels: 10 mg simvastatin (low), 20 mg simvastatin (medium), and 40 mg simvastatin (medium) as the reference group. Patients prescribed 60 mg and 80 mg simvastatin (high) were excluded from the analysis due to the limited number of patients in those dosage categories.
In the ITT analysis, we examined patients without a concurrent history of common comorbidities drugs for diabetes, RA, asthma, or COPD (ATC code see Supplementary Table S1). Additionally, in the PP analysis, we evaluated patients without common comorbidities drugs and further stratified the analysis based on patients without drug switches or drug add-ons, encompassing both PPIA and PPA. The same analytical methods employed in the primary analysis were used to estimate the overall relative effectiveness and relative effectiveness within subgroups in these cohorts.
Results
Baseline Characteristics for ITT and PP Analysis
Between January 1996 and December 2020 in Netherland, a total of 18375 patients initiated with any of the five types of statins or fibrates with most starting (79.6%) simvastatin monotherapy (Table 1). The average follow-up time was 7.3 years (sd 5.2). Males were more common than females with 55.7% and the mean age of was 56.2 years (sd 11.5). Except for simvastatin and fibrates users, the vast majority of patients had their first prescription of an antihyperlipidemic drug from 2000 to 2010. In patients with a history of drug use for any of the comorbidities, diabetes was the most common comorbidity with a prevalence of 23.9%.
Table 1.
Baseline Characteristics for Population in ITT Analysis Who Used Anti-Hyperlipidemic Drugs Monotherapy
| Demographics | Patients in ITT Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Simvastatin | Atorvastatin | Rosuvastatin | Pravastatin | Fluvastatin | Fibrates | P§ | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| N=18375 | N=14624 (79.6)* | N=2062 (11.2)* | N=773 (4.2)* | N=689 (3.7)* | N=107 (0.6)* | N=120 (0.7)* | ||
| Average follow up years† | 7.3±5.2 | 6.9 ±4.8 | 8.8 ±6.4 | 8.0 ±5.7 | 9.1 ±6.1 | 11.4 ±7.6 | 8.0 ±6.0 | / |
| Sex:male | 10234 (55.7) | 8180 (55.9) | 1125 (54.6) | 405 (52.4) | 367 (53.3) | 62 (57.9) | 95 (79.2) | <0.001 |
| Age†† (years) | 56.2 ±11.5 | 56.5 ±11.4 | 54.5 ±12.0 | 55.1 ±12.1 | 56.0 ±11.5 | 55.4 ±9.4 | 45.9 ±10.3 | <0.001‡ |
| 18–39 | 1445 (7.9) | 1061 (7.3) | 219 (10.6) | 80 (10.3) | 49 (7.1) | 5 (4.7) | 31 (25.8) | <0.001 |
| 40–69 | 14,667 (79.8) | 11,703 (80.0) | 1625 (78.8) | 605 (78.3) | 552 (80.1) | 95 (88.8) | 87 (72.5) | |
| ≥70 | 2263 (12.3) | 1860 (12.7) | 218 (10.6) | 88 (11.4) | 88 (12.8) | 7 (6.5) | 2 (1.7) | |
| Calendar-year periods | <0.001¢ | |||||||
| 1996–2000 | 813 (4.4) | 501 (3.4) | 159 (7.7) | 0 (0) | 80 (11.6) | 48 (44.9) | 25 (20.8) | |
| 2000–2010 | 7538 (41.0) | 5197 (35.5) | 1298 (62.9) | 472 (61.1) | 474 (68.8) | 54 (50.5) | 43 (35.8) | |
| 2010–2020 | 10,024 (54.6) | 8926 (61.0) | 605 (29.3) | 301 (38.9) | 135 (19.6) | 5 (4.7) | 52 (43.3) | |
| Comorbidities drug history£ | ||||||||
| Diabetes drug: Yes | 4398 (23.9) | 3633 (24.8) | 398 (19.3) | 130 (16.8) | 194 (28.2) | 20 (18.7) | 23 (19.2) | <0.001 |
| RA drug: Yes | 168 (0.9) | 143 (1.0) | 17 (0.8) | 5 (0.6) | 3 (0.4) | 0 (0) | 0 (0) | 0.613¢ |
| Asthma/COPD drug: Yes | 1408 (7.7) | 1168 (8.0) | 143 (6.9) | 47 (6.1) | 41 (6.0) | 2 (1.9) | 7 (5.8) | 0.011 |
Notes: *Row percentage, others are all column percentage. § P value: significance value of the Chi-squared test or anova test, which showed the difference of distribution of patients who used three anti-hyperlipidemic monotherapies at baseline in different subgroups of covariates. †Use mean ± sd to describe average follow-up years. †† Use mean ± sd to describe continuous age. ‡ Welch anova test to describe whether patients of different classes of anti-hyperlipidemic monotherapy were different in age (Heterogeneity of variance). ¢ Fisher’s Exact Test. £ At least two prescriptions of any of these three diseases two years before index date.
Supplementary Table S2 and S3 showed the baseline characteristic for PPIA and PPA. The average follow-up times for each cohort of patients were 3.6 years (sd: 3.3) and 5.1 years (sd: 3.2), respectively. Similar baseline characteristics distribution were observed in these two tables as in Table 1.
Incidence Rate
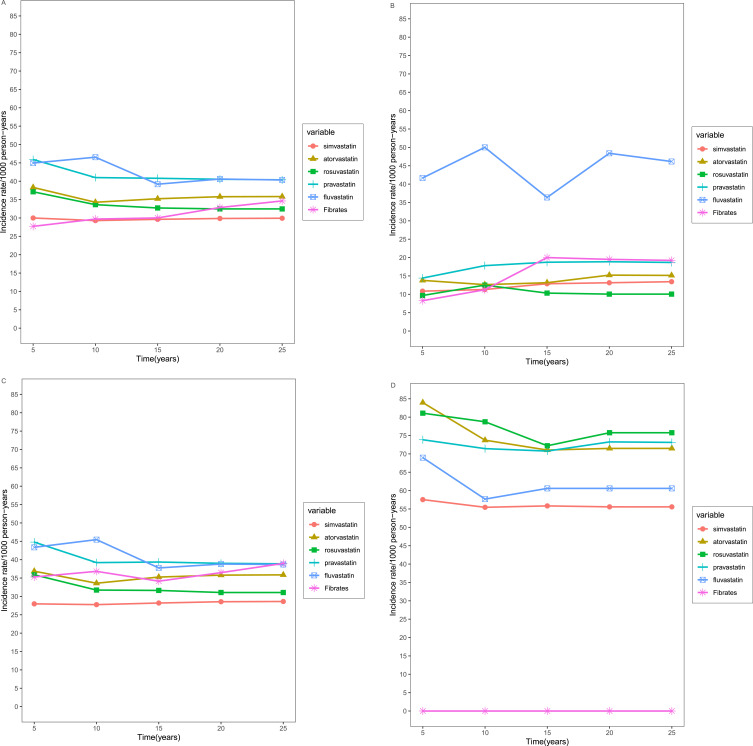
Over the 25-year follow-up period, fluvastatin and pravastatin users exhibited the highest incidence rate of CDT among the five observed time periods (Figure 1A), while simvastatin users had the lowest incidence rate. Even after age stratification, fluvastatin users still have a higher incidence rate in the 18 to 39 (Figure 1B) and 40 to 69 age groups (Figure 1C), whereas rosuvastatin users had the highest rate of CDT among the elderly population (Figure 1D). With the exception of patients who used fibrates, simvastatin users consistently maintained the lowest incidence rate (Supplementary Table S4; Figure 1).
Figure 1.
Incidence rates of CDT of six types of anti-hyperlipidemic drug stratified by age. (A) Total; (B) 18–39 year group; (C) 40–69 year group; (D) ≥70 year group.
Cox Regression Analysis
After IPTW, Cox regression analysis revealed that atorvastatin exhibited higher hazards compared to simvastatin in both ITT and PP analysis, as observed in the ITT analysis [Hazard ratio (HR): 1.27, 95% Confidence interval (CI): 1.15 to 1.40]. The same trend was observed in PPIA and PPA (1.47, 1.29 to 1.68; 1.41, 1.18 to 1.69). Except for ITT, pravastatin exhibited higher hazards compared to simvastatin (Table 2).
Table 2.
Cox Regression Analysis of Acute CDT
| Acute CDT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients in ITT Analysis (N=18375) | PPIA (N=18375) | PPA (N=11247) | ||||||||||
| Anti-hyperlipidemic Monotherapies | Crude HR (95% CI) | P | IPTW Adjusteda HR (95% CI) | P | Crude HR (95% CI) | P | IPTW Adjustedb HR (95% CI) | P | Crude HR (95% CI) | P | IPTW Adjustedc HR (95% CI) | P |
| Reference: simvastatin | ||||||||||||
| Exposure | ||||||||||||
| Atorvastatin | 1.21 (1.11,1.33) | <0.001 | 1.27 (1.15,1.40) | <0.001 | 1.43 (1.26,1.63) | <0.001 | 1.47 (1.29,1.68) | <0.001 | 1.36 (1.14,1.62) | <0.001 | 1.41 (1.18,1.69) | <0.001 |
| Rosuvastatin | 1.10 (0.95,1.27) | 0.217 | 1.17 (1.00,1.38) | 0.053 | 1.17 (0.94,1.46) | 0.152 | 1.23 (0.98,1.55) | 0.080 | 1.00 (0.71,1.40) | 0.995 | 1.02 (0.72,1.44) | 0.928 |
| Pravastatin | 1.38 (1.21,1.57) | <0.001 | 1.14 (0.96,1.35) | 0.139 | 1.41 (1.14,1.74) | 0.001 | 1.41 (1.14,1.74) | 0.001 | 1.57 (1.21,2.04) | <0.001 | 1.56 (1.20,2.04) | <0.001 |
| Fluvastatin | 1.36 (1.02,1.82) | 0.036 | 1.73 (0.82,3.67) | 0.151 | 0.98 (0.51,1.89) | 0.956 | 1.00 (0.50,1.98) | 0.989 | 1.26 (0.56,2.81) | 0.575 | 1.29 (0.56,2.97) | 0.549 |
| Fibrates | 1.15 (0.81,1.64) | 0.431 | 1.49 (0.88,2.54) | 0.137 | 0.56 (0.25,1.26) | 0.161 | 0.64 (0.27,1.48) | 0.293 | 0.68 (0.22,2.10) | 0.498 | 0.77 (0.24,2.49) | 0.668 |
Notes: aIPTW model, Exposure: five types of Anti- hyperlipidemic monotherapy and simvastatin, numerator: ~ 1, denominator = ~ sex + age + diabetes drug history + RA drug history + asthma or COPD drug history + calendar year. bIPTW model, Exposure: five types of Anti-hyperlipidemic monotherapy and simvastatin, numerator: ~ sex + age + calendar year, denominator = ~ drugs for diabetes + drugs for RA+ drugs for asthma or COPD + adherence in each 180 days + sex + age + calendar year. cIPTW model, Exposure: five types of Anti-hyperlipidemic monotherapy and simvastatin, numerator: ~ sex + age + calendar year, denominator = ~ drugs for diabetes + drugs for RA+ drugs for asthma or COPD + sex + age + calendar year.
Subgroup Analysis
In the ITT analysis, atorvastatin had higher HR than simvastatin in both male and female patients. Among patients who had drugs history for diabetes or RA, atorvastatin exhibited significantly higher hazards (Supplementary Table S5; Supplementary Figure S1).
In PPIA, atorvastatin had higher hazards compared to simvastatin in patients with both sexes, with and without initial diabetes or RA or asthma/COPD drug use, the values were even higher in patients with initial three drugs mentioned above. Furthermore, pravastatin showed a higher hazard compared to simvastatin in patients with initial diabetes drug use (Supplementary Table S6; Supplementary Figure S2). In PPA, the comparison between atorvastatin and simvastatin showed the same trend as in PPIA (Supplementary Table S7; Supplementary Figure S3).
Sensitivity Analysis
Equivalent Dosing Comparison
After IPTW, in the ITT analysis, 20 mg pravastatin exhibited greater hazards than 10 mg simvastatin; 40 mg pravastatin exhibited higher hazards compared to both 20 mg simvastatin and 40 mg simvastatin (Supplementary Table S8).
In the PP analysis, the primary results remained consistent. After IPTW, in both PPIA and PPA cohorts, 40 mg pravastatin demonstrated higher hazards compared to 20 mg and 40 mg simvastatin. Moreover, higher hazard was observed for 10 mg atorvastatin compared to 20 mg simvastatin. The 10 mg atorvastatin and 20 mg atorvastatin both had higher hazards compared to 40 mg simvastatin in both PPIA and PPA (Supplementary Table S9).
Other Cohorts
The ITT analysis demonstrated that atorvastatin still had higher hazard compared to simvastatin before and after IPTW in patients without common comorbidities drug history, fluvastatin had higher hazard than simvastatin after IPTW (Supplementary Table S10).
PP analysis revealed that among patients who did not utilize common comorbidities drugs or undergo drug switch or drug add-on, atorvastatin and pravastatin demonstrated higher hazards when compared to simvastatin (Supplementary Table S11).
Discussion
Atorvastatin and pravastatin users had higher rates of CDT over a long-term follow-up period than simvastatin monotherapy users in the prevention of cardiovascular events.
Atorvastatin and Pravastatin Users Had Higher Rates of CDT Than Simvastatin Users in ITT and/or PP Analysis
Simvastatin, pravastatin and atorvastatin are three the most prescribed statins.31 Despite their shared classification as statins, there are still differences in their mechanisms of action. Simvastatin and atorvastatin exhibit higher oral bioavailability compared to pravastatin.32 These features may influence their relative effectiveness in primary preventing CVD events. Atorvastatin has been demonstrated to have a stronger effect on reducing LDL-C levels and a longer-lasting impact.31,33 In our study, we found that simvastatin users had a lower rate of CDT compared to atorvastatin users and pravastatin users. The comparison between simvastatin users and pravastatin users can be attributed to its mechanism of action. The comparison between atorvastatin and simvastatin was different compared with many studies8,9 that showed atorvastatin had better performance than simvastatin. This difference may be related to guideline recommendations in the Netherlands, where atorvastatin is more likely to be recommended for high-risk patients to facilitate LDL-C reductions of 40% or more.7 However, we used IPTW to balance the uneven distribution between simvastatin and atorvastatin, which means the difference may be less related to the guideline preference.
Atorvastatin Users Showed Higher Rates of CDT Than Simvastatin Users in Most Subgroups Analysis
There was not much difference in HR between male and female patients, sex may not influence the comparison between atorvastatin and simvastatin. The hazards in patients with three common comorbidities drug history or with initial three common comorbidities were much higher than in patients without.
For example, in patient with RA drugs history or with initial RA drug use, atorvastatin had higher hazards compared with simvastatin, and interaction effect showed that RA drug history or initial RA drug use may worsen the effect of atorvastatin compared to simvastatin, vice versa. The combination of MTX and simvastatin seems to be more effective in improving overall cardiovascular health compared to the combination of MTX and atorvastatin. The use of MTX to treat RA can reduce the risk of cardiovascular disease,16 while simvastatin has a positive effect on the body’s anti-inflammatory mechanism,34 but whether it is because of the synergistic or additive effect MTX has with simvastatin or the effectiveness difference between simvastatin and atorvastatin remains uncertain.
Some studies showed that statins can worsen glycemic control, especially for atorvastatin,35,36 maybe this can explain why atorvastatin and pravastatin performed even worse than simvastatin in patients with diabetes drug history or with initial diabetes drug use. Notably, inhaled corticosteroids could potentially reduce the risk of coronary heart disease in patients with COPD,37 however, it also cannot be proved that whether Inhaled steroids the same effect worked on worsening atorvastatin or improve simvastatin due to effect size of interaction effect.
Sensitivity Analysis Improve the Robustness of Our Results
The primary results of sensitivity analysis remained consistent. Even after excluding potential biases caused by patients deviating from their original treatment choice or using drugs for other conditions, simvastatin continued to demonstrate superiority over atorvastatin and pravastatin in Dutch patients. This observation reinforces the recommendation for simvastatin as the preferred option.
Potential Strengths and Limitations
In our study, we employed both ITT and PP analyses, which allowed us to address different aspects of treatment outcomes. The ITT analysis provided an assessment of the overall effectiveness of the interventions, regardless of treatment adherence or protocol deviations. On the other hand, the PP analysis focused on patients who strictly adhered to the treatment protocol, providing insights into the potential benefits of optimal adherence. By incorporating both approaches, we were able to obtain a more comprehensive understanding of the outcomes and their interpretations.
Furthermore, we took into account the time-varying nature of medication adherence in PP analysis, which provided a dynamic reflection of patients’ real-world drug usage patterns over time. This approach can enhance the accuracy of our findings.
To mitigate potential indication bias, we implemented rigorous inclusion and exclusion criteria in our study design. These criteria were designed to minimize the influence of confounding factors related to patients’ baseline characteristics and indications for treatment.
As previously mentioned, individuals using pravastatin exhibited a heightened risk of CVD at the start of the study conducted by Heintjes et al.10 Atorvastatin is more likely to be recommended for high-risk patients.7 This uneven distribution of CVD risk among different exposures disrupted the balance necessary for accurate comparisons of the effects of various statins. The differential selection of statins based on patient characteristics can introduce biases in research studies examining their effects. Nonetheless, we employed IPTW to mitigate this selection bias by equalizing the uneven distribution of measured characteristics. IPTW helps eliminate the disparities and ensure a more balanced comparison among the different statins. In addition to this, in both PPA and PPIA analysis, the results stayed the same, adherence will not influence the effect between pravastatin and atorvastatin when compared to simvastatin. Furthermore, the EQD analysis also showed atorvastatin and pravastatin performed worse than simvastatin in different dosage categories. All results suggested that the differences between pravastatin and atorvastatin, compared with simvastatin, were less related to baseline selection bias.
The use of subgroup analysis as a strategy to reduce residual confounding is a notable strength. By focusing on specific subgroups within a study population, we can control for potential confounders more effectively, identify effect modifiers, and enhance the internal validity of their findings.
In terms of the robustness of our results, we conducted analyses separately for PPIA and PPA. This approach allowed us to assess the consistency of our findings across different subgroups and evaluate the impact of adherence on the outcomes. Additionally, we performed primary analysis and sensitivity analyses before and after employing IPTW, and the results remained consistent. This consistency indicates that our findings are robust and not overly influenced by specific analytical approaches.
However, it is important to acknowledge the limitations of our study. First, we focused on a single-drug compound rather than comparing entire classes of drugs. This narrow focus may limit the generalizability of our findings to other medications within the same therapeutic class. Second, the measurement of adherence itself can introduce bias, as it relies on prescription refill data, which may not accurately reflect actual medication intake. Third, despite our efforts to control for confounding variables, unmeasured confounders, such as other disease for which our exposure medicine is used as common treatment may still exist and could potentially influence the observed associations. Fourth, while acknowledging the multifactorial nature of assigning high-risk status (atorvastatin in our study), it is important to note that utilizing statistical correction, specifically through propensity scores based on the limited determinants available in the provided pharmacy database (only prescription information, lack of information on lifestyle, etc.)., may be insufficient for comprehensive adjustment. The unbalanced number of patients in each exposure groups will also affect the comparison. Furthermore, we still face the confounding caused by co-morbidities and co-medications.38 More clinical evidence may be needed to prove it in the future.
Summary
Overall, the superior performance of simvastatin in both the general population and patients with certain comorbidities, as demonstrated in our main and sensitivity analyses, offers valuable insights for practical medication guidelines in the Netherlands, despite some limitations.
Conclusion
Atorvastatin and pravastatin users had higher long-term rates of cardiovascular events compared to simvastatin monotherapy in primary prevention, the difference may be attributed to the confounding by severity, but also possibly due to differences in drug mechanisms or patient response. These findings could influence current guideline recommendations, suggesting a potential preference for simvastatin in primary prevention, underscoring the need for further research to explore long-term impacts and underlying mechanisms, especially in diverse populations.
Acknowledgments
We thank the pharmacies that supplied data to the University Groningen IADB.nl database.
Funding Statement
Xuechun Li is funded by the China Scholarship Council (file no: 202106070028).
Abbreviations
ATC code, Anatomical therapeutical chemical code; CDT, Cardiac drug therapy; CVD, Cardiovascular diseases; COPD, Chronic obstructive pulmonary disease; CI, Confidence interval; EQD, equivalent doses; HR, Hazard ratio; ITT, intention to treat; IPTW, Inverse Probability Treatment Weighting; LDL-C, LDL cholesterol; MTX, Methotrexate; PP, per protocol; PPA, PP analysis in adherent patients; PPIA, PP analysis in all patients-independent of adherence; RA, Rheumatoid arthritis; sd, Standard deviations.
Patient and Public Involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data Sharing Statement
The study remains in progress and the data are not currently available for sharing.
Ethics Approval Statement
This study is based on established database IADB.nl. Data are collected in accordance with the national and European guidelines on privacy requirements for handling human data. The authors have no ethical conflicts to disclose. Ethics approval is not needed and required for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Steenhuis reports personal fees from Menzis Zorgverzekeraar N.V., outside the submitted work; Other authors report no conflicts of interest in this work.
References
- 1.Organization WH. Cardiovascular diseases (CVDs): World Health Organization. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)). Accessed June 11, 2021.
- 2.Statistiek CBv. Deaths; underlying cause of death (shortlist), sex, age: statistics Netherlands. Available from: https://www.cbs.nl/en-gb/figures/detail/7052eng#. Accessed February 14, 2024.
- 3.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care. 2013;40(1):195–211. doi: 10.1016/j.pop.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–913. doi: 10.1038/nature06796 [DOI] [PubMed] [Google Scholar]
- 5.Foody JM, Joyce AT, Rudolph AE, et al. Persistence of atorvastatin and simvastatin among patients with and without prior cardiovascular diseases: a US managed care study. Curr Med Res Opin. 2008;24(7):1987–2000. doi: 10.1185/03007990802203279 [DOI] [PubMed] [Google Scholar]
- 6.Blais JE, Tong GKY, Pathadka S, et al. Comparative efficacy and safety of statin and fibrate monotherapy: a systematic review and meta-analysis of head-to-head randomized controlled trials. PLoS One. 2021;16(2):e0246480. doi: 10.1371/journal.pone.0246480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genootschap NH, En Innovatie K. Praktische Handleiding bij de NHG-Standaard CVRM (2019) [Practical Manual for the NHG-Standard CVRM (2019)]. Huisartsen Genootschap. 2019. [Google Scholar]
- 8.Helin-Salmivaara A, Lavikainen P, Aarnio E, et al. Sequential cohort design applying propensity score matching to analyze the comparative effectiveness of atorvastatin and simvastatin in preventing cardiovascular events. PLoS One. 2014;9(3):e90325. doi: 10.1371/journal.pone.0090325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foody JM, Joyce AT, Jeffers BW, et al. A large observational study of cardiovascular outcomes associated with atorvastatin or simvastatin therapy in hypertensive patients without prior cardiovascular disease. Am J Ther. 2011;18(2):110–116. doi: 10.1097/MJT.0b013e3181cf12d2 [DOI] [PubMed] [Google Scholar]
- 10.Heintjes EM, Hirsch MW, van der Linden MW, et al. LDL-C reductions and goal attainment among naive statin users in the Netherlands: real life results. Curr Med Res Opin. 2008;24(8):2241–2250. doi: 10.1185/03007990802264487 [DOI] [PubMed] [Google Scholar]
- 11.Blais JE, Ye X, Wan EYF, et al. Effectiveness of simvastatin versus gemfibrozil for primary prevention of cardiovascular events: a retrospective cohort study of 223,699 primary care patients. Clin Drug Investig. 2022;42(11):987–997. doi: 10.1007/s40261-022-01208-9 [DOI] [PubMed] [Google Scholar]
- 12.Neumann A, Maura G, Weill A, et al. Comparative effectiveness of rosuvastatin versus simvastatin in primary prevention among new users: a cohort study in the French national health insurance database. Pharmacoepidemiol Drug Saf. 2014;23(3):240–250. doi: 10.1002/pds.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J. 2006;151(2):273–281. doi: 10.1016/j.ahj.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Thomson NC, Charron CE, Chaudhuri R, et al. Atorvastatin in combination with inhaled beclometasone modulates inflammatory sputum mediators in smokers with asthma. Pulm Pharmacol Ther. 2015;31:1–8. doi: 10.1016/j.pupt.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Cui JY, Zhou RR, Han S, et al. Statin therapy on glycemic control in type 2 diabetic patients: a network meta-analysis. J Clin Pharm Ther. 2018;43(4):556–570. doi: 10.1111/jcpt.12690 [DOI] [PubMed] [Google Scholar]
- 16.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49(2):295–307. doi: 10.1093/rheumatology/kep366 [DOI] [PubMed] [Google Scholar]
- 17.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser ST, Schuiling-Veninga CC, Bos JH, et al. The population-based prescription database IADB.nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):285–292. doi: 10.1586/erp.13.20 [DOI] [PubMed] [Google Scholar]
- 19.Oktora MP, Denig P, Bos JHJ, et al. Trends in polypharmacy and dispensed drugs among adults in the Netherlands as compared to the United States. PLoS One. 2019;14(3):e0214240. doi: 10.1371/journal.pone.0214240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Bijlsma MJ, Bos JHJ, et al. Long-term comparative effectiveness of antihypertensive monotherapies in primary prevention of cardiovascular events: a population-based retrospective inception cohort study in the Netherlands. BMJ Open. 2023;13(8):e068721. doi: 10.1136/bmjopen-2022-068721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shau WY, Lai CL, Huang ST, et al. Statin adherence and persistence on secondary prevention of cardiovascular disease in Taiwan. Heart Asia. 2019;11(2):e011176. doi: 10.1136/heartasia-2018-011176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijlsma MJ, Janssen F, Hak E. Estimating time-varying drug adherence using electronic records: extending the proportion of days covered (PDC) method. Pharmacoepidemiol Drug Saf. 2016;25(3):325–332. doi: 10.1002/pds.3935 [DOI] [PubMed] [Google Scholar]
- 23.Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29(3):610–618. doi: 10.1097/HJH.0b013e328342ca97 [DOI] [PubMed] [Google Scholar]
- 24.Pouwels KB, Voorham J, Hak E, et al. Identification of major cardiovascular events in patients with diabetes using primary care data. BMC Health Serv Res. 2016;16:110. doi: 10.1186/s12913-016-1361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfian SD, Denig P, Coelho A, et al. Pharmacy-based predictors of non-adherence, non-persistence and reinitiation of antihypertensive drugs among patients on oral diabetes drugs in the Netherlands. PLoS One. 2019;14(11):e0225390. doi: 10.1371/journal.pone.0225390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9(3):e025806. doi: 10.1136/bmjopen-2018-025806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw. 2011;43(13):1–23. [Google Scholar]
- 28.Therneau T, Crowson C, Atkinson E. Using time dependent covariates and time dependent coefficients in the cox model. Survival Vignettes. 2017;2(3):1–25. [Google Scholar]
- 29.Steenhuis D, de Vos S, Bos J, et al. Role of traditional cardiovascular risk factors after initiation of statin therapy: a pharmlines inception cohort study. Cardiovasc Ther. 2022;2022:6587165. doi: 10.1155/2022/6587165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feingold KR. Triglyceride lowering drugs. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- 31.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial). Am J Cardiol. 2003;92(2):152–160. doi: 10.1016/S0002-9149(03)00530-7 [DOI] [PubMed] [Google Scholar]
- 32.Hirota T, Fujita Y, Ieiri I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin Drug Metab Toxicol. 2020;16(9):809–822. doi: 10.1080/17425255.2020.1801634 [DOI] [PubMed] [Google Scholar]
- 33.Pramanik S, Das AK, Chakrabarty M, et al. Efficacy of alternate-day versus everyday dosing of atorvastatin. Indian J Pharmacol. 2012;44(3):362–365. doi: 10.4103/0253-7613.96326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan J, Sun J, Huang L, et al. Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res. 2014;92(5):634–640. doi: 10.1002/jnr.23329 [DOI] [PubMed] [Google Scholar]
- 35.Hoogwerf BJ. Statins may increase diabetes, but benefit still outweighs risk. Cleve Clin J Med. 2023;90(1):53–62. doi: 10.3949/ccjm.90a.22069 [DOI] [PubMed] [Google Scholar]
- 36.Angelidi AM, Stambolliu E, Adamopoulou KI, et al. Is atorvastatin associated with new onset diabetes or deterioration of glycemic control? Systematic review using data from 1.9 million patients. Int J Endocrinol. 2018;2018:8380192. doi: 10.1155/2018/8380192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin J, Yoon HY, Lee YM, et al. Inhaled corticosteroids in COPD and the risk for coronary heart disease: a nationwide cohort study. Sci Rep. 2020;10(1):18973. doi: 10.1038/s41598-020-74854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinbongard P, Bøtker HE, Ovize M, et al. Co-morbidities and co-medications as confounders of cardioprotection-Does it matter in the clinical setting? Br J Pharmacol. 2020;177(23):5252–5269. doi: 10.1111/bph.14839 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study remains in progress and the data are not currently available for sharing.